Abstract
Objectives
We examined the presence and correlates of Black/White racial disparities in adherence to guidelines for colorectal cancer screening (CRCS).
Methods
The sample included 328 Black and 1827 White patients age 50–75 from 24 VA medical facilities who responded to a mailed survey with phone follow-up (response rate: 73% for Blacks and 89% for Whites). CRCS adherence and race were obtained through surveys and supplemented with administrative data. Logistic regressions estimated the contribution of demographic, health, cognitive, and environmental factors to racial disparities in adherence to CRCS guidelines.
Results
In unadjusted analyses, Blacks had slightly lower rates of adherence to CRCS guidelines than Whites (72% versus 77%, p < 0.05). This racial disparity in CRCS adherence was explained by race differences in demographic, health, and environmental factors but not by cognitive factors. Tests for interactions revealed that the association of race with adherence varied significantly across levels of income, education, and marital status. In particular, among those who were married with higher levels of education, CRCS adherence was significantly higher for Whites; whereas among those who were unmarried, with low levels of education, adherence was significantly higher for Blacks.
Conclusion
We found that disparities in CRCS are greatly attenuated in the VA system and both Whites and Blacks have substantially higher rates of CRCS than the national average. These results point to the success of the VA at implementing CRCS system-wide. Our findings also suggest additional initiatives may be needed for unmarried low income white men and higher income black men.
KEY WORDS: colorectal cancer, cancer screening, disparities, minority health, Veterans
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death in this country.1 Compared with Whites, Blacks are more likely to present with Stage IV disease and are less likely to survive.2 These disparities are partially attributable to lower rates of CRC screening (CRCS) by Blacks,3–5 which has been shown to significantly reduce CRC mortality.6–9
To understand what factors contribute to CRCS adherence, researchers have drawn on health behavior theories and have examined such factors as cognitions about screening, social and medical environmental factors, and demographic and health-related factors.10–12 Few studies, however, have explored the extent to which racial disparities in adherence to CRCS are a function of these underlying factors, leaving unanswered questions about the best approaches to promote screening among populations with greater disease burden.13–20 This study contributes to the broader literature on race differences in CRCS by examining the relative contribution of demographic and health factors, cognitive factors, and environmental factors to racial disparities in CRCS in a nationally representative survey of Veterans Health Affairs (VHA) patients aged 50–75.
The VHA is a particularly interesting system within which to examine racial disparities in CRCS because it provides equal access to care to all qualifying veterans, and mandates that all patients be assigned to a primary care provider, both of which diminish barriers to screening that might disproportionally affect racial minority and socioeconomically disadvantaged patients.21 Additionally, since the mid-1990s the VHA has made demonstrable advances in improving the quality of preventive care, including CRCS.22–24 With 80% of eligible patients receiving guideline-concordant CRCS,25 CRCS rates in the VHA are notably higher than the national average of less than 60%.26 Because quality improvement has been associated with reductions in disparities,27,28 it is plausible that racial disparities in adherence to CRCS could be nonexistent in the VHA. On the other hand, racial disparities in CRCS adherence could exist within the VHA, because racial disparities in care continue to exist in this setting across a broad range of clinical areas and types of services29 and because Black patients may have fewer cognitive and environmental resources that contribute to adherence. Existing VA studies provide conflicting evidence about the presence of racial disparities in CRCS, and have significant limitations. For example, one study based upon a nationally representative sample of VA patients found lower rates of CRCS among Blacks compared with Whites. This study relied entirely on administrative data, however, and therefore could not adjust for important covariates such CRC knowledge.30 Another published study, which found higher rates of screening among Black compared with White patients, also was based solely on administrative data and was conducted in a single VA site.31 Given the limitations and conflicting findings of prior VA studies addressing this issue, additional studies examining race disparities in CRCS in the VA are warranted.
In this study, we tested the hypothesis that: 1) Blacks would be less likely to be adherent to CRCS than Whites; 2) race differences would persist even after controlling for demographic and health-related factors; and 3) any significant association between Black race and CRCS would be explained by: a) less favorable cognitions about screening; and b) less social and medical environmental support for screening.
METHODS
Conceptual Framework
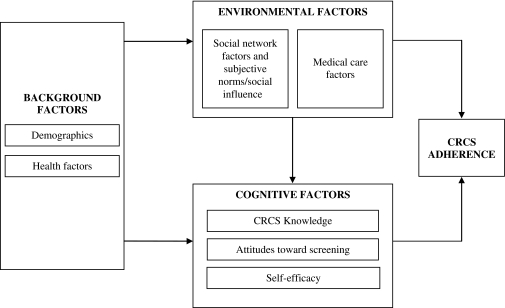
Our hypotheses and analysis plan were guided by a conceptual framework (Fig. 1) based on the Theory of Planned Behavior,32 Social Cognitive Theory,33 and the Health Belief Model.34 The framework assumes that individual demographic and health factors influence adherence through their association with environmental and cognitive factors. Cognitive factors such as knowledge and beliefs about the outcomes associated with CRC screening and the desirability or undesirability of those outcomes (i.e., attitudes toward screening), and perceptions about one’s ability (or self efficacy) to engage in screening, will influence CRC screening adherence by shaping motivation to be screened. These cognitive factors have been shown to vary by race35–41 and hence, may contribute to racial differences in CRCS. The framework assumes that medical and social environmental factors can affect adherence by determining whether an individual has access to screening (i.e., is offered a screening procedure and resources for conducting the procedure are locally available) or by influencing the accessibility of screening (by determining the availability of things such as transportation to and from a colonoscopy appointment). There is evidence that the environmental factors that support screening may be less prevalent among Blacks (e.g., physician recommendation for screening, healthcare access, and social support).42–49
Figure 1.
