ABSTRACT
BACKGROUND
Geriatric conditions, collections of symptoms common in older adults and not necessarily associated with a specific disease, increase in prevalence with advancing age. These conditions are important contributors to the complex health status of older adults. Diabetes mellitus is known to co-occur with geriatric conditions in older adults and has been implicated in the pathogenesis of some conditions.
OBJECTIVE
To investigate the prevalence and incidence of geriatric conditions in middle-aged and older-aged adults with diabetes.
DESIGN
Secondary analysis of nationally-representative, longitudinal health interview survey data (Health and Retirement Study waves 2004 and 2006).
PARTICIPANTS
Respondents 51 years and older in 2004 (n = 18,908).
MAIN MEASURES
Diabetes mellitus. Eight geriatric conditions: cognitive impairment, falls, incontinence, low body mass index, dizziness, vision impairment, hearing impairment, pain.
KEY RESULTS
Adults with diabetes, compared to those without, had increased prevalence and increased incidence of geriatric conditions across the age spectrum (p < 0.01 for each age group from 51-54 years old to 75–79 years old). Differences between adults with and without diabetes were most marked in middle-age. Diabetes was associated with the two-year cumulative incidence of acquiring new geriatric conditions (odds ratio, 95% confidence interval: 1.8, 1.6–2.0). A diabetes-age interaction was discovered: as age increased, the association of diabetes with new geriatric conditions decreased.
CONCLUSIONS
Middle-aged, as well as older-aged, adults with diabetes are at increased risk for the development of geriatric conditions, which contribute substantially to their morbidity and functional impairment. Our findings suggest that adults with diabetes should be monitored for the development of these conditions beginning at a younger age than previously thought.
KEY WORDS: diabetes mellitus, geriatric conditions, middle-age
INTRODUCTION
Background
Geriatric conditions, collections of symptoms common in older adults and not necessarily associated with a specific disease, increase in prevalence with age1–3. Geriatric conditions are associated with functional impairment in older adults and contribute to their complex health status.1
Diabetes mellitus co-occurs with geriatric conditions in older adults,4–16 and the American Geriatrics Society has included screening for and management of geriatric conditions (or syndromes) in its guidelines for improving the care of older adults with diabetes.17 Diabetes is theorized to be associated with early or accelerated aging and has been implicated in the pathogenesis of some geriatric conditions (e.g., falls).4,9,12,14,15,17
There is no consensus regarding the definitions and understanding of the terms geriatric condition and geriatric syndrome.1 Previous work by the authors investigated seven conditions - cognitive impairment, falls, incontinence, low body mass index, dizziness, vision impairment, and hearing impairment.1 The American Geriatric Society’s “Guidelines for Improving the Care of the Older Person with Diabetes Mellitus” also included pain.17 Here, we investigate whether these eight “geriatric” conditions occur at younger ages in adults with diabetes.
Objective
The goal of this study was to compare the prevalence and incidence of geriatric conditions in middle-aged and older-aged adults with diabetes to that of middle- and older-aged adults without diabetes. We hypothesized that adults with diabetes, compared to those without, had increased prevalence and increased incidence of geriatric conditions (in aggregate and individually).
METHODS
Design
We performed secondary analysis of population-based, longitudinal health interview survey data.
Participants
Study data are from the 2004 and 2006 waves of the Health and Retirement Study (HRS), a nationally representative, biennial longitudinal health interview survey of a cohort of adults age 51 years and older in the United States.18,19 The HRS is sponsored by the National Institute on Aging and performed by the Institute for Social Research at the University of Michigan. The survey is designed to study adults as they transition into retirement.
The HRS was approved by the Health Sciences Institutional Review Board at the University of Michigan. The data used for this analysis are publicly available and contain no unique identifiers, thus assuring respondent anonymity.
In the 2004 wave of the HRS, 18,908 respondents were 51 years and older; they represented 82.0 million adults 51 years and older in the United States in that year. For each respondent unable to be interviewed, usually because of medical and/or cognitive problems, a proxy (n = 1,719), frequently the spouse (n = 1,077), was enlisted to answer questions for that respondent.
Main Measures
Diabetes Mellitus
Respondents who reported in 2004 that a physician had diagnosed them with diabetes or high blood sugar were categorized as having diabetes. The HRS does not distinguish between type 1 and type 2 diabetes.
