Abstract
OBJECTIVES
To evaluate the comparative effectiveness of antiviral drugs in adults with chronic hepatitis B monoinfection for evidence-based decision-making.
METHODS
A systematic review of randomized controlled clinical trials (RCTs) published in English. Results after interferon and nucleos(t)ides analog therapies were synthesized with random-effects meta-analyses and number needed to treat (NNT).
RESULTS
Despite sustained improvements in selected biomarkers, no one drug regimen improved all intermediate outcomes. In 16 underpowered RCTs, drug treatments did not reduce mortality, liver cancer, or cirrhosis. Sustained HBV DNA clearance was achieved in one patient when two were treated with adefovir (NNT from 1 RCT = 2 95%CI 1;2) or interferon alfa-2b (NNT from 2 RCTs = 2 95%CI 2;4), 13 with lamivudine (NNT from 1 RCT = 13 95%CI 7;1000), and 11 with peginterferon alfa-2a vs. lamivudine (NNT from 1 RCT = 11 95%CI 7;25). Sustained HBeAg seroconversion was achieved in one patient when eight were treated with interferon alfa-2b (NNT from 2 RCTs = 8 95%CI 5;33) or 10—with peginterferon alfa-2b vs. interferon alfa-2b (NNT from 1 RCT = 10 95%CI 5;1000). Greater benefits and safety after entecavir vs. lamivudine or pegylated interferon alfa-2b vs. interferon alfa-2b require future investigation of clinical outcomes. Adverse events were common and more frequent after interferon. Treatment utilization for adverse effects is unknown.
CONCLUSIONS
Individual clinical decisions should rely on comparative effectiveness and absolute rates of intermediate outcomes and adverse events. Future research should clarify the relationship of intermediate and clinical outcomes and cost-effectiveness of drugs for evidence-based policy and clinical decisions.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-010-1569-5) contains supplementary material, which is available to authorized users.
KEY WORDS: antiviral agents/adverse effects, antiviral agents/therapeutic use, hepatitis B/therapy, treatment outcome, cost-benefit analysis, decision trees
INTRODUCTION
Chronic hepatitis B infection (CHB) afflicts over 1 million adults in the United States alone and presents a significant public health problem1. Treatment goals include prevention of cirrhosis, liver decompensation, and hepatocellular carcinoma2,3 that cause an estimated 2,000 to 4,000 deaths annually; over $1 billion is spent per year on hepatitis B-related hospitalizations. Although we do not have good evidence that antiviral drugs can prevent overall mortality, liver-specific mortality, or development of hepatocellular carcinoma in patients with chronic hepatitis B2,4, therapeutic nihilism is difficult to justify as this is a potentially dangerous disease5. Physicians and patients have to make decisions based on benefits of antiviral drugs on virologic, biochemical, and histologic markers including hepatitis B virus (HBV) deoxyribonucleic acid (DNA) clearance, HBeAg loss or seroconversion, decreases in alanine aminotransferase (ALT) levels, and improvement in liver histology1,2,4,6. The National Institutes of Health (NIH) consensus development conference synthesized evidence from available research suggesting feasible intermediate endpoints3,7 and harms of interferon8 and nucleos(t)ides analog therapies9.
The aim of this report is to present a comprehensive analysis of the benefits and harms of antiviral drug therapies for HBeAg positive CHB mono infection in terms of available intermediate outcomes. In addition to the systematic review on the management of chronic HBV infection,2,4 we have created a comprehensive evidence map with pooled risk differences, the number needed to treat to achieve favorable changes in biomarkers, number of events that are attributable to the drugs, and absolute rates of clinical events after available treatments. We included only the results from RCTs because such studies prove the causal effects of the treatments10. Comparative effectiveness of antiviral drugs should provide the basis for evidence-based decision making in clinical settings11,12. This review was commissioned as background material for the NIH Consensus Development Conference on Management of Chronic Hepatitis B in Adults2.
METHODS
In our analytical framework we examined pooled rates, relative risks, and absolute risk differences in intermediate outcomes and clinical harms after antiviral drugs. We then calculated attributable events13,14.
We searched MEDLINE® via PubMed®, the Cochrane Library15, and Medwatch16 to find randomized controlled clinical trials of adults with CHB published in English between 1989 and September 2008 that reported mortality, incidence of hepatocellular carcinoma, liver failure, prevalence and incidence of cirrhosis, HBeAg or HBsAg presence or seroconversion, viral load of HBV deoxyribonucleotide acid (HBV DNA), ALT levels, histological necroinflammatory and fibrosis scores17, and adverse events after antiviral drugs, including interferon alfa-2b, pegylated interferon alfa-2a, lamivudine, adefovir, entecavir, telbivudine, and tenofovir. We used the following definitions for intermediate biomarkers:
Complete response (resolved hepatitis B) included HBsAg loss or seroconversion in combination with undetectable HBV DNA and normal ALT.
ALT normalization.
Virological outcomes included HBsAg and HBeAg clearance or seroconversion, and HBV DNA clearance.
Histological outcomes included improvement in necroinflammatory scores without worsening in fibrosis scores.
Resistance was defined as worsening of histological scores or persistent HBV DNA load, or rates of genetic mutations.
We prioritized HBsAg loss or seroconversion (diagnostic marker of resolved hepatitis) and improvement in histological necroinflammatory scores without worsening in fibrosis scores (diagnostic markers of liver cirrhosis)3,18. We focused on sustained improvement in biomarkers at follow up off the treatments3,19. Our review focuses on HBeAg-positive patients since we previously reported effects of antiviral drugs on HBeAg-negative patients4.
We analyzed study quality using the following criteria: subject selection, length and loss of follow-up, adjustment for confounding factors in observational studies and intention to treat principle in clinical trials, masking treatment status, randomization scheme and adequacy, allocation concealment, and justification of sample sizes in RCTs10,20.
The protocol for the meta-analyses was created according to recommendations for meta-analysis of RCTs21,22. Meta-analysis was used to assess the consistency of the association between treatments and outcomes using the DerSimonian–Laird inverse-variance weighting method, and random effects models23. We chose the random effects models to incorporate differences across trials in patient populations, baseline rates of the outcomes, dosing of drugs, and other factors in the pooled analysis. For pooled relative risks and absolute risk differences we excluded trials with no events in both groups and added a correction coefficient of 0.5 in the trials with no events only in one group. Statistical heterogeneity was assessed by calculating the percent of the total variance due to between-study variability (I2 statistic) and Chi square tests24,25. Higher I2 values indicate greater between-study heterogeneity. All calculations were performed with STATA software 10.1 (The Statistics/Data Analysis StataCorp. Stata statistical software: Release 9.2. College Station, Texas). We tested the consistency of the results by comparing the direction and strength of the association. We assumed that publication bias was present and did not conduct formal statistical tests to measure it26.
We assessed the level of evidence based on modified GRADE Working Group criteria27,28. We assigned low level of evidence to data from small RCTs, or from RCTs with serious flaws in design/analysis, and from post hoc subgroup analysis. We assigned moderate level of evidence when a single large multinational study or several small RCTs reported consistent effects of the same drugs. We assigned a high level of evidence to data from multiple high quality studies that reported consistent sustained effects at least 6 months post therapy29.
We used pooled absolute risk difference to calculate the number needed to treat and the number of events attributable to treatment per 1000 patients29,30. We calculated means and 95% confidence intervals for number needed to treat as reciprocal to pooled absolute risk difference when absolute risk difference was significant 31. We calculated means and 95% confidence intervals for treatment eventsper 1000 treated, multiplying pooled absolute risk difference by 1000. For clinical decision making we also calculated pooled rates of the outcomes and all reported in the trials adverse effects32 with random effects models using MetaAnalyst software33.
We based the estimated medication cost needed to achieve one specified event on recommendations for reported weekly doses and length of treatment found in the Red Book: Pharmacy’s Fundamental Resource®34-36.
Role of the Funding Source
The Agency for Healthcare Research and Quality suggested the initial questions and provided copyright release for this manuscript. The funding source had no role in the literature searches, data analysis, conduct of the study, preparation of the review, or interpretation of the results. The funding source reviewed and approved the submitted manuscript without revisions.
RESULTS
We identified 93 relevant articles representing RCTs of interferon alfa-2b37-68, peginterferon alfa-2a69-73, adefovir74-85, peginterferon alfa-2b86-97, entecavir98-103, lamivudine40,43,71,72,84,104-119, or telbivudine85,104,120,121. We also reviewed one recently published study of tenofovir122. The studies enrolled predominately HBeAg-positive individuals. We excluded eleven reports that described outcomes in HBeAg-negative patients47,50,52,55,57,67,69,71,74-76,119. We identified 16 RCTs (4431 patients) that analyzed the effects of antiviral drugs on clinical outcomes and concluded that drug treatments did not improve clinical outcomes of chronic hepatitis B infection. Clinical outcomes were rarely reported, and the trials were underpowered to detect differences in mortality, liver cancer or cirrchosis2,4. The results of meta-analyses with pooled relative risk, absolute risk differences, and weights from random effects models are presented in Online Appendix 1.
We summarized the results of the RCTs into an evidence map (Table 1). No one treatment improved all outcomes, and there was limited evidence on comparative effects.
Table 1.