Conceptual framework.
Study Population/Sampling Frame
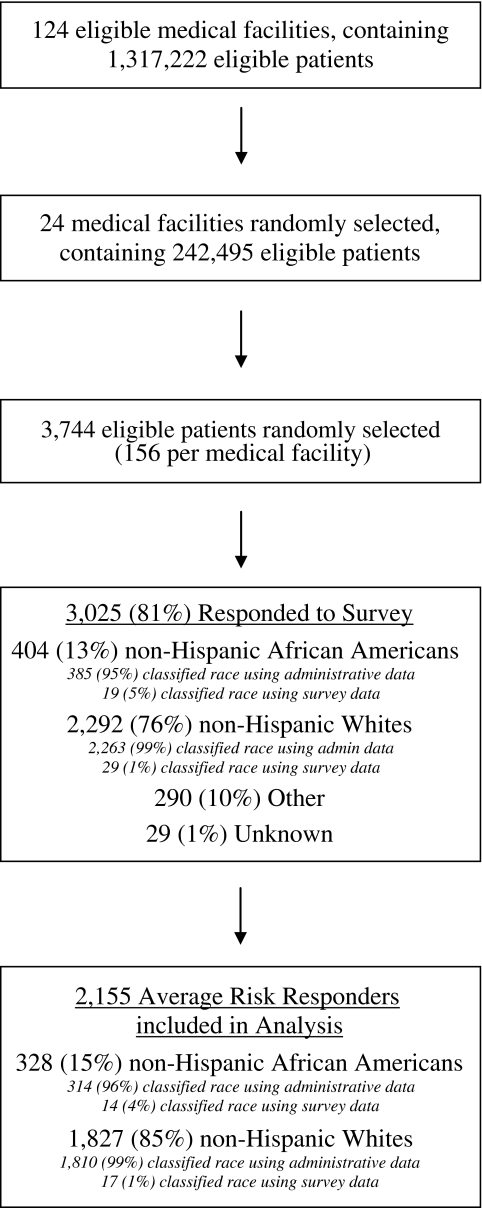
The study population included male and female veterans, age 50–75, with one or more primary care visits between January 2005 and December 2006, at one of 124 VA medical centers participating in a 2003 organizational survey on CRCS and diagnostic practices. VA employees, deceased patients, and anyone enrolled in VA adult day care or nursing home facilities, or diagnosed with CRC, dementia, or Alzheimer’s were excluded. To derive the study sample, the 124 eligible medical centers were grouped into 12 strata according to the size of the eligible patient population and the proportion of Black patients within the site (Fig. 2). Two sites were then randomly selected from each stratum (yielding 24 facilities) and a simple random sample of 156 patients was selected from each sampled site (total sample = 3,744). Of the 3,744 eligible patients, 3,025 responded to the survey (response rate 81% overall, 73% for Blacks, 89% for Whites). This analysis was restricted to the 1827 non-Hispanic White and the 328 non-Hispanic Black patients (referred to as “White” and “Black”) who were at “average risk” for CRC (i.e., no documented inflammatory bowel disease or colorectal polyps diagnosis).
Figure 2.
Subject flow diagram.
Data Collection
The initial survey mailing included a cover letter, a questionnaire, and a $2 cash incentive. A reminder postcard was mailed approximately one week after the first survey mailing. A second survey mailing (with no incentive) was mailed to those who did not return a questionnaire within three to four weeks of the first mailing. Phone administration of the survey was attempted with all participants who did not return a questionnaire within three weeks of the second survey mailing. The 15-page questionnaire (available at http://www.hsrd.minneapolis.med.va.gov/PDF/SCREEN_NationalSurvey.pdf) included measures related to CRCS: patient demographic and health factors and specific cognitive and environmental factors. The Minneapolis VA Medical Center's Subcommittee for Human Studies approved the study's protocol.
Dependent Variables
At the time of the survey, CRCS guidelines recommended men and women aged 50 and older have either a fecal occult blood test (FOBT) annually, sigmoidoscopy or double contrast barium enema (DCBE) every five years, colonoscopy every 10 years, or a combination of annual FOBT and sigmoidoscopy every five years. A patient was classified as adherent if either their self-report (consisting of three items from the CRCS adherence questionnaire developed by the National Cancer Institute50) or VA administrative claims data indicated they were adherent to recommended CRCS guidelines. We used this combined measure of adherence, because it allowed us to avoid loss of observations to missing self-reported data, and to include procedures received outside the VA health care system by study participants (which are likely to vary by race since 67% of Whites versus 53% of Blacks in our sample have a non-VA source of care). Previous research conducted with veterans found acceptable levels of validity for self-reported CRCS behavior using these self-reported measures and did not find racial differences in the validity of self report.51 Specific details of this measure are provided by Partin et al., 2010.52
Patient race Patient race was assessed by self-report. Eighty-six individuals (2.8%) left this field blank. We used administrative data to determine the race of 49 of these and imputed the race of the remaining 37 for whom administrative data were not available.