Geriatric Conditions
The HRS provides information on the eight geriatric conditions. Survey questions about the individual conditions include those that can be used to indicate the severity or activity of each condition (with the exception of dizziness).1 Our analysis focused on the more severe/active form of the conditions, which we specified as follows:
Falls: falls resulting in injury and/or three or more falls in the previous two years
Incontinence: incontinence episodes 15 or more days each month
Low body mass index (BMI) (based on self-report height and weight): <18.5 kg/m2
Dizziness: dizziness or lightheadedness as a persistent/troublesome problem
Vision impairment: poor eyesight or blindness despite use of corrective lenses
Hearing impairment: poor hearing despite use of hearing aides
Pain: often troubled with severe pain
(We also expanded the definition of each condition to include moderate/less active forms, which we examined in sensitivity analyses.)
The HRS assesses cognitive function in one of two ways.20–23 For self-respondents, cognitive function is evaluated using a performance-based measure, a modified version of the Telephone Interview for Cognitive Status (TICS),24 a validated cognitive screening instrument patterned on the Mini-Mental State Examination25 and specifically designed for population-based studies. We defined moderate/severe cognitive impairment as a score of 7 or below on the 35-point cognitive scale for those 65 years and older and as a score of 4 or below on the 27-point cognitive scale for those 51 to 64 years old. These cut-points have previously been used by researchers because the proportion of people that they identify as having serious cognitive impairment is consistent with other estimates of the prevalence of dementia. Detailed information on the cognitive measures that compose the modified TICS is available on the HRS website (http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf).
Respondents unable to complete the interview are each assigned a proxy respondent by a trained interviewer according to study protocol. We determined the presence and degree of cognitive impairment for these respondents by combining the proxy’s assessment of the respondent’s memory with the interviewer’s assessment of the respondent’s cognitive function.23
Mortality
For respondents who had died, exit interviews were conducted with their designated proxy respondents. HRS mortality data are cross-referenced with the National Center for Health Statistics National Death Index (NDI).
Chronic Diseases
We included the following chronic diseases in our analyses: hypertension, heart disease, chronic lung disease, cancer, stroke, musculoskeletal conditions, and psychiatric problems. Respondents reported whether or not a physician had diagnosed them with each disease; follow-up questions about the diseases included those indicating their activity or severity. We limited each disease to its severe/active form (e.g., receiving treatment for the disease).1,21 For instance, we limited stroke to those requiring medication for stroke (or its complications) and/or with remaining problems (e.g., weakness in arms/legs, difficulty speaking/swallowing, etc.). We limited psychiatric problems to those respondents taking medication and/or receiving psychiatric or psychological treatment.
Sociodemographic Factors
Sociodemographic variables included age, gender, race (Caucasian, African-American, Hispanic), marital status (married, unmarried), educational attainment, net worth (total household assets minus current debt, in quartiles),18 and living situation (with others, alone, in long-stay nursing facility). (Note that a small number of respondents answered the race/ethnicity question as “other;” these respondents were grouped with Caucasians in the analyses.)
STATISTICAL ANALYSIS
To adjust for the complex sample design of the HRS, the differential probability of selection, and non-response, all analyses were weighted and adjusted using the statistical package STATA (Release 9.0, Stata Corp, College Station, TX). Thus, we were able to take advantage of the nationally representative data set to produce national population estimates.
Respondents were analyzed in terms of their diabetes status in 2004. Geriatric conditions were analyzed both in aggregate (numbers of conditions) and individually. We used 2004 data to estimate the prevalence of geriatric conditions. We used 2004 and 2006 data to estimate the two-year cumulative incidence of acquiring new geriatric condition(s); we used the two years between the two waves of data as the period of time during which all of the individuals in the population sample could be considered to be at risk for the outcome (i.e., a new geriatric condition).26 Of note, HRS data on the geriatric conditions do not include their exact time of onset; hence, HRS data do not support the calculation of incidence rates for the conditions. (Also, the HRS does not survey respondents 64 years and younger about falls, so the prevalence and the incidence of falls could not be determined for these ages. Respondents were not questioned about dizziness in the 2006 wave, so its incidence in 2006 could not be determined.)
We calculated the two-year cumulative incidence (from 2004 to 2006) of acquiring at least one additional geriatric condition as follows: The population at risk in 2006 included all respondents alive in that year (the denominator); we excluded the 1,243 respondents who died following the 2004 interview. For this population at risk, we determined the number of respondents who had an increase in their number of geriatric conditions in 2006 as compared to 2004 (the numerator).
We similarly calculated two-year cumulative incidence for the individual geriatric conditions (except dizziness). For each of these seven analyses, the population at risk in 2006 included all respondents who were alive in 2006 and who did not report the indicated condition in 2004 (the denominator). For each analysis, we then determined the number of respondents who newly reported having the geriatric condition in 2006 (the numerator).