Absolute Risk Difference in Tested Intermediate Outcomes After Antiviral Drugs for Chronic Hepatitis B in Adults
| Comparison | HBsAg [RCTs/patients] | HBeAg [RCTs/patients] | HBV DNA clearance [RCTs/patients] | Histology improved [RCTs/patients] | ALT normal [RCTs/patients] | Relapse/Mutation [RCTs/patients] |
|---|---|---|---|---|---|---|
| Adefovir vs. placebo | SC: NS [1/120] L | Loss: 0.11 (0.06; 0.16) [2/995] M | 0.38 (0.23; 0.53) [4/1002] H | Fibrosis: 0.20 (0.14; 0.26) [2/699] M | 0.40 (0.30; 0.49) [5/1342] | NS [2/1055] L |
| SC: 0.05 (0.01; 0.09) | 20.41 (6.79; 61.32) [4/1002] | Necroinflammation scores: | 2.97 (2.38; 3.69) H | NS* [1/140] L | ||
| [2/700] H | 0.59 (0.46; 0.72)* [1/120] L | 0.26 (0.17; 0.34) [3/819] H | 0.26 (0.19; 0.33)* [2/600] M | |||
| LAM vs. placebo | Loss: NS [1/175] L | Loss: 0.13 (0.04; 0.22) [4/1349] M | 0.48 (0.31; 0.66) [7/1305]H | Necroinflammation: | 0.22 (0.13; 0.31) [7/1602]M | YMDD mutation: 0.43 (0.38; 0.48) [2/826] H |
| NS* [3/1068] L | 0.15 (0.05; 0.24)* [2/318] M | 3.79 (2.71; 5.30) H | 2.09 (1.60; 2.74) M | 2.42 (1.94; 3.01) M | ||
| SC: 0.05 (0.001; 0.10) [6/1638] H | 0.08 (0.00; 0.15)* [1/136] L | 0.25 (0.13; 0.38) [4/580] M | 0.21 (0.04; 0.38)* [1/136] L | |||
| 1.70 (1.05; 2.74) | ||||||
| NS * [2/318] L | ||||||
| Adefovir + LAM vs. LAM | Loss: NS [1/39] L | Loss: 0.12 (0.03; 0.21) [2/134] M | 0.25 (0.10; 0.39) | 0.32 (0.13; 0.52) [2/13] M | YMDD:-0.33 (-0.50; -0.17) [1/95] L | |
| SC: NS [2/134] L | [2/134] L | Wild type mutation: NS [1/95] L | ||||
| Adefovir + LAM vs. adefovir | Loss: NS [1/39] L | Loss: NS [1/39] L | NS:[1/39] L | NS [1/39] L | ||
| SC: NS[1/39] L | ||||||
| Entecavir vs. LAM | Loss: NS [2/1117] M | Loss: NS [3/1112] L | 0.23 (0.11; 0.35) [4/1636] | Necroinflammation: 0.14 (0.04; 0.24) [3/1633] M | 0.22 (0.11; 0.32) [6/2423] | NS [0/1347] L |
| SC: NS [1/408] L | SC: NS [3/1185] M | 1.64 (1.22; 2.22) | Fibrosis: NS [2/995] M | 1.62 (1.28; 2.06) H | -0.16 (-0.20; -0.12)* [1/709] L | |
| L/M NS*1/709] L | ||||||
| LAM vs. adefovir | Loss: NS [1/38] L | -0.26 (-0.47; -0.06) [1/38] L | -0.42 (-0.67; -0.18) [1/38] L | |||
| SC:NS [1/38] L | ||||||
| LAM vs. telbivudine | Loss: NS [1/63] L | -0.30 (-0.55; -0.04) [1/63] L | NS [1/85] L | NS [1/63] L | ||
| SC: NS [1/63] L | ||||||
| Telbivudine vs. adefovir | Loss: NS [1/135] L | 0.28 (0.12; 0.44) | NS [1/135] L | |||
| SC: 6.03 (2.20; 16.52) [1/136] L | [1/136] L | |||||
| Telbivudine + LAM vs. LAM | Loss: NS[1/60] L | NS [1/60] L | NS [1/101] L | NS [1/60] L | ||
| SC: NS [1/60] L | ||||||
| Telbivudine + LAM vs. telbivudine | Loss: NS [1/85] L | NS [1/85] L | NS [1/101] L | NS [1/85] L | ||
| SC: NS [1/85] L | ||||||
| Interferon alfa-2b vs. placebo | Loss: NS [3/166] M | Loss: 0.55 (0.29; 0.81) | 0.45 (0.22; 0.68) | Total scores: NS* [1/40] L | 0.31 (0.17; 0.44)* [2/131] M | Relapse: NS* [5/378] H |
| NS* [4/247] L | [1/40] L | [1/34] L | HAI scores:0.24 (0.00; 0.48) [1/72] L | |||
| SC: NS* [2/82] L | 2.52 (1.55; 4.10) | 0.44 (0.27; 0.60)* [3/168] L | ||||
| 0.28 (0.07; 0.50)* [3/351] M | ||||||
| SC:NS [1/40] L | ||||||
| 0.12 (0.03; 0.21) * [2/304] M | ||||||
| Interferon alfa-2b + lamivudine vs. placebo | Loss: 0.06 (0.00; 0.13) [1/119] L | Loss: NS [1/118] L | 0.48 (0.33; 0.63) [1/119] L | HAI scores | NS [1/119] L | YMDD mutation |
| NS* [1/119] L | NS* [2/450] M | NS* [1/119] L | NS [1/119] L | NS [1/118] L | ||
| SC: NS [1/119] L | ||||||
| NS* [2/450] L | ||||||
| Interferon alfa-2b + corticosteroid vs. no treatment | Loss: 0.11 (0.02; 0.20) [2/103] M | 0.25 (0.04; 0.46) [1/34] L | 0.25 (0.06; 0.43)* [1/87] L | Relapse | ||
| NS* [2/121] M | NS* [1/87] L | |||||
| Interferon alfa-2b vs. LAM | Loss: NS [1/151] L | NS [1/76] L | Knodell scores: NS* [1/151] L | NS [1/151] L | YMDD mutation | |
| NS* [2/625] M | NS* [1/151] L | NS* [2/151] L | -0.23 (-0.33; -0.14)* [1/151] L | |||
| SC: NS[1/151] L | ||||||
| NS* [3/776] M | ||||||
| Interferon Alfa 2b + LAM vs. interferon alfa-2b | Loss: NS [1/144] L | NS [1/144] L | HAI scores | NS [1/144] L | YMDD mutation | |
| NS* [2/347] M | NS* [2/278] L | 0.54 (0.28; 0.79) [1/48] L | NS* [2/192] L | NS* [1/144] L | ||
| SC: NS [1/144] L | Knodell scores | |||||
| NS* [3/482] L | NS* [1/144] L | |||||
| Interferon alfa-2b + LAM vs. LAM | Loss: | Loss: NS [3/414] M | NS [7/786] H | HAI scores | NS [5/626] M | Relapse: NS [4/326] H |
| NS [2/262] L | NS* [5/1167] M | NS* [4/365] M | NS [3/327] M | NS* [6/751] M | NS* [2/158] L | |
| NS* [3/495] L | SC: NS [4/565] H | necroinflammation NS [2/389] L | YMDD mutation: | |||
| NS* [3/490] M | Knodell scores | -0.18 (-0.35; -0.01) [6/721] M | ||||
| NS* [1/157] L | 0.42 (0.16; 1.09) M | |||||
| -0.23 (-0.32; -0.14)* [1/157] L | ||||||
| Interferon alfa-2b + corticosteroid vs. IFN alfa-2b | Loss: NS [2/125] M | Loss: NS [2/77] L | NS [2/77] L | NS* [3/170] M | Relapse: NS* [2/141] L | |
| NS* [3/141] L | NS* [3/122] L | NS* [6/322] H | ||||
| SC: NS* [2/85] L | ||||||
| Peginterferon alfa-2a vs. LAM | Loss: 0.08 (0.01; 0.16) M | -0.15 (-0.22; -0.07) [1/543] M | Necroinflammation | -0.29 (-0.42; -0.17) [2/905] | YMDD mutation | |
| 0.13 (0.05; 0.20)* [1/543] M | 0.12 (0.02; 0.22) [1/552]* L | 0.57 (0.46; 0.70) [2/905] M | -0.25 (-0.31; -0.20) [1/543] L | |||
| SC: NS [1/543] L | 0.09 (0.04; 0.14) [1/543]* L | Fibrosis: NS* [1/552] L | 0.13 (0.07; 0.20)* [2/905] H | |||
| 0.13 (0.06; 0.20) [1/814]* M | HAI: NS [2/1366]* M | |||||
| Peginterferon alfa-2a + LAM vs. LAM | Loss: NS [1/543] L | 0.29 (0.21; 0.37) [1/543] M | Total scores: NS [2/1366]* H | -0.20 (-0.29; -0.10) [2/905] H | YMDD mutation | |
| 0.07 (0.00; 0.15)* [1/543] M | 0.09 (0.04; 0.13) [1/543]* L | 0.13 (0.06; 0.19) [2/905]* H | -0.22 (-0.28; -0.16) [1/543] L | |||
| SC: NS [1/543] L | ||||||
| 0.08 (0.01; 0.15) *[1/814] L | ||||||
| Peginterferon alfa-2a + LAM vs. peginterferon alfa-2a | Loss: NS [1/542] L | 0.44 (0.36; 0.51) [1/542] M | Total scores: NS [1/96]* L | NS [1/542] L | YMDD mutation: 0.03 (0.01; 0.06) [1/542] L | |
| NS [1/542]* M | NS[1/542]* L | NS [1/542]* L | ||||
| SC: NS [1/542] L | ||||||
| NS [1/814]* L | ||||||
| Peginterferon alfa-2b vs. interferon alfa-2b | SC: NS* [1/230] | Loss: 0.10 (0.00; 0.21) [1/230]* L | NS [1/230]* L | |||
| Peginterferon alfa-2b + LAM vs. LAM | Negative HBVDNA + HBsAg SC | Loss: 0.34 (0.16; 0.52) [1/100] M | NS [1/100] L | HAI scores | NS [1/100] L | NS: [1/100]* L |
| 0.32 (0.14; 0.50) [1/100] | SC: 0.32 (0.14; 0.50) | NS [1/100]* L | NS: [1/100] L | YMDD mutation: NS [1/100] L | ||
| [1/100] L | ||||||
| NS: [1/100]* L | ||||||
| Peginterferon alfa-2b + LAM vs. peginterferon alfa-2b | Loss: NS [1/307] | Loss: 0.12 (0.01; 0.22) [1/307] M | fibrosis scores: NS [1/307]* L | 0.14 (0.03; 0.24) [1/307] L | YMDD mutation: 0.09 (0.04; 0.14) [1/307] L | |
| NS [2/614]* M | necroinflammation scores: NS [1/307] L | NS [1/307]* L | ||||
| SC: NS [1/307]L | ||||||
| NS: [1/307]* L |
SC = seroconversion; NS = not significant; italic = relative risk; * = outcomes off treatments; LAM = lamivudine
Level of evidence: L = low; M = moderate; H = high
Only interferon therapies resulted in HBsAg loss, which is one of the criteria of resolved hepatitis B. One RCT demonstrated a significant increase in HBsAg loss at the end of administration of interferon alfa-2b60 and after interferon alfa-2b with corticosteroid. Pooled analysis of two RCTs that compared steroid pretreatment followed by interferon alfa-2b to no antiviral drugs found a significant increase in HBsAg loss at the end of treatment46,60. Combined therapy of interferon alfa-2b with lamivudine resulted in HBsAg loss and HBV DNA clearance in one RCT43. Peginterferon alfa-2b combined with lamivudine when compared to lamivudine alone increased HBV DNA clearance and HBsAg seroconversion95. All treatments failed to increase rates of post-treatment HBsAg loss at follow up off drug administration (range 8-48 weeks off treatment)37,43,59,63,67,113,116.