Demographic/health factors Age (50-64 versus 65-75), gender, and comorbidities were abstracted from VA medical records, and education (≤ high school, some college, ≥ college graduate), income (≤ $20,000, $20,001-40,000, >$40,000), family history (any relatives with colon cancer), and overall health (mean value for a five-category measure ranging from excellent to poor health) were obtained from the questionnaire. Comorbidities were summarized using the Charlson Comorbidity Index53,54 and a measure of mental health diagnoses that categorized individuals as: (a) no mental health diagnosis, (b) single psychiatric diagnosis (ICD-9 codes 290-302 and 306-311) (c) single substance abuse related diagnosis (ICD-9 codes 303-305), or (d) dual diagnosis (psychiatric and substance abuse).
Cognitive factors Two cognitive factors were assessed: CRCS knowledge and attitudes toward screening. CRC knowledge was assessed from two questions assessing familiarity with CRC screening principles and screening guidelines.55 Indicators of correct responses to these questions (i.e., 50 reported as the screening initiation age; strongly agree or agree that someone can have CRC without symptoms) were used in the analyses. Attitudes toward screening were assessed using the following scales developed by Vernon and colleagues:56 salience (four items, Cronbach’s alpha = 0.89), fears (three items, α= 0.69), susceptibility (four items, α= 0.75), screening efficacy (two items, α= 0.65), and self-efficacy (four items, α= 0.80).
Environmental Factors Social environmental factors examined included the four-item tangible support (α = 0.93) and the eight-item emotional/informational support (α = 0.97) subscales of the Medical Outcomes Study (MOS) social support scale, marital status (married, unmarried),57 and the previously validated four-item social influence scale (a measure of subjective norms, α = 0.69).56,58 The MOS tangible and emotional/informational support scales both have five-point Likert response options and their standardized scores range from 1 to 5, with higher scores indicating greater support. Medical environmental factors examined in this study included: (1) patient self-report of receipt of recommendation for CRCS from a physician, (2) whether the patient received care entirely within the VA or had an additional source of care (referred to as “dual use”), and (3) a measure of facility complexity. Facility complexity level is a summary of seven variables representing volume of veterans served by the facility, availability of intensive care units, levels of teaching and research, and patient severity. Facilities are given a score based on these characteristics and are classified into five complexity levels (1 = least complex) based on availability of these resources.59 This measure was dichotomized into low (levels 1 and 2) and high complexity (levels 3–5) for analyses.
Data Analysis and Power
Using the Hsieh et al. method,60 we determined that we had at least 85% power to detect a 10-percentage point difference in the screening adherence rates between Blacks (n = 328) and Whites (n = 1827) in our study sample (N = 2155). We used logistic regression to examine the unadjusted associations between race and CRCS adherence. Next we ran separate logistic regression analyses to examine the extent to which the following sets of factors (identified a priori based on our conceptual model) attenuated the association between race and CRCS adherence: demographic/health-related, cognitive, and social/medical environmental factors. We then tested for interactions between race and items comprising these three sets of factors. The logistic regression models were fit using SAS 9.2 Proc Survey Logistic and were weighted to account for oversampling and stratification. Each model included a random effect for facility to account for the possible interdependence of patients within each site.
Only 3.7% of the data values for the three groups of factors included in these models were missing. However, 1298 (60.2%) of the 2,155 patients in the sample had non-missing values for at least one of the factors. To avoid biases and power reductions that would result from dropping cases with non-missing values from the analyses, we used multiple imputation procedures61,62 and replaced each missing value with five randomly drawn values resulting in five imputed, complete versions of the original data set. We combined the results from separately analyzing these five data sets in order to obtain estimated standard errors reflecting not only the variation from sampling, but also the uncertainty due to imputation, thus preventing biased narrower confidence intervals.
We also adjusted for survey non-response, which was of particular concern in this study because Blacks were less likely to respond to the survey compared with Whites. We fit a logistic regression model to calculate propensity scores (estimated response probabilities) for all veterans in the sample using demographic and clinical covariates from administrative data available for both the respondents and non-respondents. The veterans were ordered according to their propensity scores and then divided into quintiles, all the responders and non-responders in each quintile having similar scores with assumed similar CRCS adherence behavior.63 In each quintile, the sampling weights of the respondents were increased by a factor compensating for the non-respondents to produce sampling weights adjusted for the non-respondents (i.e., the sum of these weights over all the respondents in the sample equaling the size of the target population). These non-response-adjusted sampling weights were then used in conjunction with the logistic regression models analyzing the association of race with CRCS adherence to obtain results adjusted for survey non-response.64
RESULTS
Sample Characteristics
Compared with Whites, Blacks tended to be younger and to have fewer years of education, lower levels of income, poorer health, more medical comorbidities, and were more likely to have diagnoses of substance abuse, psychiatric illness or both (Table 1). Blacks also had slightly higher scores on measures of CRCS salience and CRC susceptibility than Whites, but were less likely to correctly answer the CRC knowledge questions. Black were less likely than Whites to be married and had lower levels of emotional and tangible support, but had higher levels of social influence than Whites. There were no racial differences in physician recommendation for CRCS. Blacks were less likely to have a source of care outside the VA and were more likely to receive care in a high complexity facility.
Table 1.