We used multivariate logistic regression modeling to examine the association between diabetes (independent variable) and the two-year risk of acquiring at least one new geriatric condition (dependent variable). We sequentially introduced groups of variables into the model: age, other demographic variables, and chronic diseases. We also introduced a diabetes-age interaction into the models. From the logistic models, we obtained estimates of odds ratios for the association between diabetes and the two-year risk of acquiring new geriatric conditions.
Of the 18,908 respondents in the population sample in 2004, 1,243 (6.6%) died prior to the 2006 wave, and 921 (4.9%) were lost to follow-up in 2006. We performed extensive sensitivity analyses including and excluding the 921 respondents lost to follow-up. For the analyses in this paper, these 921 respondents were treated as being alive in 2006 and as not having new geriatric conditions. Thus, they were included in the population at risk (the denominator) in calculating two-year cumulative incidence.
KEY RESULTS
Table 1 shows (column 1) the characteristics of the study population, weighted to be representative of adults age 51 years and older in the United States in the 2004. Table 1 also shows (columns 2–5) the characteristics of the four subgroups of the population, according to whether or not respondents had diabetes and whether or not they had one or more geriatric conditions (severe form). We compare our subgroup of interest, older adults having diabetes and having one or more geriatric conditions, to the other subgroups. Overall, 3,506 respondents, representing 13.6 million nationally, reported having diabetes. Among those with diabetes, adults who were older, female, unmarried, and of decreased socioeconomic status were more likely to have geriatric conditions.
Table 1.
Characteristics of the Study Population, Overall and by Presence of Diabetes and Geriatric Conditions
| Weighted Percentage* | ||||||
|---|---|---|---|---|---|---|
| Entire 2004 Wave | Diabetes, ≥1 Geriatric Conditions | Diabetes, No Geriatric Conditions | No Diabetes, ≥1 Geriatric Conditions | No Diabetes, No Geriatric Conditions | ||
| n = 18,908, representing 82.0 million | n = 1,611, representing 5.9 million | n = 1,895, representing 7.7 million | n = 4,973, representing 20.0 million | n = 10,429, representing 49.0 million | ||
| Overall Prevalence | 7.2 | 9.4 | 23.8 | 59.6 | ||
| Age (years) | 51-60 | 42.4 | 25.8 | 41.6 | 27.5 | 50.5 |
| 61-70 | 26.8 | 29.2 | 31.2 | 23.5 | 27.2 | |
| 71-80 | 19.7 | 27.5 | 20.7 | 25.8 | 16.1 | |
| >80 | 11.1 | 17.5 | 6.5 | 23.2 | 6.3 | |
| P value | <0.001 | <0.001 | <0.001 | |||
| Gender | Female | 54.4 | 58.3 | 46.0 | 64.0 | 51.4 |
| P value | <0.001 | 0.005 | <0.001 | |||
| Race | Caucasian | 83.9 | 74.3 | 76.4 | 83.8 | 86.2 |
| African-American | 9.3 | 15.3 | 14.8 | 8.9 | 7.8 | |
| Hispanic | 6.9 | 10.5 | 8.8 | 7.3 | 5.9 | |
| P value | 0.32 | <0.001 | <0.001 | |||
| Marital Status | Unmarried | 34.5 | 44.5 | 32.1 | 46.4 | 28.9 |
| P value | <0.001 | 0.24 | <0.001 | |||
| Education (years) | <12 | 20.7 | 39.9 | 22.9 | 29.2 | 14.6 |
| 12 | 33.0 | 32.2 | 34.0 | 35.1 | 32.1 | |
| >12 | 46.3 | 27.9 | 43.2 | 35.7 | 53.3 | |
| P value | <0.001 | <0.001 | <0.001 | |||
| Net Worth (dollars) | ≤40,000 | 22.9 | 41.6 | 26.4 | 31.7 | 16.6 |
| 40,001–155,000 | 23.9 | 27.3 | 28.1 | 24.3 | 22.7 | |
| 155,001–420,000 | 25.7 | 17.6 | 26.1 | 22.7 | 27.8 | |
| >420,000 | 27.5 | 13.6 | 19.4 | 21.3 | 32.9 | |
| P value | <0.001 | <0.001 | <0.001 | |||
| Living Situation | With others | 77.0 | 71.7 | 81.1 | 67.6 | 80.6 |
| Alone | 21.0 | 22.4 | 18.4 | 27.0 | 19.2 | |
| Nursing Facility | 1.9 | 5.9 | 0.5 | 5.4 | 0.3 | |
| P value | <0.001 | 0.008 | <0.001 | |||
*Weighted percentages were derived using Health and Retirement Study (HRS) respondent population weights to adjust for differential probability of selection into the sample and differential non-response.