Monotherapy with oral antiviral drugs resulted in HBV DNA clearance and HBeAg seroconversion compared to placebo. For instance, lamivudine at the end of monotherapy compared to placebo increased the rates of HBeAg loss40,43,113,117 and HBeAg seroconversion,40,43,113,117,118,123 but it also increased rate of YMDD mutations (mutation in amino acid sequence tyrosine, methionine, aspartate, aspartate)43,109. Adefovir improved fibrosis and necroinflammatory scores74,75,77, and HBeAg loss77,78 and seroconversion77,78.
Evidence on the comparative effectiveness of antiviral drugs was limited. Some antiviral drugs demonstrated superior effects on intermediate markers when compared to each other but no one regime demonstrated superior benefits on all biomarkers. For instance, lamivudine compared to peginterferon alfa-2a improved HBV DNA clearance72 but reduced HBeAg loss72. Entecavir compared to lamivudine improved necroinflammation in three studies98,99,102 with no differences on fibrosis scores in two studies that examined this biomarker99,102. Entecavir compared to lamivudine also increased the rates of HBV DNA clearance98-100,103, with no differences in HBsAg99,103 and HBeAg loss99,100,102, or HBsAg103 and HBeAg99,100,102 seroconversion. Low levels of evidence suggested greater effects of telbivudine on HBV DNA clearance when compared to lamivudine104, or to adefovir85 with no differences in other outcomes. Moderate evidence from two RCTs suggested better effects from combined therapy with adefovir plus lamivudine on HBeAg loss82,84 and ALT normalization82,84 when compared to lamivudine alone, but not adefovir alone. Limited evidence from one recently published RCT122 suggested superior effects from tenofovir compared to adefovir on viral suppression, normalized ALT levels and loss of HBsAg at the end of 48 weeks of treatment.
Evidence about the comparative effectiveness of combined therapies is very limited. We found only one study that demonstrated peginterferon alfa-2a combined with lamivudine, compared to monotherapy with either lamivudine or peginterferon alfa-2a improved HBV DNA clearance72.
Sustained Effects Few of the drugs evaluated demonstrated sustained benefits superior to placebo. Interferon alfa-2b improved sustained HBV DNA and HBeAg clearance40,59,63, seroconversion40,59, and ALT normalization60,63. Adefovir improved sustained ALT normalization75,78 and HBV DNA clearance75.Pegylated interferon alfa-2a demonstrated superior sustained effects when compared to lamivudine. Pegylated interferon alfa-2a vs. lamivudine improved sustained HBV DNA72 and HBeAg clearance72, seroconversion72, and ALT normalization71,72. Pegylated interferon alfa-2a plus lamivudine improved sustained HBV DNA72 and HBeAg seroconversion72 and ALT normalization71,72 compared to lamivudine, but not pegylated interferon alfa-2 monotherapy.
Number Needed to Treat The number needed to treat to achieve one event of improved biomarker was fewer than ten for most of the treatments (Table 2). Examining how many patients should be treated to improve liver histology (diagnostic markers of liver cirrhosis), we found that effectiveness is comparable among antiviral drugs. For instance, monotherapy with interferon alfa-2b was needed in four patients to achieve reduction in liver histological scores in one of them67. We would need to treat two patients with combined therapy of interferon alfa-2b and lamivudine to achieve improvement in histological scores in one of them44. Adefovir administration in four74,75,77 or entecavir in seven patients98,99,102 would result in reduced necroinflammation in one patient. Peginterferon alfa-2a administered in eight patients would result in sustained depression of necroinflammation in one patient when compared to lamivudine71. Adefovir is the only drug that reduced fibrosis scores; administration to four patients would result in reduction of fibrosis scores in one patient74,77. Sustained HBV DNA clearance was associated with favorable clinical outcomes in observational studies18,19,124. According to our analysis, sustained HBV DNA clearance in one patient would be possible if two were treated with interferon alfa-2b or adefovir or 13 with lamivudine. Sustained HBeAg loss in one patient would be achieved if four were treated with interferon alfa-2b. We would need to treat eight patients with interferon alfa-2b to have one case of sustained HBeAg seroconversion. Sustained ALT normalization could be seen in one patient when three were treated with interferon alfa-2b or four with adefovir. Viral resistant YMDD mutations would be detected in one from two treated with lamivudine patients.
Table 2.
Number Need to Treat to Have One Additional Event After Antiviral Treatments of Chronic Hepatitis B infection; Results from Randomized Controlled Clinical Trials
| Drug | Outcome | Number needed to treat (95%CI) |
|---|---|---|
| End of treatment | ||
| Monotherapy vs. placebo | ||
| Adefovir | Reduced fibrosis [2/699] M | 5(4;7) |
| Reduced necroinflammatory scores [3/819] H | 4(3;6) | |
| HBeAg loss [2/995] M 110 | 9(6;17) | |
| Seroconversion [2/700] H | 20(11;100) | |
| Lamivudine | HBeAg loss [4/1349] M | 8(5;25) |
| YMDD mutations [2/826]H | 2(2;3) | |
| Interferon alfa-2b | Histological scores [1/72] L | 4(2;1000) |
| HBV DNA clearance [1/34] L | 2(1;5) | |
| HBeAg loss [1/40] L | 2(1;3) | |
| Combined therapy vs. placebo | ||
| Interferon alfa-2b+lamivudine | HBsAg Loss [1/119] L | 17(8;333) |
| HBV DNA clearance [1/119] L | 2(2;3) | |
| Interferon alfa-2b+ corticosteroid | HBsAg Loss [2/103] M | 9(5;50) |
| HBV DNA clearance [1/34] L | 4(2;25) | |
| Monotherapy vs. active control | ||
| LAM vs. peginterferon alfa-2a | HBV DNA clearance [1/543] M | 7(5;14) |
| YMDD mutation [1/543] L | 4(3;5) | |
| HBeAg Loss [1/543] M | -13(-100;-6) | |
| Entecavir vs. lamivudine | Reduced necroinflammation [3/1633] M | 7(4;25) |
| Adefovir vs. lamivudine | HBV DNA clearance [1/38] L | 4(2;167) |