Distribution of Demographic/Health, Cognitive, and Environmental Factors, Among Survey Respondents, Overall and by Race
| Factors | Respondentsan = 2155 | Black n = 328 | White n = 1827 | P-value |
|---|---|---|---|---|
| Demographic/Health | ||||
| Age: 50-64 | 61% | 78% | 58% | < 0.001 |
| 65-75 | 39% | 22% | 43% | |
| Gender (male) | 96% | 96% | 97% | 0.461 |
| Education: High school or less | 48% | 49% | 48% | 0.029 |
| Some college | 34% | 38% | 34% | |
| College graduate or more | 18% | 13% | 19% | |
| Income: $20,000 or less | 37% | 52% | 35% | < 0.001 |
| $20,001-$40,000 | 33% | 31% | 33% | |
| $40,001 or more | 30% | 18% | 32% | |
| Overall health: Excellent | 5% | 4% | 5% | < 0.001 |
| Very good | 20% | 12% | 21% | |
| Good | 35% | 36% | 34% | |
| Fair | 30% | 34% | 30% | |
| Poor | 11% | 14% | 10% | |
| Family history of CRC | 15% | 14% | 15% | 0.653 |
| Charlson Comorbidity Index (mean) | 1.8 | 2.2 | 1.7 | 0.001 |
| Substance abuse diagnosis | 37% | 52% | 34% | < 0.001 |
| Psychiatric diagnosis | 49% | 64% | 47% | < 0.001 |
| Dual substance/psychiatric diagnosis | 23% | 39% | 21% | < 0.001 |
| Cognitive | ||||
| Knowledge (percentage correct) | ||||
| Initial CRC screening age (50 yrs) | 58% | 47% | 60% | < 0.001 |
| CRC onset w/o symptoms | 68% | 62% | 69% | 0.018 |
| CRC salience (mean) | 4.2 | 4.3 | 4.2 | < 0.001 |
| CRC fears (mean) | 3.4 | 3.4 | 3.4 | 0.419 |
| CRC susceptibility (mean) | 2.7 | 2.8 | 2.7 | 0.008 |
| Screening efficacy (mean) | 4.0 | 4.0 | 4.0 | 0.977 |
| Self efficacy (mean) | 3.7 | 3.8 | 3.7 | 0.065 |
| Environmental | ||||
| Married | 60% | 38% | 64% | < 0.001 |
| Social influence (mean) | 3.6 | 3.7 | 3.6 | < 0.001 |
| MOS Emotional Support (mean) | 3.8 | 3.5 | 3.8 | < 0.001 |
| MOS Tangible Support (mean) | 3.9 | 3.5 | 3.9 | < 0.001 |
| MD recommendation for screening | 85% | 84% | 85% | 0.817 |
| Dual use (VA and non-VA medical care) | 62% | 53% | 67% | < 0.001 |
| VA facility complexity score: low | 50% | 24% | 55% | < 0.001 |
| High | 50% | 76% | 46% | |
aSample respondents: 2155 average-risk, non-Hispanic Black and non-Hispanic White veterans who completed the survey questionnaire. Tests for significance at the p ≤ 0.05 level included chi-square tests for categorical variables and t-tests for continuous variables (i.e., means)
Race and Adherence to CRCS Guidelines
The predictors of CRCS adherence in our model included several demographic/health related factors (age, overall health, Charlson Comorbidity score), cognitive factors (CRC salience, CRC fears, screening efficacy, self-efficacy) and environmental factors (marital status, social influence, low facility complexity, and provider recommendation for screening, which was the strongest predictor in our model). Table 2 shows the association between race and CRCS adherence prior to adjustment (Model 1), and after adjustment for: demographic/health-related factors (Model 2), cognitive factors (Model 3), environmental factors (Model 4), and all factors (Model 5). Before adjusting for demographic/health, cognitive, and environmental factors, Blacks were significantly less likely to be adherent to CRCS guidelines than Whites (72% versus 77%, p < 0.05). The effect of race on adherence to CRCS was not statistically significant when adjusting for demographic/health related factors (Model 2) and environmental factors (Model 4), but remained significant in the model that adjusted for cognitive factors (Model 3).
Table 2.