Proportions are related to the columns and not the rows; the columns for each variable (not the rows) add to 100%. For example, of those respondents having diabetes and ≥ 1 geriatric conditions, 74.3% are Caucasian, 15.3% are African-American, and 10.5% are Hispanic
P value from the χ2 test for the association between the indicated variable and having diabetes/≥1 geriatric conditions. (Respondents in the diabetes/≥1 geriatric conditions subgroup are compared to respondents in each of the other three subgroups for the indicated variable)
Population estimates are rounded to the nearest 100,000
A small number of respondents (n = 429) answered the race/ethnicity question as “other;” these respondents were grouped with Caucasians in the analyses
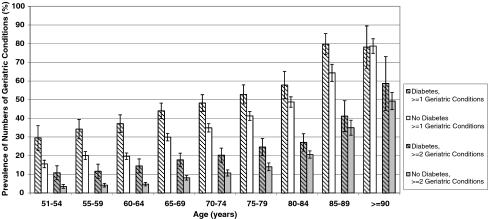
Figure 1 depicts the prevalence of having at least one geriatric condition (severe form) by age (grouped in 5-year increments) for adults with and without diabetes; it also shows the prevalence of having at least two geriatric conditions by age for adults with and without diabetes. We found that adults with diabetes had substantially increased prevalence of geriatric conditions, beginning in and most pronounced in middle-age, compared to adults without diabetes (p < 0.01 for each age group from 51–54 years old to 75–79 years old).
Figure 1.
Prevalence of Numbers of Geriatric Conditions by Age and Diabetes Status. Weighted percentages were derived using Health and Retirement Study (HRS) respondent population weights to adjust for differential probability of selection into the sample and differential non-response. Prevalence results are for 2004. 95% confidence intervals are indicated.
We also determined the prevalence of the eight individual geriatric conditions (severe form) across the age spectrum (Table 2, white columns). All conditions, except low BMI, generally showed substantially and statistically significant greater prevalence in adults with diabetes, compared to those without diabetes.
Table 2.
Prevalence and Incidence of Geriatric Conditions by Age and Diabetes Status
| Weighted Percentage* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| 51-60 (n = 5,150) | 61-70 (n = 6,617) | 71-80 (n = 4,435) | >80 (n = 2,706) | |||||
| Prevalence† | Incidence‡ | Prevalence† | Incidence‡ | Prevalence† | Incidence‡ | Prevalence† | Incidence‡ | |
| Cognitive Impairment | ||||||||
| Diabetes | 2.8 | 1.3 | 3.9 | 2.4 | 5.0 | 4.9 | 16.1 | 9.9 |
| No Diabetes | 1.1 | 0.9 | 1.1 | 0.8 | 3.9 | 2.8 | 14.6 | 9.0 |
| P value | 0.002 | 0.37 | <0.001 | <0.001 | 0.19 | 0.008 | 0.55 | 0.67 |
| Falls | ||||||||
| Diabetes | – | – | 18.4§ | 15.4§ | 21.2 | 15.1 | 29.8 | 23.2 |
| No Diabetes | – | – | 11.9§ | 7.9§ | 14.1 | 12.5 | 24.2 | 22.8 |
| P value | – | – | <0.001§ | <0.001§ | <0.001 | 0.08 | 0.03 | 0.93 |
| Incontinence | ||||||||
| Diabetes | 6.2 | 3.5 | 8.4 | 6.7 | 12.5 | 6.7 | 19.5 | 12.9 |
| No Diabetes | 2.7 | 1.9 | 5.2 | 3.8 | 8.5 | 6.1 | 16.9 | 11.7 |
| P value | <0.001 | 0.02 | <0.001 | <0.001 | 0.001 | 0.43 | 0.24 | 0.63 |
| Dizziness | ||||||||
| Diabetes | 19.3 | – | 18.9 | – | 18.3 | – | 15.5 | – |
| No Diabetes | 9.3 | – | 9.7 | – | 12.4 | – | 18.1 | – |
| P value | <0.001 | – | <0.001 | – | <0.001 | – | 0.70 | – |
| Low BMI | ||||||||
| Diabetes | 0.6 | 0.3 | 0.4 | 0.3 | 0.3 | 0.5 | 4.1 | 0.9 |
| No Diabetes | 1.0 | 0.4 | 1.3 | 0.5 | 2.5 | 1.8 | 6.7 | 3.3 |
| P value | 0.18 | 0.86 | 0.006 | 0.39 | <0.001 | 0.005 | 0.08 | 0.01 |
| Vision Impairment | ||||||||
| Diabetes | 7.3 | 4.4 | 8.8 | 4.4 | 11.9 | 6.0 | 18.9 | 9.1 |