| ALT normalization [1/38] L | 2(1;6) | |
| Telbivudine vs. lamivudine | HBV DNA clearance [1/136] L | 3(2;250) |
| HBV DNA clearance [1/136] L | 4(2;8) | |
| Combined therapy vs. active control | ||
| Adefovir + LAM vs. LAM | HBV DNA clearance [2/134] L | 4(3;10) |
| HBeAg Loss [2/134] M | 8(5;33) | |
| ALT normalization [2/13] M | 3(2;8) | |
| YMDD mutation [1/95] L | -3(-6;-2) | |
| Interferon alfa-2b + LAM vs. interferon alfa-2b | Improved HAI scores [1/48] L | 2(1;4) |
| Interferon alfa-2b+ LAM vs. LAM | YMDD mutation [6/721] M | -6(-100;-3) |
| Peginterferon alfa-2a+LAM vs. LAM | HBV DNA clearance [1/543] M | 3(3;5) |
| YMDD mutation [1/543] L | -5(-10;-3) | |
| Peginterferon alfa-2a+LAM vs. peginterferon alfa-2a | HBV DNA clearance [1/542] M | 2(2;3) |
| YMDD mutation [1/542] L | 33(17;100) | |
| Peginterferon alfa-2b+LAM vs. LAM | HBV DNA clearance combined with HBsAg seroconversion [1/100]L | 3(2;7) |
| HBeAg Loss [1/100] M | 3(2;6) | |
| HBeAg seroconversion [1/100] L | 3(2;7) | |
| Peginterferon alfa-2b+LAM vs. peginterferon alfa-2b | HBeAg Loss [1/307] M | 8(5;100) |
| ALT normalization [1/307] L | 7(4;33) | |
| YMDD mutation [1/307] L | 11(7;25) | |
| Off treatment | ||
| Monotherapy vs. placebo | ||
| Adefovir | HBV DNA clearance [1/120] L | 2(1;2) |
| ALT normalization [2/600] M | 4(3;5) | |
| Lamivudine | HBV DNA clearance [1/136] L | 13(7;1000) |
| ALT normalization [1/136] L | 5(3;25) | |
| Interferon alfa-2b | HBV DNA clearance [3/168] L | 2(2;4) |
| HBeAg loss [3/351] M | 4(2;14) | |
| HBeAg seroconversion [2/304] M | 8(5;33) | |
| ALT normalization [2/131] M | 3(2;6) | |
| Combined therapy vs. placebo | ||
| Interferon alfa-2b+ corticosteroid | ALT normalization [1/87] L | 4(2;17) |
| Monotherapy vs. active control | ||
| Interferon alfa-2b vs. LAM | YMDD mutation [1/151] L | -4(-7;-3) |
| Peginterferon alfa-2a vs. LAM | Reduced necroinflammation [1/552] L | 8(5;50) |
| HBV DNA clearance [1/543] L | 11(7;25) | |
| HBeAg loss [1/543] M | 8(5;17) | |
| ALT normalization [2/905] H | 8(5;14) | |
| Peginterferon alfa-2b vs. interferon alfa-2b | HBeAg Loss [1/230] L | 10(5;1000) |
| Entecavir vs. LAM | Relapse [1/709] L | 6(5;8) |
| Combined therapy vs. active control | ||
| I nterferon alfa-2b+ LAM vs. LAM | Mutation [1/157] L | -4(-7;-3) |
| Peginterferon alfa-2a+LAM vs. LAM | HBV DNA clearance [1/543] L | 11(8;25) |
| HBeAg loss [1/543] M | 14(7;1000) | |
| HBeAg seroconversion [1/814] L | 13(7;100) | |
| ALT normalization [2/905]H | 8(5;17) | |
abbreviation: LAM- lamivudine; negative number needed to treat demonstrate reduced rates of the events after active vs. control treatments
Medication Cost Finite courses of interferon were more expensive than oral drugs. Among interferon formulations, the cost of pegylated interferon alfa-2b administration was lower when compared to interferon alfa-2b or pegylated interferon alfa-2a. We estimate an average cost per patient34 of about $16,176 for 48 weeks of pegylated interferon alfa-2b administration, versus $60,390 for 24 weeks of interferon alfa-2b administration, or $97,065 for 48 weeks of pegylated interferon alfa-2a administration. Treatments with oral antiviral drugs are less expensive, averaging around $17,302 for 96 weeks of adefovir administration, $1,565 for 52 weeks of lamivudine administration, $8,274 for 52 weeks of entecavir administration, and $7,644 for 52 weeks of telbivudine administration.Sustained HBeAg loss in one patient can cost $161,760 if treated with peginterferon alfa-2b, or $215,681 if treated with interferon alfa-2b, and treatment with peginterferon alfa-2a alone or combined with lamivudine will cost to three to four times more. Sustained HBeAg seroconversion in one patient would cost more than $500,000 with interferon alfa-2b and more than $770,000 with peginterferon alfa-2a combined with lamivudine. Actual costs may be less due to possible price discounts, but relative differences would remain the same.
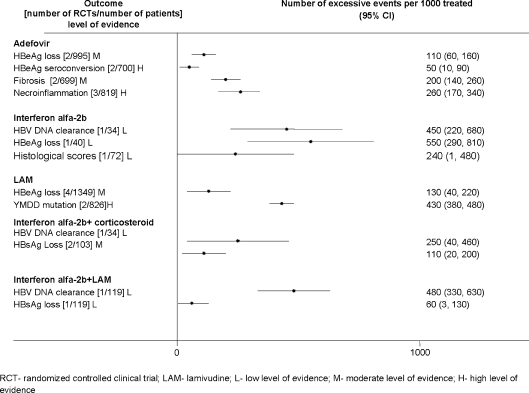
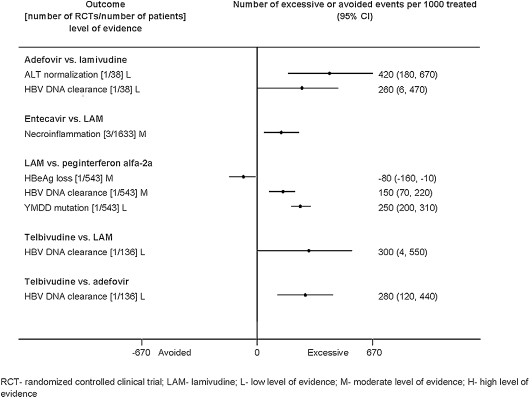
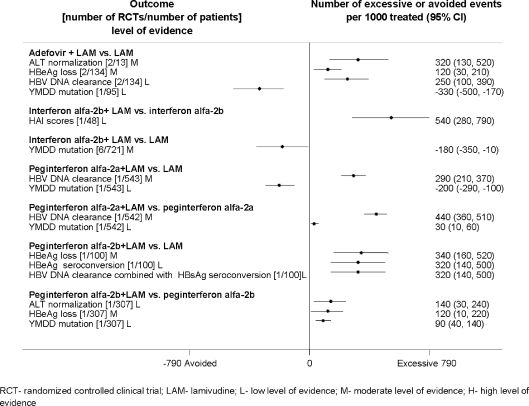
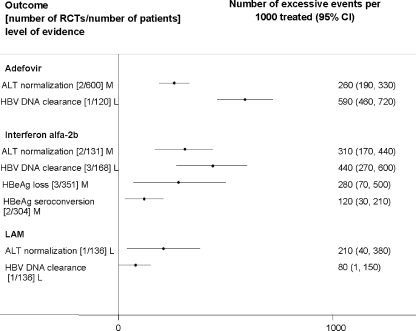
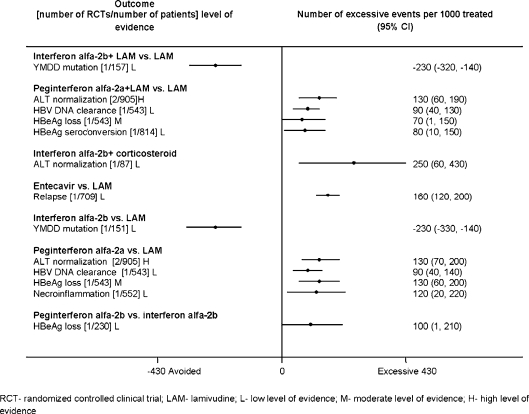
Attributable Events The number of events that were attributable to treatment with antiviral drugs varied across treatments (Figs. 1, 2, 3, 4, 5). Compared to placebo, interferon alfa-2b alone or combined with lamivudine resulted in greater improvement in histological scores and HBV DNA clearance (Fig. 1). HBV DNA clearance was greater after adefovir treatment when compared to lamivudine and greater after telbivudine when compared to lamivudine or adefovir (Fig. 2). Improvement in histological scores was attributable only to interferon alfa-2b combined with lamivudine therapy when compared to interferon monotherapy (Fig. 3). One RCT demonstrated that 320 cases of resolved hepatitis B per 1000 treated would be attributable to combined administration of peginterferon alfa-2b with lamivudine vs. lamivudine alone. Sustained HBV DNA clearance in more than 400 per 1,000 treated was attributable to adefovir or interferon alfa-2b administration when compared to placebo (Fig. 4). Sustained intermediate outcomes could be attributed to the examined treatments when compared to active control in less than 300 per 1,000 treated patients (Fig. 5).
Figure 1.
Results from randomized controlled clinical trials that compared active treatments to placebo at the end of the treatment.
Figure 2.
Results from randomized controlled clinical trials that compared active mono-treatments at the end of the treatment.
Figure 3.
Results from randomized controlled clinical trials that compared combined therapy vs. monotherapy at the end of the treatment.
Figure 4.
Sustained results from randomized controlled clinical trials that compared active treatments to placebo at follow up off the treatment.
Figure 5.
Sustained results from randomized controlled clinical trials that compared active treatments at follow up off the treatment.