Odds Ratios from Logistic Regressions Relating CRCS Adherence to Race, Controlling for No Other Factors, Demographic/Clinical Factors, Cognitive Factors, Environmental Factors, All Factors, and Interactions.1,2
| Predictor | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|
| Intercept | 1.45 | 0.04** | 0.28** | 0.01** | 0.01** | |
| Race (Black vs. White) | 0.74** | 0.91 | 0.66** | 0.84 | 0.89 | 3.99**3 |
| Demographic/Health | ||||||
| Age (65–75 vs. 50-74) | 1.70** | 1.85** | 1.86** | |||
| Education (some college vs. ≤ hs) | 0.94 | 0.96 | 1.24 | |||
| Education (≥ college grad vs. ≤ hs) | 1.33 | 1.26 | 1.54 | |||
| Income ($20–$40 K vs. < $20 K) | 1.36 | 1.24 | 0.95 | |||
| Income (> $40 K vs. < $20 K) | 1.37 | 1.14 | 1.01 | |||
| Family hx of disease (vs. absence) | 1.06 | 0.80 | 0.79 | |||
| Overall health (1 unit increment) | 1.17** | 1.09 | 1.09* | |||
| Charlson score (1 unit increment) | 1.07* | 1.07* | 1.08* | |||
| Substance dx (vs. absence) | 0.69 | 0.70 | 0.73 | |||
| Psychiatric dx (vs. absence) | 1.17 | 1.12 | 1.15 | |||
| Dual subst/psych dx (vs. absence) | 0.97 | 1.11 | 1.05 | |||
| Cognitive | ||||||
| Knowledge (correct vs. incorrect) | ||||||
| Initial CRC screening age | 0.92 | 1.01 | 1.00 | |||
| CRC onset w/o symptoms | 1.11 | 1.07 | 1.06 | |||
| CRC salience (1 unit increment) | 1.56** | 1.78** | 1.82** | |||
| CRC fears (1unit increment) | 1.20* | 1.16 | 1.16* | |||
| CRC susceptibility (1 unit incr.) | 1.12 | 1.18 | 1.17 | |||
| Screening efficacy (1 unit incr.) | 1.17* | 1.19* | 1.23* | |||
| Self efficacy (1 unit increment) | 1.36** | 1.36** | 1.33** | |||
| Environmental | ||||||
| Married (vs. not married) | 1.28 | 1.28 | 1.59** | |||
| Social influence (1unit increment) | 1.31** | 0.74** | 0.73** | |||
| MOS Emotional Sup (1 unit incr) | 1.09 | 0.98 | 0.97 | |||
| MOS Tangible Supp (1 unit incr) | 1.02 | 0.98 | 0.98 | |||
| MD rec for screening | 2.98** | 3.19** | 3.16** | |||
| Dual use (VA /nonVA care) | 1.11 | 0.98 | 0.96 | |||
| Low facility complexity (vs. abs) | 1.21 | 1.26* | 1.26* | |||
| Interactions3 | ||||||
| Race x Married | 0.37** | |||||
| Race x Income (< $20 K) | 0.42** | |||||
| Race x Income (> $40 K) | 0.55 | |||||
| Race x Education (some college) | 0.36** | |||||
| Race X Education (≥ college grad) | 0.38 | |||||
1. The odds ratios and p-values have been adjusted for item nonresponse (using multiple imputation: 5 imputed data sets) and survey nonresponse. 2. C-statistic = 51.8% (model 1: race only), 62.6% (model 2: demographic/clinical factors), 58.6% (model 3: cognitive factors), 70.4% (model 4: environmental factors), and 72.3% (model 5: all factors combined); 3. The simple effect of race on CRC screening adherence for the unmarried, medium-income ($20-$40 K), low-educated (≤ high school) veterans; *p ≤ 0.05; **p ≤ 0.01
Interactions Between Race and CRCS Adherence Correlates
Tests for interactions revealed that the association of race with adherence varied significantly across levels of income, education, and marital status, after controlling for other demographic, cognitive, and environmental factors (all interactions were significant at p < 0.05). Model 6, presented in Table 2, adds these interactions to the total model. There was a fairly consistent pattern of results such that, among those who were married with higher levels of education, CRCS adherence was significantly higher for Whites; whereas among those who were unmarried, with low levels of education, adherence was significantly higher for Blacks. For example, among unmarried veterans with a high school degree or less, CRCS adherence rates were 74% for Blacks and 63% for Whites among those making $20,000 or less, and were 88% for Blacks and 64% for Whites among those making between $20,000 and $40,000. Among married veterans with a college degree making over $40,000, CRCS adherence rates were 58% for Blacks and 82% for Whites.
DISCUSSION
We found that Blacks were five percentage points less likely to be adherent to CRCS than Whites. This gap is smaller in magnitude than most prior studies, which have documented Black/White differences in CRCS ranging from 6% to 18%,14,16,18,19,65–67 but is consistent with more recent results from national surveys and a prior VA study that used a nationally representative sample.68 In addition, this observed disparity was no longer present when we adjusted for demographic and health factors. It is also noteworthy that we found no racial difference in physician recommendations for CRCS (84% for Blacks, 85% for Whites) which, as we found, is a strong correlate of CRCS.
The high rates of CRCS adherence among Black and White veterans (72% and 77%, respectively) are considerably higher than national rates among Blacks and Whites during a similar period (48.6% and 56.8%).69 These high screening rates are likely to be due, in part, to various VHA efforts initiated over the past ten years to increase adherence to CRCS, including the implementation of clinical reminders as part of the electronic medical record and an incentivized audit-and-feedback system of performance measures.21–24
This study builds upon prior studies examining racial disparities in adherence to CRCS, only a few of which have examined the extent to which cognitive and environmental factors contribute to disparities.19,66 Counter to our hypotheses, race differences in adherence to CRCS were explained by race differences in demographic/health-related factors and social/medical environmental factors, but not by cognitive factors. Although more research is needed to fully understand this pattern of results, it is likely that race differences in utilization of the VA as a source of care is a contributing factor. For example, one potential explanation for the relatively low rates of screening among disadvantaged White veterans is that these individuals are more likely to live in rural areas than their Black counterparts and therefore may have more difficulty accessing VA care.70,71 Also, the dominant modes of CRC screening differ within and outside the VA. FOBT is the dominant mode of screening within the VA whereas screening by colonoscopy is more common outside the VA. We conducted additional analyses and found that, among those who were adherent to CRCS, Black veterans had significantly higher rates of FOBT (60% vs. 53%, p = 0.025) and lower rates of colonoscopy compared with White veterans (47% vs. 57%, p = 0.012). It may be the case that higher SES Black veterans, who are more likely than their lower SES Black counterparts to receive care outside the VA, are less likely to be offered a colonoscopy than whites receiving care outside the VA. Alternatively, higher SES blacks may be less likely than their white counterparts to find the option of colonoscopy acceptable (perhaps because it is an invasive procedure or because they are more likely to be unmarried and hence lack the necessary support required for the procedure).