| No Diabetes | 2.8 | 1.7 | 3.0 | 2.0 | 5.0 | 3.6 | 14.2 | 7.9 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.007 | 0.03 | 0.44 |
| Hearing Impairment | ||||||||
| Diabetes | 3.1 | 2.8 | 6.4 | 4.7 | 10.6 | 6.0 | 14.4 | 12.5 |
| No Diabetes | 2.0 | 1.5 | 3.5 | 2.3 | 6.5 | 4.0 | 14.5 | 8.8 |
| P value | 0.13 | 0.03 | <0.001 | <0.001 | <0.001 | 0.05 | 0.98 | 0.11 |
| Pain | ||||||||
| Diabetes | 9.5 | 7.2 | 10.5 | 6.8 | 9.0 | 6.4 | 7.6 | 8.1 |
| No Diabetes | 4.1 | 2.9 | 4.4 | 3.2 | 4.7 | 4.2 | 6.2 | 4.4 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 | 0.17 | 0.02 |
*Weighted percentages were derived using Health and Retirement Study (HRS) respondent population weights to adjust for differential probability of selection into the sample and differential non-response.
P value from the χ2 test for the association between the indicated variable and diabetes status
†Prevalence (2004) of indicated geriatric condition among respondents with diabetes and without diabetes
‡Two-year cumulative incidence (2004–2006) of indicated geriatric condition among respondents with diabetes and without diabetes
§Falls prevalence and incidence were determined for adults 65–70 years old (n = 3,953).
BMI: body mass index
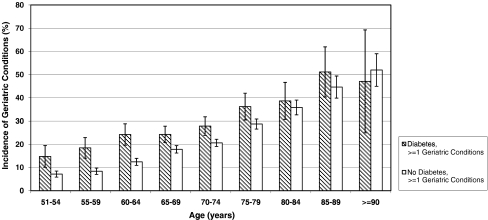
Figure 2 depicts the two-year cumulative incidence of acquiring at least one new geriatric condition (severe form) by age (grouped in 5 year increments) for adults with and without diabetes. Adults with diabetes had substantially increased incidence, again beginning in and most pronounced in middle-age, compared to adults without diabetes (p < 0.01 for each age group from 51–54 years old to 75–79 years old).
Figure 2.
Two-Year Cumulative Incidence of Geriatric Conditions (≥1) by Age and Diabetes Status. Weighted percentages were derived using Health and Retirement Study (HRS) respondent population weights to adjust for differential probability of selection into the sample and differential non-response. Incidence results are for 2006. Confidence intervals of 95% are indicated.
We similarly determined the two-year cumulative incidence of the seven individual geriatric conditions (severe form) across the age range (Table 2, gray columns). All conditions, except low BMI, demonstrated statistically significantly greater incidence in adults with diabetes, compared to those without diabetes, for persons younger than 80 years of age. In contrast, adults age 80 years and older with diabetes, compared to those without diabetes, had greater incidence only of pain.
Next, we examined the association between diabetes and the two-year risk of acquiring new geriatric conditions (Table 3). The odds ratio for this association was statistically significant and remained so in models adjusting for age, other demographic characteristics, and chronic diseases. Testing the model for interactions, we found a statistically significant diabetes-age interaction; the association of diabetes with new geriatric conditions varied with age. As age increased, the strength of the association of diabetes with new geriatric conditions decreased. As a further step (data not shown), we calculated the odds for models with interaction terms (Model 3), for comparison with models without interaction terms (Model 2). For example, for adults age 51–60 years with diabetes, the odds of developing new geriatric conditions is 1.96 times as large as the odds for those without diabetes developing new conditions. In contrast, for adults age 71–80 years with diabetes, the odds of developing new conditions is only 1.25 times as large as the odds for those without diabetes developing new conditions.
Table 3.