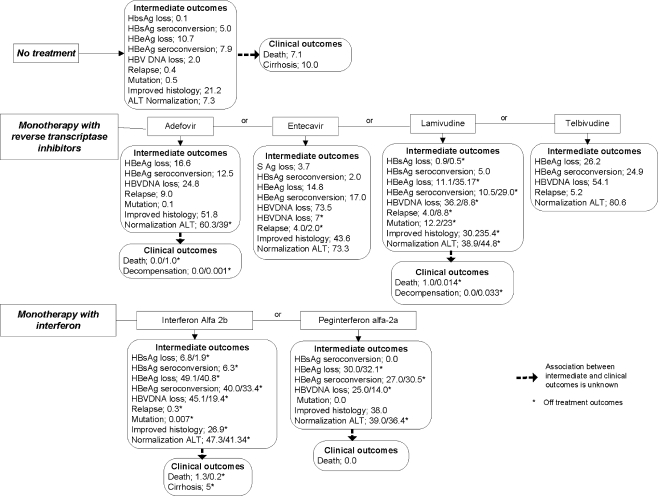
Absolute Rates of the Outcomes Physicians and patients make individual decisions based on known average probabilities of clinically important benefits and harms125. Probabilities vary depending on individual patient characteristics that can influence the expected treatment effects126,127. We have synthesized the probabilities of clinical and intermediate outcomes in HBeAg positive patients that participated in RCTs (Fig. 6). We hesitated to use indirect statistical comparisons that have not been examined in head-to-head RCTs. For example, mortality has never been examined in placebo controlled RCTs of pegylated interferon alfa-2a; therefore, lower rates of death after active drug (0%) vs. placebo (7.1%) could not provide good evidence of better survival. However, the rates of patient outcomes after placebo could provide a reasonable estimation of baseline risk in adults with CHB. Published studies examined the effects of antiviral drugs only on selected outcomes; none of the RCTs reported all outcomes. Analyzing the rates of all outcomes from different RCTs can give a more complete estimation of drug effects. Combined treatments did not result in better outcomes when compared to monotherapy. Relapse and treatment failure were more common after active treatments than after placebo128,129. The most common adverse events during antiviral therapy included flu-like symptoms after interferon, fatigue, headache, abdominal pain, nausea, diarrhea, or laboratory abnormalities, which were reported in over 50% of patients2,4. Absolute rates of adverse events (in >10% of the patients) after placebo and active treatments are presented in Online Appendix Table 2. Direct comparisons in published RCTs reported non significant differences in serious adverse events and withdrawal rates when compared to placebo2. Laboratory toxicity after adefovir restricted usage of the drug in patients with impaired renal function2. Entecavir was better tolerated than lamivudine. Dose modifications due to neutropenia and thrombocytopenia were necessary in 50% of patients after interferon treatment4. Withdrawal rates were 24% higher after interferon-alfa-2b than with no treatment4. Pegylated interferon-alfa-2a combined with lamivudine resulted in greater discontinuation versus placebo or lamivudine alone4. Patients had serious adverse events more often after combined therapy of lamivudine with interferon-alfa-2b or pegylated interferon-alfa-2a than after lamivudine alone4. Long-term adverse drug events include reduced bone mineral density after tenofovir and moderate serum creatine phosphokinase elevations after telbivudine130.
Figure 6.
Rates (%) of clinical and intermediate outcomes in HBe antigen positive patients (pooled from randomized controlled clinical trials with random effects models).
DISCUSSION
For our original report, we created a comprehensive evidence map which compares absolute rates of the outcomes, number needed to treat, number attributable to treatment events, and cost of treatment. The map provides the most complete information about the comparative effectiveness of antiviral drugs and can be used for decision-making in clinical settings.
At the present time, decision-making in clinical settings is based on the published guidelines. Current guidelines recommend finite treatment with pegylated interferon alfa or nucleos(t)ides analogs based on rates of HBV DNA clearance and HBs Ag seroconversion131,132. The recommendations of the European Association for the Study of the Liver are based on the rates of HBeAg seroconversion and HBV DNA clearance from individual studies and do not differentiate benefits, harms, and cost among different interferon alfa or nucleos(t)ides analogs131.
Our present analysis and the evidence map we created gives clinicians data on the comparative effectiveness of each therapy on intermediate outcomes. It includes the number needed to treat to achieve the selected outcome. The evidence map also identifies gaps in our knowledge about sustained effects from antiviral drugs on clinical outcomes, liver histology, resolved HBV infection, and consistent improvement in virologic and biochemical outcomes. The map suggests that future research should investigate a balance between long term benefits and harms from both mono and combined therapy.
The next step in our analyses was to estimate the number needed to treat in order to inform clinicians about the effectiveness of antiviral drugs. For instance, sustained HBV DNA clearance in one patient can be achieved when two are treated with interferon alfa- 2b or adefovir, but they would need to treat 13 with lamivudine.
Clinicians can then compare harms. Viral resistant YMDD mutations would be detected in one of two patients treated with lamivudine43,109. Adefovir is contraindicated in patients with impaired renal function2. Around 21% of patients treated with interferon alfa-2b would require reduction in dose due to adverse events37.
Clinicians now have actionable information about anticipated benefits and harms from antiviral drugs and laboratory parameters to monitor during and after the treatments. Our analyses also provide patients with information about rates of improvement in intermediate markers (Fig. 6) and harms of each available treatment (Online Appendix 2). Patients should be informed that no good evidence exists about preventive effects of the drugs against liver cancer or cirrhosis, the rates of sustained HBV DNA loss are 14% after peginterferon alfa-2A and 19.4% after interferon alfa-2B but both drugs caused fever in 57-61% of the patients respectively.
The costs of oral antiviral drugs are lower than that of interferon, but a more complete cost estimation should include the price of treatment of adverse effects, e.g. cost of long term osteopenia after treatment with tenofovir, or elevations in serum creatine phosphokinase after telbivudine130.
Our analysis uncovered a lack of good evidence from RCTs that antiviral drugs prevent cirrhosis, liver decompensation, cancer, or mortality. Few studies were specifically designed to assess these clinical outcomes; most were intended to assess the relatively short-term effects of therapies on intermediate virological, histological, or biochemical outcomes. Observational studies suggested a strong positive association between viral load and liver fibrosis with clinical outcomes124. Low or undetectable DNA, however, did not eliminate the risk of liver cancer or cirrhosis124. Furthermore, observational studies could not provide a valid estimation of drug-induced reductions in viral level in association to improved clinical outcomes124. Therefore, we hesitated to predict drug prevention of clinical outcomes based on observed effects on biomarkers. Previously published cost-effectiveness analyses relied on assumptions from available natural history data133,134. We avoided such assumptions and used the results from RCTs that could establish causality between drug administration on intermediate outcomes. Additional costs for patients with treatment failure, relapse, or adverse events are unknown.
Limitations
As noted above, almost all studies were designed to assess the relatively short-term comparative effectiveness of the drugs on intermediate laboratory markers, not clinical outcomes. We could not find high quality evidence that antiviral drugs prevent liver carcinoma or death. One large RCT of 651 patients with advanced liver disease reported that lamivudine compared to placebo reduced the rates of the combined end point of disease progression, which was defined as the first occurrence of any of the following: an increase of at least two points in the Child–Pugh score, spontaneous bacterial peritonitis with proven sepsis, renal insufficiency, bleeding, gastric or esophageal varices, development of hepatocellular carcinoma, or death related to liver disease (34/436 in lamivudine vs. 38/215 in placebo group, hazard ratio 0.45, P = 0.001)109. However, the effects on liver cancer was either borderline (p = 0.047) or not significant (p = 0.052) when the authors excluded five patients who developed hepatocellular carcinoma during the first year of the study109. We prioritized laboratory measures based on observational research3,124 and expert consensus about validity of intermediate outcomes.
Treatment effects in population subgroups, including patients with drug resistance, patients with HBeAg negative CHB, age, gender, and other subgroups, are reported elsewhere2,4. We could not find evidence about comorbidities and concomitant treatments with respect to comparative effectiveness, harms, and cost.
Key Messages and Conclusions
A moderate level of evidence suggests positive sustained effects of antiviral drugs on intermediate biomarkers of CHB infection, liver histology, and function. No one drug regimen improved all biomarkers. There was no high quality evidence of the effects on clinical outcomes. Cost and attributable events vary across outcomes and drugs. Low to moderate evidence suggested lower cost and better effects from pegylated interferon alfa-2b when compared to interferon alfa-2b and entecavir when compared to lamivudine, but sustained effects on liver histology and function are unknown. Adverse events after antiviral drug use are common in adults with CHB. Individual clinical decisions should be made based on number needed to treat, cost, and absolute rates of positive outcomes and adverse events with respect to patient autonomy. Future research should clarify the cost-effectiveness of antiviral drugs on clinical outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Pooled analysis used to calculate number needed to treat (1/pooled absolute risk difference) and number of attributable per 1000 events (1000*pooled absolute risk difference) (DOC 596 kb)
Rates (%) of the most common (>10%) adverse effects after antiviral drugs in adults with chronic hepatitis B (Pooled with random effect models from randomized controlled clinical trials) (DOC 109 kb)
Acknowledgements
We would like to thank AHRQ Task Order Officer Shilpa Amin, MD, MBSC for her guidance throughout the project. We also want to thank librarians Judith Stanke and Dr. Del Reed for their contributions to the literature search; Maureen Carlyle and Marilyn Eells for their excellent technical assistance in preparation of the full evidence report and this manuscript; Rebecca Schultz for her assistance in formatting the tables, and Nancy Russell, MLS for her assistance with proofreading the manuscript.
Grant Support This project was funded under Contract No. 290-02-0009 from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The authors of this report are responsible for its content. Statements in the paper should not be construed as endorsement by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Conflict of Interest None disclosed.