There are several limitations to this study. Because the combined measure is based in part on self-report, and some over reporting of adherence does occur in this population,51 this measure may have led to some overestimation of adherence. However, our adherence estimates (from data collected in 2007) correspond closely to the 2007 adherence estimate of 78% derived from the VA national performance measurement system (based on medical records only).72 Additionally, the results of this study—particularly high rates of screening and the “reversal” of disparities that we found among disadvantaged patients—might not apply outside of the VA, which has made great strides at implementing CRCS system-wide.21
These results have several implications for research, policy, and practice. First, other healthcare systems can build on the VA’s success in promoting CRCS. Second, while the use of FOBT as the primary screening mode in VA has been successful, it is critical to ensure that positive FOBTs are appropriately followed up with diagnostic colonoscopy and to ensure that Black patients are as likely as white patients to receive appropriate follow-up. Two recent studies conducted in the VA, including one study based on a nationally representative cohort of VA patients,30,73 suggest that, although follow-up rates are similar for Black and White veterans, they are disappointingly low for both groups. Finally, there is a need for additional research to understand the “reverse” disparities we found among less advantaged, White veterans. If it is the case that living in a rural setting accounts for low rates of CRCS among disadvantaged White veterans, it will be critical to develop strategies to improve screening in this population.70,71 Additional research is also needed to understand why Black veterans are less likely to be screened by colonoscopy than their White counterparts.
Acknowledgements
This project was supported by three grants from VA Health Services Research & Development: Investigator Initiated grant #IIR 04-042-2 (Partin); Career Development Award #CDTA 03-174 (Fisher); and Merit Review Entry Program award #MRP 04-412-1 (Burgess).
Conflict of Interest None disclosed.
References
- 1.Cancer Facts and Figures 2007. Atlanta: American Cancer Society; 2007.
- 2.Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104(3):629–639. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 3.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006;24(14):2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 4.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med Clin North Am. 2005;89(4):771–793. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Berry J, Bumpers K, Ogunlade V, et al. Examining racial disparities in colorectal cancer care. J Psychosoc Oncol. 2009;27(1):59–83. doi: 10.1080/07347330802614840. [DOI] [PubMed] [Google Scholar]
- 6.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124(2):544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100(3):515–523. doi: 10.1111/j.1572-0241.2005.41829.x. [DOI] [PubMed] [Google Scholar]
- 8.Palmer RC, Schneider EC. Social disparities across the continuum of colorectal cancer: a systematic review. Cancer Causes Control. 2005;16(1):55–61. doi: 10.1007/s10552-004-1253-3. [DOI] [PubMed] [Google Scholar]
- 9.Gornick ME, Eggers PW, Riley GF. Associations of race, education, and patterns of preventive service use with stage of cancer at time of diagnosis. Health Serv Res. 2004;39(5):1403–1427. doi: 10.1111/j.1475-6773.2004.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38(5):536–550. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 11.McQueen A, Vernon SW, Myers RE, Watts BG, Lee ES, Tilley BC. Correlates and predictors of colorectal cancer screening among male automotive workers. Cancer Epidemiol Biomark Prev. 2007;16(3):500–509. doi: 10.1158/1055-9965.EPI-06-0757. [DOI] [PubMed] [Google Scholar]
- 12.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19(4):339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 13.Fisher DA, Dougherty K, Martin C, Galanko J, Provenzale D, Sandler RS. Race and colorectal cancer screening: a population-based study in North Carolina. N C Med J. 2004;65(1):12–15. [PubMed] [Google Scholar]
- 14.Seeff LC, Nadel MR, Klabunde CN, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100(10):2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 15.James TM, Greiner KA, Ellerbeck EF, Feng C, Ahluwalia JS. Disparities in colorectal cancer screening: a guideline-based analysis of adherence. Ethn Dis. 2006;16(1):228–233. [PubMed] [Google Scholar]
- 16.Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168(12):1317–1324. doi: 10.1001/archinte.168.12.1317. [DOI] [PubMed] [Google Scholar]
- 17.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41(1):23–29. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 national health interview survey. Cancer Epidemiol Biomark Prev. 2008;17(7):1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 19.O'Malley AS, Forrest CB, Feng S, Mandelblatt J. Disparities despite coverage: gaps in colorectal cancer screening among Medicare beneficiaries. Arch Intern Med. 2005;165(18):2129–2135. doi: 10.1001/archinte.165.18.2129. [DOI] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Schellhase KG, Sparapani RA, Laud PW, Neuner JM. Disparities in colon cancer screening in the Medicare population. Arch Intern Med. 2007;167(3):258–264. doi: 10.1001/archinte.167.3.258. [DOI] [PubMed] [Google Scholar]
- 21.Chao HH, Schwartz AR, Hersh J, et al. Improving colorectal cancer screening and care in the veterans affairs healthcare system. Clin Colorectal Cancer. 2009;8(1):22–28. doi: 10.3816/CCC.2009.n.004. [DOI] [PubMed] [Google Scholar]
- 22.Evans DC, Nichol WP, Perlin JB. Effect of the implementation of an enterprise-wide electronic health record on productivity in the Veterans Health Administration. Health Econ Policy Law. 2006;1(Pt 2):163–169. doi: 10.1017/S1744133105001210. [DOI] [PubMed] [Google Scholar]
- 23.Kizer KW. The "new VA": a national laboratory for health care quality management. Am J Med Qual. 1999;14(1):3–20. doi: 10.1177/106286069901400103. [DOI] [PubMed] [Google Scholar]
- 24.Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the veterans affairs health care system on the quality of care. N Engl J Med. 2003;348(22):2218–2227. doi: 10.1056/NEJMsa021899. [DOI] [PubMed] [Google Scholar]
- 25.Performance OoQa. Measure master report for national quarter 2 FY2009: Veteran's Health Administration; 2009.