Association Between Diabetes and the Two-Year Risk of Acquiring New Geriatric Conditions (≥1)
| Odds Ratio (95% CI) | ||||
|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | Model 4§ | |
| Diabetes | 1.8 (1.6–2.0) | 1.5 (1.3–1.7) | 2.0 (1.4–2.7) | 1.7 (1.3–2.4) |
| Age (years) | ||||
| 61–70 | 2.1 (1.8–2.4) | 2.1 (1.8–2.5) | 2.0 (1.7–2.4) | |
| 71–80 | 3.3 (2.9–3.8) | 3.6 (3.2–4.2) | 3.3 (2.9–3.8) | |
| >80 | 6.3 (5.3–7.5) | 7.1 (5.9–8.6) | 6.5 (5.4–7.8) | |
| Gender | ||||
| Female | 1.2 (1.1–1.4) | 1.2 (1.1–1.4) | 1.2 (1.1–1.3) | |
| Race | ||||
| African-American | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) | 0.9 (0.8–1.1) | |
| Hispanic | 0.9 (0.7–1.0) | 0.9 (0.7–1.0) | 0.9 (0.8–1.1) | |
| Marital Status | ||||
| Married | 0.8 (0.8–1.0) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | |
| Education (years) | ||||
| 12 | 0.7 (0.7–0.8) | 0.7 (0.7–0.8) | 0.8 (0.7–0.8) | |
| ≥12 | 0.6 (0.6–0.7) | 0.6 (0.6–0.7) | 0.7 (0.6–0.8) | |
| Net Worth (dollars) | ||||
| 40,001–155,000 | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | |
| 155,001–420,000 | 0.6 (0.5–0.6) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | |
| >420,000 | 0.5 (0.5–0.6) | 0.5 (0.5–0.6) | 0.6 (0.5–0.7) | |
| Chronic Diseases | ||||
| Hypertension | 1.0 (0.9–1.1) | |||
| Heart Disease | 1.3 (1.2–1.4) | |||
| Chronic Lung Disease | 1.3 (1.1–1.7) | |||
| Cancer | 1.2 (0.8–1.6) | |||
| Stroke | 1.7 (1.4–2.1) | |||
| Musculoskeletal | 1.4 (1.2–1.5) | |||
| Psychiatric | 1.7 (1.4–1.9) | |||
| Diabetes-Age Interaction (years) | ||||
| 61–70 | 0.9 (0.6–1.3) | 0.9 (0.6–1.3) | ||
| 71–80 | 0.6 (0.5–0.9) | 0.7 (0.5–1.0) | ||
| >80 | 0.5 (0.3–0.8) | 0.5 (0.4–0.8) | ||
Odds ratios were derived using Health and Retirement Study (HRS) respondent population weights to adjust for differential probability of selection into the sample and differential non-response.
*Unadjusted
†Adjusted for six sociodemographic characteristics
‡Adjusted for six sociodemographic characteristics and diabetes-age interaction
§Adjusted for six sociodemographic characteristics, seven chronic diseases, and diabetes-age interaction.
Referent groups are: Age, 51-60 years; Gender, male; Race, Caucasian; Marital Status, unmarried; Education, <12 years; Net Worth, ≤40,000 dollars; Chronic Diseases, not having the indicated disease; and Diabetes-Age Interaction, 51–60 years – diabetes
CI: confidence interval.
Last, we performed extensive sensitivity analyses to test our findings (data not shown). First, we repeated the analyses, using the moderate/less active form of the geriatric conditions in place of the severe form. Second, we repeated the analyses examining geriatric conditions in aggregate, excluding conditions not always regarded as geriatric in nature (e.g., pain, dizziness). In these analyses, no substantial differences were discovered in the nature of our findings.
CONCLUSIONS
This study examines the prevalence and incidence of geriatric conditions, in aggregate and individually, among middle- and older-aged adults with and without diabetes. To our knowledge, this is the first study employing nationally representative data to do so. Confirming our hypotheses, we showed that adults with diabetes had increased prevalence and incidence of geriatric conditions, compared to adults without diabetes. These findings were most pronounced in middle-age, revealing a diabetes-age interaction (as age increased, the association of diabetes with new geriatric conditions decreased) and suggesting that adults with diabetes may begin to accumulate “geriatric” conditions in middle-age.