Footnotes
Corporate names, city, and state of manufacturers of brand-name materials
STATA software (The Statistics/Data Analysis StataCorp. Stata statistical software: Release 9.2. College Station, Texas, USA)
Adefovir (Hepsera, Gilead Sciences, Foster City, California)
Entecavir (Baraclude, Bristol-Myers Squibb, New York)
Interferon Alfa 2b (Intron A, Schering-Plough, Milan, Italy)
Interferon Alfa 2b (Intron A, Schering Plough, Kenilworth, New Jersey)
Interferon Alfa 2b (Intron A, Shering-Plough, Athens, Greece)
Interferon Alfa 2b (Intron A, Scherag, South Africa)
Interferon Alfa 2b (Schering-Plough, Shanghai, China)
Interferon Alfa 2b (Intron A, Essex, Switzerland)
Lamivudine (Glaxo Wellcome, Suzhou, China)
Lamivudine (Zeffix, GlaxoSmithKline, Greenford, United Kingdom)
Lamivudine (Epivir-HBV Glaxo-Wellcome Inc, Research Triangle Park, North Carolina)
Lamivudine (GSK, Athens, Greece)
Lamivudine (Zeffix, GlaxoSmithKline, Middlesex, UK)
Peginterferon alfa-2a (PEGASYS, F. Hoffmann-La Roche Ltd., Basel, Switzerland)
Peginterferon alfa-2b (PegIntron, Schering-Plough Corp., Kenilworth, New Jersey)
Telbivudine Idenix Pharmaceuticals Inc. (Cambridge, Massachusetts)
Tenofovir DF Gilead Sciences
References
- 1.Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology. 2009;49(5 Suppl):S4–S12. doi: 10.1002/hep.22946. [DOI] [PubMed] [Google Scholar]
- 2.Wilt TJ, Shamliyan T, Shaukat A, et al. Management of chronic hepatitis B. Evid Rep Technol Assess (Full Rep) 2008;174:1–671. [PMC free article] [PubMed] [Google Scholar]
- 3.Feld JJ, Wong DK, Heathcote EJ. Endpoints of therapy in chronic hepatitis B. Hepatology. 2009;49(5 Suppl):S96–S102. doi: 10.1002/hep.22977. [DOI] [PubMed] [Google Scholar]
- 4.Shamliyan TA, MacDonald R, Shaukat A, et al. Antiviral therapy for adults with chronic hepatitis B: a systematic review for a National Institutes of Health Consensus Development Conference. Ann Intern Med. 2009;150(2):111–124. doi: 10.7326/0003-4819-150-2-200901200-00101. [DOI] [PubMed] [Google Scholar]
- 5.Reichen J. ACP Journal Club. Review: evidence is insufficient to evaluate effectiveness of antiviral therapies for chronic hepatitis B. Ann Intern Med. 2009;150(10):JC5–JC6. doi: 10.7326/0003-4819-150-10-200905190-02006. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health (U.S.). NIH consensus development conference management of hepatitis B. [Bethesda, Md.: National Institutes of Health; 2008: Retieved October 1, 2010 http://videocast.nih.gov/launch.asp?14714 Retrieved October 1, 2010 http://videocast.nih.gov/launch.asp?14712 Retrieved October 1, 2010 http://videocast.nih.gov/launch.asp?14719
- 7.Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49(5 Suppl):S72–S84. doi: 10.1002/hep.22884. [DOI] [PubMed] [Google Scholar]
- 8.Perrillo R. Benefits and risks of interferon therapy for hepatitis B. Hepatology. 2009;49(5 Suppl):S103–S111. doi: 10.1002/hep.22956. [DOI] [PubMed] [Google Scholar]
- 9.Dienstag JL. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology. 2009;49(5 Suppl):S112–S121. doi: 10.1002/hep.22920. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J, Green S, Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley-Blackwell; 2008. [Google Scholar]
- 11.Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med. 2009;151(3):203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. [DOI] [PubMed] [Google Scholar]
- 12.Slutsky J, Atkins D, Chang S, Sharp BA. AHRQ series paper 1: comparing medical interventions: AHRQ and the effective health-care program. J Clin Epidemiol. 2010;63(5):481–483. doi: 10.1016/j.jclinepi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Helfand M, Balshem H. AHRQ series paper 2: principles for developing guidance: AHRQ and the effective health-care program. J Clin Epidemiol. 2010;63(5):484–490. doi: 10.1016/j.jclinepi.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 Suppl):21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 15.Cochrane Collaboration. The Cochrane library. Retrieved October 1, 2010 from http://www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME
- 16.United States. Food and Drug Administration. MedWatch. MedWatch online voluntary reporting form (3500). [Rockville, Md.]: Food and Drug Administration, MedWatch; 2002. Retrieved October 1, 2010 from http://purl.access.gpo.gov/GPO/LPS39966 and retrieved October 1, 2010 from http://purl.access.gpo.gov/GPO/LPS81658
- 17.Fontaine H, Petitprez K, Roudot-Thoraval F, Trinchet JC. Guidelines for the diagnosis of uncomplicated cirrhosis. Gastroenterol Clin Biol. 2007;31(5):504–509. doi: 10.1016/S0399-8320(07)89420-6. [DOI] [PubMed] [Google Scholar]
- 18.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45(2):507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 19.Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: an update for clinicians. Mayo Clin Proc. 2007;82(8):967–975. doi: 10.4065/82.8.967. [DOI] [PubMed] [Google Scholar]
- 20.West S, King V, Carey TS, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess. 2002;47:1–11. [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 22.Whitehead A. Meta-analysis of Controlled Clinical Trials. Chichester: John Wiley & Sons; 2002. [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Stat Med. Feb 6 2006. [DOI] [PubMed]
- 25.Knapp G, Biggerstaff BJ, Hartung J. Assessing the amount of heterogeneity in random-effects meta-analysis. Biom J. 2006;48(2):271–285. doi: 10.1002/bimj.200510175. [DOI] [PubMed] [Google Scholar]
- 26.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53(2):207–216. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 27.Atkins D, Briss PA, Eccles M, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 2005;5(1):25. doi: 10.1186/1472-6963-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care. London: NetLibrary, Inc. BMJ Books; 2001. [Google Scholar]
- 30.Ebrahim S. The use of numbers needed to treat derived from systematic reviews and meta-analysis. Caveats and pitfalls. Eval Health Prof. 2001;24(2):152–164. doi: 10.1177/01632780122034858. [DOI] [PubMed] [Google Scholar]
- 31.Altman DG. Confidence intervals for the number needed to treat. Br Med J (Clinical research) 1998;317(7168):1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou R, Aronson N, Atkins D, et al. AHRQ series paper 4: assessing harms when comparing medical interventions: AHRQ and the effective health-care program. J Clin Epidemiol. 2010;63(5):502–512. doi: 10.1016/j.jclinepi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Red book 2008 pharmacy’s fundamental reference. 2008 ed. Montvale, N.J.: Thomson; 2008.
- 35.You JH, Chan FW. Pharmacoeconomics of entecavir treatment for chronic hepatitis B. Expert Opin Pharmacother. 2008;9(15):2673–2681. doi: 10.1517/14656566.9.15.2673. [DOI] [PubMed] [Google Scholar]
- 36.Jones J, Shepherd J, Baxter L, et al. Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation. Health Technol Assess. 2009;13(35):1–172. doi: 10.3310/hta13350. [DOI] [PubMed] [Google Scholar]
- 37.Janssen HL, Gerken G, Carreno V, et al. Interferon alfa for chronic hepatitis B infection: increased efficacy of prolonged treatment. The European Concerted Action on Viral Hepatitis (EUROHEP) Hepatology. 1999;30(1):238–243. doi: 10.1002/hep.510300113. [DOI] [PubMed] [Google Scholar]
- 38.Schalm SW, Heathcote J, Cianciara J, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46(4):562–568. doi: 10.1136/gut.46.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbaro G, Zechini F, Pellicelli AM, et al. Long-term efficacy of interferon alpha-2b and lamivudine in combination compared to lamivudine monotherapy in patients with chronic hepatitis B. An Italian multicenter, randomized trial. J Hepatol. 2001;35(3):406–411. doi: 10.1016/s0168-8278(01)00145-3. [DOI] [PubMed] [Google Scholar]
- 40.Perrillo RP, Lai CL, Liaw YF, et al. Predictors of HBeAg loss after lamivudine treatment for chronic hepatitis B. Hepatology. 2002;36(1):186–194. doi: 10.1053/jhep.2002.34294. [DOI] [PubMed] [Google Scholar]
- 41.Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology. 2002;36(6):1425–1430. doi: 10.1053/jhep.2002.37139. [DOI] [PubMed] [Google Scholar]
- 42.Chung YH, Song BC, Lee GC, et al. Individualization of interferon therapy using serum hepatitis B virus DNA to reduce viral relapse in patients with chronic hepatitis B: a randomized controlled trial. Eur J Gastroenterol Hepatol. 2003;15(5):489–493. doi: 10.1097/01.meg.0000059120.41030.52. [DOI] [PubMed] [Google Scholar]
- 43.Schiff ER, Dienstag JL, Karayalcin S, et al. Lamivudine and 24 weeks of lamivudine/interferon combination therapy for hepatitis B e antigen-positive chronic hepatitis B in interferon nonresponders. J Hepatol. 2003;38(6):818–826. doi: 10.1016/s0168-8278(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 44.Yalcin K, Degertekin H, Yildiz F, Celik Y. Comparison of 12-month courses of interferon-alpha-2b-lamivudine combination therapy and interferon-alpha-2b monotherapy among patients with untreated chronic hepatitis B. Clin Infect Dis. 2003;36(12):1516–1522. doi: 10.1086/375085. [DOI] [PubMed] [Google Scholar]
- 45.Niederau C, Heintges T, Niederau M, Stremmel W, Strohmeyer G. Prospective randomized controlled trial of sequential treatment with corticoids and alpha-interferon versus treatment with interferon alone in patients with chronic active hepatitis B. Eur J Med. 1992;1(7):396–402. [PubMed] [Google Scholar]
- 46.Robson SC, Brice E, Rensburg C, Kannemeyer J, Hift RJ, Kirsch RE. Safety and efficacy of interferon alpha-2b following prednisone withdrawal in the treatment of chronic viral hepatitis B. A case-controlled, randomised study. S Afr Med J. 1992;82(5):317–320. [PubMed] [Google Scholar]