- 26.Enhancing use and quality of colorectal cancer screening February 2–4 2010: final panel statement. Maryland: Bethesda; 2010. [Google Scholar]
- 27.Sequist TD, Adams A, Zhang F, Ross-Degnan D, Ayanian JZ. Effect of quality improvement on racial disparities in diabetes care. Arch Intern Med. 2006;166(6):675–681. doi: 10.1001/archinte.166.6.675. [DOI] [PubMed] [Google Scholar]
- 28.Sehgal AR. Impact of quality improvement efforts on race and sex disparities in hemodialysis. Jama. 2003;289(8):996–1000. doi: 10.1001/jama.289.8.996. [DOI] [PubMed] [Google Scholar]
- 29.Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and Ethnic Disparities in the VA Health Care System: A Systematic Review. Journal of General Internal Medicine. 2008. [DOI] [PMC free article] [PubMed]
- 30.Etzioni DA, Yano EM, Rubenstein LV, et al. Measuring the quality of colorectal cancer screening: the importance of follow-up. Dis Colon Rectum. 2006;49(7):1002–1010. doi: 10.1007/s10350-006-0533-2. [DOI] [PubMed] [Google Scholar]
- 31.Dolan NC, Ferreira MR, Fitzgibbon ML, et al. Colorectal cancer screening among African-American and white male veterans. Am J Prev Med. 2005;28(5):479–482. doi: 10.1016/j.amepre.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Ajzen I. The Theory of Planned Behavior. Organ Behav Hum Decis Process. 1991;50:179–211. doi: 10.1016/0749-5978(91)90020-T. [DOI] [Google Scholar]
- 33.Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health. 1998;13:623–649. doi: 10.1080/08870449808407422. [DOI] [Google Scholar]
- 34.Rosenstock I, Kirscht J. The health belief model and personal health behavior.
- 35.Weinrich SP, Weinrich MC, Boyd MD, Johnson E, Frank-Stromborg M. Knowledge of colorectal cancer among older persons. Cancer Nurs. 1992;15(5):322–330. doi: 10.1097/00002820-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Jepson C, Kessler LG, Portnoy B, Gibbs T. Black-white differences in cancer prevention knowledge and behavior. Am J Public Health. 1991;81(4):501–504. doi: 10.2105/AJPH.81.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf RL, Zybert P, Brouse CH, et al. Knowledge, beliefs, and barriers relevant to colorectal cancer screening in an urban population: a pilot study. Fam Community Health. 2001;24(3):34–47. doi: 10.1097/00003727-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Greiner KA, James AS, Born W, et al. Predictors of fecal occult blood test (FOBT) completion among low-income adults. Prev Med. 2005;41(2):676–684. doi: 10.1016/j.ypmed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman RK, Tabbarah M, Trauth J, Nowalk MP, Ricci EM. Predictors of lower endoscopy use among patients at three inner-city neighborhood health centers. J Urban Health. 2006;83(2):221–230. doi: 10.1007/s11524-005-9028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janz NK, Wren PA, Schottenfeld D, Guire KE. Colorectal cancer screening attitudes and behavior: a population-based study. Prev Med. 2003;37(6 Pt 1):627–634. doi: 10.1016/j.ypmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 41.McAlearney AS, Reeves KW, Dickinson SL, et al. Racial differences in colorectal cancer screening practices and knowledge within a low-income population. Cancer. 2008;112(2):391–398. doi: 10.1002/cncr.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogedegbe G, Cassells AN, Robinson CM, et al. Perceptions of barriers and facilitators of cancer early detection among low-income minority women in community health centers. J Natl Med Assoc. 2005;97(2):162–170. [PMC free article] [PubMed] [Google Scholar]
- 43.Finney Rutten LJ, Nelson DE, Meissner HI. Examination of population-wide trends in barriers to cancer screening from a diffusion of innovation perspective (1987-2000) Prev Med. 2004;38(3):258–268. doi: 10.1016/j.ypmed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Brenes GA, Paskett ED. Predictors of stage of adoption for colorectal cancer screening. Prev Med. 2000;31(4):410–416. doi: 10.1006/pmed.2000.0729. [DOI] [PubMed] [Google Scholar]
- 45.Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare. Washington: National Academy Press; 2002. Medicine; Io, ed.