Both diabetes and geriatric conditions have their greatest incidence and prevalence in older adults.17,27 The pathophysiology of diabetes, along with treatments and complications of the disease, can affect multiple organ systems. In turn, multiple organ systems can be implicated in the pathophysiology underlying many of the geriatric conditions. Diabetes, then, has the potential to contribute multiple causal factors to the development of the individual conditions. These causal factors may interact with other risk factors for the conditions, leading diabetes to play a substantial and complex role in the pathophysiology underlying the conditions. For example, diabetes complications and/or co-morbid diseases include vision impairment, hyperglycemia, dehydration, postural hypotension, chronic kidney disease, autonomic and peripheral neuropathy, foot disorders, cardiovascular disease, cerebrovascular disease, and peripheral vascular disease. These complications, impairments, and co-morbid conditions are all potential etiologic factors in falling.3,10,27–29
In some cases, diabetes may play a predominant in causing the conditions, e.g., vision impairment, in some individuals. In other cases, the pathophysiologic relationship between diabetes and geriatric conditions is less clear; for instance, the role of diabetes in causing cognitive impairment is controversial and complex.10 The role played by diabetes in the development of the conditions (direct consequence versus indirect risk factor versus only an association) highlights the underdeveloped taxonomy and conceptual framework of geriatric conditions and syndromes.
Our study found a diabetes-age interaction in the association between diabetes and the risk of developing new geriatric conditions: diabetes was a stronger risk factor for the development of the conditions in middle-age than in old age. One explanation for this interaction is that the effect of diabetes may diminish as adults without diabetes begin to manifest the aging process and develop diseases (other than diabetes) whose incidence and prevalence increase with age (e.g., cardiovascular disease). By the time that both groups reach old age, the effect of age and these other diseases may outweigh or overwhelm the effect of diabetes.
Low BMI was the exception in our analysis of geriatric conditions. Generally, BMIs in the overweight range (greater than 25 kg/m2) are associated with better health status in the older adult population, whereas low BMI is an adverse prognostic marker. In our analysis, low BMI was the only geriatric condition not having increased prevalence and incidence in adults with diabetes. These negative findings for low BMI are not unexpected. First, the relationship of diabetes and insulin resistance with weight gain is well established; thus, the association of a weight-related geriatric condition (low BMI) with diabetes can be expected to differ from the association of the other geriatric conditions (cognitive impairment, falls, incontinence, etc.) with diabetes. Second, our specification for low BMI (less than 18.5 kg/m2) yielded low rates for this condition in the overall sample (both respondents with diabetes and respondents without diabetes).
Of note, although not the focus of our study, we found that stroke and psychiatric disorders were also strongly associated with new geriatric conditions (Model 4). Similar to diabetes, these two disorders may have important and complex roles in the development of the conditions. Thus, our analysis may not be unique to diabetes.
A chief strength of the study is that it is based on a large, nationally representative survey (HRS) that provides data on geriatric conditions.1,21 The determination of cognitive impairment is performance-based. Also, the HRS samples across a broad age range, from middle-age to the oldest old, and it samples respondents living in the community and in long-stay nursing facilities. Finally, the HRS is a biennial longitudinal survey, making possible future studies that examine the association of diabetes with geriatric conditions over multiple waves and that investigate the role of other diseases.
The study has several limitations.1,21 First, the HRS uses self-report data to determine both diabetes and the geriatric conditions, and the conditions chosen for this study are limited by the questions included in the HRS survey. For example, the HRS does not have data on delirium. Second, the HRS does not distinguish between type 1 and type 2 diabetes. However, using data from the National Health and Nutrition Examination Survey (NHANES) and the National Health Interview Survey (NHIS), the Centers for Disease Control (CDC) concluded that 95% of adults with diabetes have type 2 diabetes.30 Third, the conditions chosen are not universally regarded as geriatric in nature (e.g., dizziness, pain). Last, HRS data do not support the calculation of incidence rates, leading us to instead report two-year cumulative incidence.
Middle-aged and older-aged adults with diabetes are at increased risk for the development of geriatric conditions. Although the pathophysiology underlying their development is not fully worked out, it is clear that geriatric conditions contribute substantially to the morbidity and functional impairment of adults with diabetes. The study’s findings suggest that adults with diabetes should be monitored for the development of these conditions beginning at a younger age than previously thought. Questioning patients about symptom burdens such as falling and incontinence is important because these conditions can be managed, resulting in improvement in symptoms and decrease in disability.
Acknowledgement
The authors gratefully acknowledge Cathy Emiline-Fegan for her assistance with manuscript preparation.
Funding Sources Dr. Cigolle was supported by a Ruth L. Kirschstein National Research Service Award from the National Institute on Aging (1F32AG027649-01), the NIH-NCRR KL2 Mentored Clinical Scholars Program at the University of Michigan, the Ann Arbor VA Geriatric Research, Education and Clinical Center (GRECC), and the John A. Hartford Foundation Center of Excellence in Geriatrics at the University of Michigan. Dr. Lee was supported by the Claude D. Pepper Older Americans Independence Center at the University of Michigan and the Ann Arbor VA GRECC. Dr. Langa was supported by National Institute on Aging R01 AG 027010. Dr. Blaum was supported by National Institute on Aging R01 AG021493A and the Ann Arbor VA GRECC. The National Institute on Aging provided funding for the Health and Retirement Study (U01 AG09740), data from which were used in this study.