- 47.Akarca US, Ersoz G, Gunsar F, et al. Interferon-lamivudine combination is no better than lamivudine alone in anti-HBe-positive chronic hepatitis B. Antivir Ther. 2004;9(3):325–334. [PubMed] [Google Scholar]
- 48.Jang MK, Chung YH, Choi MH, et al. Combination of alpha-interferon with lamivudine reduces viral breakthrough during long-term therapy. J Gastroenterol Hepatol. 2004;19(12):1363–1368. doi: 10.1111/j.1440-1746.2004.03464.x. [DOI] [PubMed] [Google Scholar]
- 49.Lok AS, Wu PC, Lai CL, et al. A controlled trial of interferon with or without prednisone priming for chronic hepatitis B. Gastroenterology. 1992;102(6):2091–2097. doi: 10.1016/0016-5085(92)90337-x. [DOI] [PubMed] [Google Scholar]
- 50.Economou M, Manolakopoulos S, Trikalinos TA, et al. Interferon-alpha plus lamivudine vs lamivudine reduces breakthroughs, but does not affect sustained response in HBeAg negative chronic hepatitis B. World J Gastroenterol. 2005;11(37):5882–5887. doi: 10.3748/wjg.v11.i37.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarin SK, Kumar M, Kumar R, et al. Higher efficacy of sequential therapy with interferon-alpha and lamivudine combination compared to lamivudine monotherapy in HBeAg positive chronic hepatitis B patients. Am J Gastroenterol. 2005;100(11):2463–2471. doi: 10.1111/j.1572-0241.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 52.Shi M, Wang RS, Zhang H, et al. Sequential treatment with lamivudine and interferon-alpha monotherapies in hepatitis B e antigen-negative Chinese patients and its suppression of lamivudine-resistant mutations. J Antimicrob Chemother. 2006;58(5):1031–1035. doi: 10.1093/jac/dkl385. [DOI] [PubMed] [Google Scholar]
- 53.Scotto G, Palumbo E, Fazio V, Cibelli DC, Saracino A, Angarano G. Efficacy and tolerability of lamivudine alone versus lamivudine plus alpha-interferon for treatment of chronic active hepatitis B in patients with a precore-mutant variant. Infez Med. 2006;14(3):145–151. [PubMed] [Google Scholar]
- 54.Lu HY, Zhuang LW, Yu YY, et al. Intrahepatic HBV DNA as a predictor of antivirus treatment efficacy in HBeAg-positive chronic hepatitis B patients. World J Gastroenterol. 2007;13(20):2878–2882. doi: 10.3748/wjg.v13.i20.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akyuz F, Kaymakoglu S, Demir K, et al. Lamivudine monotherapy and lamivudine plus interferon alpha combination therapy in HBeAg negative chronic hepatitis B not responding to previous interferon alpha monotherapy. Acta Gastroenterol Belg. 2007;70(1):20–24. [PubMed] [Google Scholar]
- 56.Perez V, Tanno H, Villamil F, Fay O. Recombinant interferon alfa-2b following prednisone withdrawal in the treatment of chronic type B hepatitis. J Hepatol. 1990;11(Suppl 1):S113–S117. doi: 10.1016/0168-8278(90)90175-q. [DOI] [PubMed] [Google Scholar]
- 57.Hadziyannis S, Bramou T, Makris A, Moussoulis G, Zignego L, Papaioannou C. Interferon alfa-2b treatment of HBeAg negative/serum HBV DNA positive chronic active hepatitis type B. J Hepatol. 1990;11(Suppl 1):S133–S136. doi: 10.1016/0168-8278(90)90180-y. [DOI] [PubMed] [Google Scholar]
- 58.Muller R, Baumgarten R, Markus R, et al. Treatment of chronic hepatitis B with interferon alfa-2b. J Hepatol. 1990;11(Suppl 1):S137–S140. doi: 10.1016/0168-8278(90)90181-p. [DOI] [PubMed] [Google Scholar]
- 59.Waked I, Amin M, Abd el Fattah S, Osman LM, Sabbour MS. Experience with interferon in chronic hepatitis B in Egypt. J Chemother. 1990;2(5):310–318. doi: 10.1080/1120009x.1990.11739035. [DOI] [PubMed] [Google Scholar]
- 60.Perrillo RP, Schiff ER, Davis GL, et al. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med. 1990;323(5):295–301. doi: 10.1056/NEJM199008023230503. [DOI] [PubMed] [Google Scholar]
- 61.Zarski JP, Causse X, Cohard M, Cougnard J, Trepo C. A randomized, controlled trial of interferon alfa-2b alone and with simultaneous prednisone for the treatment of chronic hepatitis B. French Multicenter Group. J Hepatol. 1994;20(6):735–741. doi: 10.1016/s0168-8278(05)80143-6. [DOI] [PubMed] [Google Scholar]
- 62.Reichen J, Bianchi L, Frei PC, Male PJ, Lavanchy D, Schmid M. Efficacy of steroid withdrawal and low-dose interferon treatment in chronic active hepatitis B. Results of a randomized multicenter trial. Swiss Association for the Study of the Liver. J Hepatol. 1994;20(2):168–174. doi: 10.1016/s0168-8278(05)80054-6. [DOI] [PubMed] [Google Scholar]
- 63.Bisceglie AM, Fong TL, Fried MW, et al. A randomized, controlled trial of recombinant alpha-interferon therapy for chronic hepatitis B. Am J Gastroenterol. 1993;88(11):1887–1892. [PubMed] [Google Scholar]
- 64.Perez V, Findor J, Tanno H, Sorda J. A controlled trial of high dose interferon, alone and after prednisone withdrawal, in the treatment of chronic hepatitis B: long term follow up. Gut. 1993;34(2 Suppl):S91–S94. doi: 10.1136/gut.34.2_suppl.s91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muller R, Baumgarten R, Markus R, et al. Low dose alpha interferon treatment in chronic hepatitis B virus infection. Gut. 1993;34(2 Suppl):S97–S98. doi: 10.1136/gut.34.2_suppl.s97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Alcorocho JM, Bartolome J, Cotonat T, Carreno V. Efficacy of prolonged interferon-alpha treatment in chronic hepatitis B patients with HBeAb: comparison between 6 and 12 months of therapy. J Viral Hepat. 1997;4(Suppl 1):27–32. doi: 10.1111/j.1365-2893.1997.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 67.Lampertico P, Ninno E, Manzin A, et al. A randomized, controlled trial of a 24-month course of interferon alfa 2b in patients with chronic hepatitis B who had hepatitis B virus DNA without hepatitis B e antigen in serum. Hepatology. 1997;26(6):1621–1625. doi: 10.1002/hep.510260634. [DOI] [PubMed] [Google Scholar]
- 68.Mutimer D, Naoumov N, Honkoop P, et al. Combination alpha-interferon and lamivudine therapy for alpha-interferon-resistant chronic hepatitis B infection: results of a pilot study. J Hepatol. 1998;28(6):923–929. doi: 10.1016/s0168-8278(98)80338-3. [DOI] [PubMed] [Google Scholar]
- 69.Bonino F, Marcellin P, Lau GK, et al. Predicting response to peginterferon alpha-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut. 2007;56(5):699–705. doi: 10.1136/gut.2005.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooksley WG, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepatitis. 2003;10(4):298–305. doi: 10.1046/j.1365-2893.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 71.Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351(12):1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 72.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352(26):2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 73.Cindoruk M, Karakan T, Unal S. Hepatic steatosis has no impact on the outcome of treatment in patients with chronic hepatitis B infection. J Clin Gastroenterol. 2007;41(5):513–517. doi: 10.1097/01.mcg.0000225586.78330.60. [DOI] [PubMed] [Google Scholar]
- 74.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348(9):800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 75.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352(26):2673–2681. doi: 10.1056/NEJMoa042957. [DOI] [PubMed] [Google Scholar]
- 76.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131(6):1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 77.Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348(9):808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 78.Zeng M, Mao Y, Yao G, et al. A double-blind randomized trial of adefovir dipivoxil in Chinese subjects with HBeAg-positive chronic hepatitis B. Hepatology. 2006;44(1):108–116. doi: 10.1002/hep.21225. [DOI] [PubMed] [Google Scholar]
- 79.Westland C, Delaney Wt, Yang H, et al. Hepatitis B virus genotypes and virologic response in 694 patients in phase III studies of adefovir dipivoxil1. Gastroenterology. 2003;125(1):107–116. doi: 10.1016/s0016-5085(03)00700-5. [DOI] [PubMed] [Google Scholar]
- 80.Westland CE, Yang H, Delaney WEt, et al. Week 48 resistance surveillance in two phase 3 clinical studies of adefovir dipivoxil for chronic hepatitis B. Hepatology. 2003;38(1):96–103. doi: 10.1053/jhep.2003.50288. [DOI] [PubMed] [Google Scholar]
- 81.Izzedine H, Hulot JS, Launay-Vacher V, et al. Renal safety of adefovir dipivoxil in patients with chronic hepatitis B: two double-blind, randomized, placebo-controlled studies. Kidney Int. 2004;66(3):1153–1158. doi: 10.1111/j.1523-1755.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 82.Perrillo R, Hann HW, Mutimer D, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126(1):81–90. doi: 10.1053/j.gastro.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 83.Akyildiz M, Gunsar F, Ersoz G, et al. Adefovir dipivoxil alone or in combination with lamivudine for three months in patients with lamivudine resistant compensated chronic hepatitis B. Dig Dis Sci. 2007;52(12):3444–3447. doi: 10.1007/s10620-006-9718-8. [DOI] [PubMed] [Google Scholar]
- 84.Peters MG, Hann HWH, Martin P, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126(1):91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 85.Chan HL, Heathcote EJ, Marcellin P, et al. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med. 2007;147(11):745–754. doi: 10.7326/0003-4819-147-11-200712040-00183. [DOI] [PubMed] [Google Scholar]
- 86.Flink HJ, Zonneveld M, Hansen BE, Man RA, Schalm SW, Janssen HL. Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. American J Gastroenterol. 2006;101(2):297–303. doi: 10.1111/j.1572-0241.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 87.Janssen HL, Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365(9454):123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 88.Buster EH, Hansen BE, Buti M, et al. Peginterferon alpha-2b is safe and effective in HBeAg-positive chronic hepatitis B patients with advanced fibrosis. Hepatology. 2007;46(2):388–394. doi: 10.1002/hep.21723. [DOI] [PubMed] [Google Scholar]