- 46.Morrow GR, Way J, Hoagland AC, Cooper R. Patient compliance with self-directed Hemoccult testing. Prev Med. 1982;11(5):512–520. doi: 10.1016/0091-7435(82)90065-2. [DOI] [PubMed] [Google Scholar]
- 47.Richardson JL, Danley K, Mondrus GT, Deapen D, Mack T. Adherence to screening examinations for colorectal cancer after diagnosis in a first-degree relative. Prev Med. 1995;24(2):166–170. doi: 10.1006/pmed.1995.1030. [DOI] [PubMed] [Google Scholar]
- 48.Thomas W, White CM, Mah J, Geisser MS, Church TR, Mandel JS. Longitudinal compliance with annual screening for fecal occult blood. Minnesota Colon Cancer Control Study. Am J Epidemiol. 1995;142(2):176–182. doi: 10.1093/oxfordjournals.aje.a117616. [DOI] [PubMed] [Google Scholar]
- 49.El-Serag HB, Petersen L, Hampel H, Richardson P, Cooper G. The use of screening colonoscopy for patients cared for by the Department of Veterans Affairs. Arch Intern Med. 2006;166(20):2202–2208. doi: 10.1001/archinte.166.20.2202. [DOI] [PubMed] [Google Scholar]
- 50.Vernon SW, Meissner H, Klabunde C, et al. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiologic research. Cancer Epidemiol Biomark Prev. 2004;13(6):898–905. [PubMed] [Google Scholar]
- 51.Partin MR, Grill J, Noorbaloochi S, et al. Validation of self-reported colorectal cancer screening behavior from a mixed-mode survey of veterans. Cancer Epidemiol Biomark Prev. 2008;17(4):768–776. doi: 10.1158/1055-9965.EPI-07-0759. [DOI] [PubMed] [Google Scholar]
- 52.Partin MR, Noorbaloochi S, Grill J, et al. The interrelationships between and contributions of background, cognitive, and environmental factors to colorectal cancer screening adherence. Cancer Causes Control. [DOI] [PubMed]
- 53.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 54.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 55.Nelson DE, Kreps GL, Hesse BW, et al. The Health Information National Trends Survey (HINTS): development, design, and dissemination. J Health Commun. 2004;9(5):443–460. doi: 10.1080/10810730490504233. [DOI] [PubMed] [Google Scholar]
- 56.Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol Biomark Prev. 1997;6(10):825–832. [PubMed] [Google Scholar]
- 57.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med (1982) 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-B. [DOI] [PubMed] [Google Scholar]
- 58.Tiro JA, Vernon SW, Hyslop T, Myers RE. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening among African Americans and Caucasians. Cancer Epidemiol Biomark Prev. 2005;14(12):2855–2861. doi: 10.1158/1055-9965.EPI-05-0217. [DOI] [PubMed] [Google Scholar]
- 59.Yano EM, Soban LM, Parkerton PH, Etzioni DA. Primary care practice organization influences colorectal cancer screening performance. Health Serv Res. 2007;42(3 Pt 1):1130–1149. doi: 10.1111/j.1475-6773.2006.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17(14):1623–1634. doi: 10.1002/(SICI)1097-0258(19980730)17:14<1623::AID-SIM871>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 61.Little RJ, Rubin DB. Statistical analysis with missing data; 2002.
- 62.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 63.Little RJA. Survey nonresponse adjustments for estimates of means. Int Stat Rev. 1986;54:139–157. doi: 10.2307/1403140. [DOI] [Google Scholar]
- 64.Smith PJ, Rao JNK, Battaglia MP, Ezzati-Rice TM, Daniels D, Khare M. Compensating for provider nonresponse using response propensity to form adjustment cells: the national immunization survey. Vital and Health Statistics. 2001;2:133. [PubMed] [Google Scholar]
- 65.Schenck AP, Klabunde CN, Davis WW. Racial differences in colorectal cancer test use by Medicare consumers. Am J Prev Med. 2006;30(4):320–326. doi: 10.1016/j.amepre.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Shokar NK, Carlson CA, Weller SC. Factors associated with racial/ethnic differences in colorectal cancer screening. J Am Board Fam Med. 2008;21(5):414–426. doi: 10.3122/jabfm.2008.05.070266. [DOI] [PubMed] [Google Scholar]
- 67.Rao RS, Graubard BI, Breen N, Gastwirth JL. Understanding the factors underlying disparities in cancer screening rates using the Peters-Belson approach: results from the 1998 National Health Interview Survey. Med Care. 2004;42(8):789–800. doi: 10.1097/01.mlr.0000132838.29236.7e. [DOI] [PubMed] [Google Scholar]
- 68.Use of colorectal cancer tests-United States, 2002, 2004, and 2006. Morbidity and Mortality Weekly Report. 2008;57(10):253–8. [PubMed]
- 69.2007 National Healthcare Disparities Report. Rockville: U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 70.Weeks WB, Kazis LE, Shen Y, et al. Differences in health-related quality of life in rural and urban veterans. Am J Public Health. 2004;94(10):1762–1767. doi: 10.2105/AJPH.94.10.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weeks WB, Wallace AE, West AN, Heady HR, Hawthorne K. Research on rural veterans: an analysis of the literature. J Rural Health. 2008;24(4):337–344. doi: 10.1111/j.1748-0361.2008.00179.x. [DOI] [PubMed] [Google Scholar]
- 72.Fihn SD. Improving quality: lessons from the Department of Veterans Affairs. Circulation. 2009;2(4):294–296. doi: 10.1161/CIRCOUTCOMES.109.884353. [DOI] [PubMed] [Google Scholar]
- 73.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomark Prev. 2006;15(6):1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]