Conflict of Interest None disclosed.
REFERENCES
- 1.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147(3):156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 2.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348–1353. doi: 10.1001/jama.273.17.1348. [DOI] [PubMed] [Google Scholar]
- 4.Araki A, Ito H. Diabetes mellitus and geriatric syndromes. Geriatr Gerontol Int. 2009;9:105–114. doi: 10.1111/j.1447-0594.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown JS, Seeley DG, Fong J, Black DM, Ensrud KE, Grady D. Urinary incontinence in older women: who is at risk? Study of Osteoporotic Fractures Research Group. Obstet Gynecol. 1996;87(5 Pt 1):715–721. doi: 10.1016/0029-7844(96)00013-0. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 7.Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med. 1999;107(2B):2S–8S. doi: 10.1016/S0002-9343(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 8.Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diab Care. 2002;25(1):61–67. doi: 10.2337/diacare.25.1.61. [DOI] [PubMed] [Google Scholar]
- 9.Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2000;160(2):174–180. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 10.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59(8):818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- 11.Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: the health and retirement study. J Am Geriatr Soc. 2009;57(3):511–516. doi: 10.1111/j.1532-5415.2008.02150.x. [DOI] [PubMed] [Google Scholar]
- 12.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS ONE. 2009;4(1):e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morley JE. The elderly Type 2 diabetic patient: special considerations. Diabet Med. 1998;15(Suppl 4):S41–46. doi: 10.1002/(SICI)1096-9136(1998120)15:4+<S41::AID-DIA747>3.3.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32–38. doi: 10.1210/jc.86.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Ueda T, Tamaki M, Kageyama S, Yoshimura N, Yoshida O. Urinary incontinence among community-dwelling people aged 40 years or older in Japan: prevalence, risk factors, knowledge and self-perception. Int J Urol. 2000;7(3):95–103. doi: 10.1046/j.1442-2042.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- 16.Vinik AI. Diabetic neuropathy: pathogenesis and therapy. Am J Med. 1999;107(2B):17S–26S. doi: 10.1016/S0002-9343(99)00009-1. [DOI] [PubMed] [Google Scholar]
- 17.Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl Guidelines):S265–280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 18.Juster FT, Suzman R. An Overview of the Health and Retirement Study. J Hum Resour. 1995;30(Supplement):S7–S56. doi: 10.2307/146277. [DOI] [Google Scholar]
- 19.Soldo BJ, Hurd MD, Rodgers WL, Wallace RB. Asset and Health Dynamics Among the Oldest Old: an overview of the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. May 1997;52 Spec No:1-20. [DOI] [PubMed]
- 20.Blaum CS, Ofstedal MB, Liang J. Low cognitive performance, comorbid disease, and task-specific disability: findings from a nationally representative survey. J Gerontol A Biol Sci Med Sci. 2002;57(8):M523–531. doi: 10.1093/gerona/57.8.m523. [DOI] [PubMed] [Google Scholar]
- 21.Cigolle CT, Langa KM, Kabeto MU, Blaum CS. Setting eligibility criteria for a care-coordination benefit. J Am Geriatr Soc. 2005;53(12):2051–2059. doi: 10.1111/j.1532-5415.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 22.Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. May 1997;52 Spec No:37-48. [DOI] [PubMed]
- 23.Langa KM, Chernew ME, Kabeto MU, et al. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J Gen Intern Med. 2001;16(11):770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111–117. [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res Nov. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Gordis L. Epidemiology. 2nd ed. Philadelphia: W. B. Saunders; 2000: 32.
- 27.Cigolle CT, Blaum CB. Diabetes and Falls. In: Sinclair A, ed. Diabetes in Old Age. 3rd ed; 2009.
- 28.Volpato S, Leveille SG, Blaum C, Fried LP, Guralnik JM. Risk factors for falls in older disabled women with diabetes: the women’s health and aging study. J Gerontol A Biol Sci Med Sci. 2005;60(12):1539–1545. doi: 10.1093/gerona/60.12.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace C, Reiber GE, LeMaster J, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diab Care. 2002;25(11):1983–1986. doi: 10.2337/diacare.25.11.1983. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed August 25, 2010.