- 89.Borg MJ, Zonneveld M, Zeuzem S, et al. Patterns of viral decline during PEG-interferon alpha-2b therapy in HBeAg-positive chronic hepatitis B: relation to treatment response. Hepatology. 2006;44(3):721–727. doi: 10.1002/hep.21302. [DOI] [PubMed] [Google Scholar]
- 90.Flink HJ, Hansen BE, Heathcote EJ, et al. Successful treatment with peginterferon alfa-2b of HBeAg-positive HBV non-responders to standard interferon or lamivudine. Am J Gastroenterol. 2006;101(11):2523–2529. doi: 10.1111/j.1572-0241.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 91.Zonneveld M, Flink HJ, Verhey E, et al. The safety of pegylated interferon alpha-2b in the treatment of chronic hepatitis B: predictive factors for dose reduction and treatment discontinuation. Aliment Pharmacol Ther. 2005;21(9):1163–1171. doi: 10.1111/j.1365-2036.2005.02453.x. [DOI] [PubMed] [Google Scholar]
- 92.Flink HJ, Sprengers D, Hansen BE, et al. Flares in chronic hepatitis B patients induced by the host or the virus? Relation to treatment response during Peg-interferon {alpha}-2b therapy. Gut. 2005;54(11):1604–1609. doi: 10.1136/gut.2004.062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zonneveld M, Zondervan PE, Cakaloglu Y, et al. Peg-interferon improves liver histology in patients with HBeAg-positive chronic hepatitis B: no additional benefit of combination with lamivudine. Liver Int. 2006;26(4):399–405. doi: 10.1111/j.1478-3231.2006.01257.x. [DOI] [PubMed] [Google Scholar]
- 94.Chan HL, Hui AY, Wong VW, Chim AM, Wong ML, Sung JJ. Long-term follow-up of peginterferon and lamivudine combination treatment in HBeAg-positive chronic hepatitis B. Hepatology. 2005;41(6):1357–1364. doi: 10.1002/hep.20695. [DOI] [PubMed] [Google Scholar]
- 95.Chan HL, Leung NW, Hui AY, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med. 2005;142(4):240–250. doi: 10.7326/0003-4819-142-4-200502150-00006. [DOI] [PubMed] [Google Scholar]
- 96.Chan HL, Tse AM, Zhang MD, et al. Genetic polymorphisms of interleukin-1-beta in association with sustained response to anti-viral treatment in chronic hepatitis B in Chinese. Aliment Pharmacol Ther. 2006;23(12):1703–1711. doi: 10.1111/j.1365-2036.2006.02948.x. [DOI] [PubMed] [Google Scholar]
- 97.Zhao H, Kurbanov F, Wan MB, et al. Genotype B and younger patient age associated with better response to low-dose therapy: a trial with pegylated/nonpegylated interferon-alpha-2b for hepatitis B e antigen-positive patients with chronic hepatitis B in China. Clin Infect Dis. 2007;44(4):541–548. doi: 10.1086/511042. [DOI] [PubMed] [Google Scholar]
- 98.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354(10):1011. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 99.Chang TT, Gish RG, Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 100.Lai CL, Rosmawati M, Lao J, et al. Entecavir is superior to lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infection. Gastroenterology. 2002;123(6):1831–1838. doi: 10.1053/gast.2002.37058. [DOI] [PubMed] [Google Scholar]
- 101.Chang TT, Gish RG, Hadziyannis SJ, et al. A dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patients. Gastroenterology. 2005;129(4):1198–1209. doi: 10.1053/j.gastro.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 102.Sherman M, Yurdaydin C, Sollano J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130(7):2039–2049. doi: 10.1053/j.gastro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Gish RG, Lok AS, Chang TT, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133(5):1437–1444. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 104.Lai CL, Leung N, Teo EK, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005;129(2):528–536. doi: 10.1016/j.gastro.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 105.Kweon YO, Goodman ZD, Dienstag JL, et al. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35(6):749–755. doi: 10.1016/s0168-8278(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 106.Ke CZ, Chen Y, Gong ZJ, et al. Dynamic changes of HBV DNA in serum and peripheral blood mononuclear cells of chronic hepatitis patients after lamivudine treatment. World J Gastroenterol. 2006;12(25):4061–4063. doi: 10.3748/wjg.v12.i25.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuen MF, Chow DH, Tsui K, et al. Liver histology of Asian patients with chronic hepatitis B on prolonged lamivudine therapy. Aliment Pharmacol Ther. 2005;21(7):841–849. doi: 10.1111/j.1365-2036.2005.02410.x. [DOI] [PubMed] [Google Scholar]
- 108.Yao GB. Management of hepatitis B in China. J Med Virol. 2000;61(3):392–397. doi: 10.1002/1096-9071(200007)61:3<392::aid-jmv19>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 109.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 110.Liaw YF, Leung NW, Chang TT, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119(1):172–180. doi: 10.1053/gast.2000.8559. [DOI] [PubMed] [Google Scholar]
- 111.Leung NW, Lai CL, Chang TT, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology. 2001;33(6):1527–1532. doi: 10.1053/jhep.2001.25084. [DOI] [PubMed] [Google Scholar]
- 112.Dienstag JL, Goldin RD, Heathcote EJ, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124(1):105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 113.Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341(17):1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 114.Honkoop P, Man RA, Niesters HG, et al. Quantitative hepatitis B virus DNA assessment by the limiting-dilution polymerase chain reaction in chronic hepatitis B patients: evidence of continuing viral suppression with longer duration and higher dose of lamivudine therapy. J Viral Hepatitis. 1998;5(5):307–312. doi: 10.1046/j.1365-2893.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 115.Nevens F, Main J, Honkoop P, et al. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology. 1997;113(4):1258–1263. doi: 10.1053/gast.1997.v113.pm9322520. [DOI] [PubMed] [Google Scholar]
- 116.Chan HL, Wang H, Niu J, Chim AM, Sung JJ. Two-year lamivudine treatment for hepatitis B e antigen-negative chronic hepatitis B: a double-blind, placebo-controlled trial. Antivir Ther. 2007;12(3):345–353. [PubMed] [Google Scholar]
- 117.Yao G, Wang B, Cui Z, Yao J, Zeng M. A randomized double-blind placebo-controlled study of lamivudine in the treatment of patients with chronic hepatitis B virus infection. Chin Med J (Engl) 1999;112(5):387–391. [PubMed] [Google Scholar]
- 118.Kim YJ, Kim BG, Jung JO, Yoon JH, Lee HS. High rates of progressive hepatic functional deterioration whether lamivudine therapy is continued or discontinued after emergence of a lamivudine-resistant mutant: a prospective randomized controlled study. J Gastroenterol. 2006;41(3):240–249. doi: 10.1007/s00535-005-1750-5. [DOI] [PubMed] [Google Scholar]
- 119.Tassopoulos NC, Volpes R, Pastore G, et al. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B.Lamivudine Precore Mutant Study Group. Hepatology. 1999;29(3):889–896. doi: 10.1002/hep.510290321. [DOI] [PubMed] [Google Scholar]
- 120.Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357(25):2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 121.Hou J, Yin YK, Xu D, et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trial. Hepatology. 2008;47(2):447–454. doi: 10.1002/hep.22075. [DOI] [PubMed] [Google Scholar]
- 122.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 123.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339(2):61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 124.Taylor BC, Yuan JM, Shamliyan TA, Shaukat A, Kane RL, Wilt TJ. Clinical outcomes in adults with chronic hepatitis B in association with patient and viral characteristics: a systematic review of evidence. Hepatology. 2009;49(5 Suppl):S85–S95. doi: 10.1002/hep.22929. [DOI] [PubMed] [Google Scholar]
- 125.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users' guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 126.McAlister FA, Straus SE, Guyatt GH, Haynes RB. Users' guides to the medical literature: XX. Integrating research evidence with the care of the individual patient. Evidence-Based Medicine Working Group. JAMA. 2000;283(21):2829–2836. doi: 10.1001/jama.283.21.2829. [DOI] [PubMed] [Google Scholar]
- 127.Chalmers I. Well informed uncertainties about the effects of treatments. Br Med J (Clinical research) 2004;328(7438):475–476. doi: 10.1136/bmj.328.7438.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology. 2009;49(5 Suppl):S174–S184. doi: 10.1002/hep.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(5 Suppl):S156–S165. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 130.Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology. 2009;49(5 Suppl):S185–S195. doi: 10.1002/hep.22885. [DOI] [PubMed] [Google Scholar]
- 131.EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. Feb 2009;50(2):227-242. [DOI] [PubMed]
- 132.Belongia EA, Costa J, Gareen IF, et al. NIH consensus development statement on management of hepatitis B. NIH Consens State Sci Statements. 2008;25(2):1–29. [PubMed] [Google Scholar]
- 133.Dusheiko GM. Cost-effectiveness of oral treatments for chronic hepatitis B. J Hepatol. Aug 6 2009. [DOI] [PubMed]
- 134.Buti M, Brosa M, Casado MA, Rueda M, Esteban R. Modeling the cost-effectiveness of different oral antiviral therapies in patients with chronic hepatitis B. J Hepatol. May 20 2009. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Pooled analysis used to calculate number needed to treat (1/pooled absolute risk difference) and number of attributable per 1000 events (1000*pooled absolute risk difference) (DOC 596 kb)
Rates (%) of the most common (>10%) adverse effects after antiviral drugs in adults with chronic hepatitis B (Pooled with random effect models from randomized controlled clinical trials) (DOC 109 kb)