Abstract
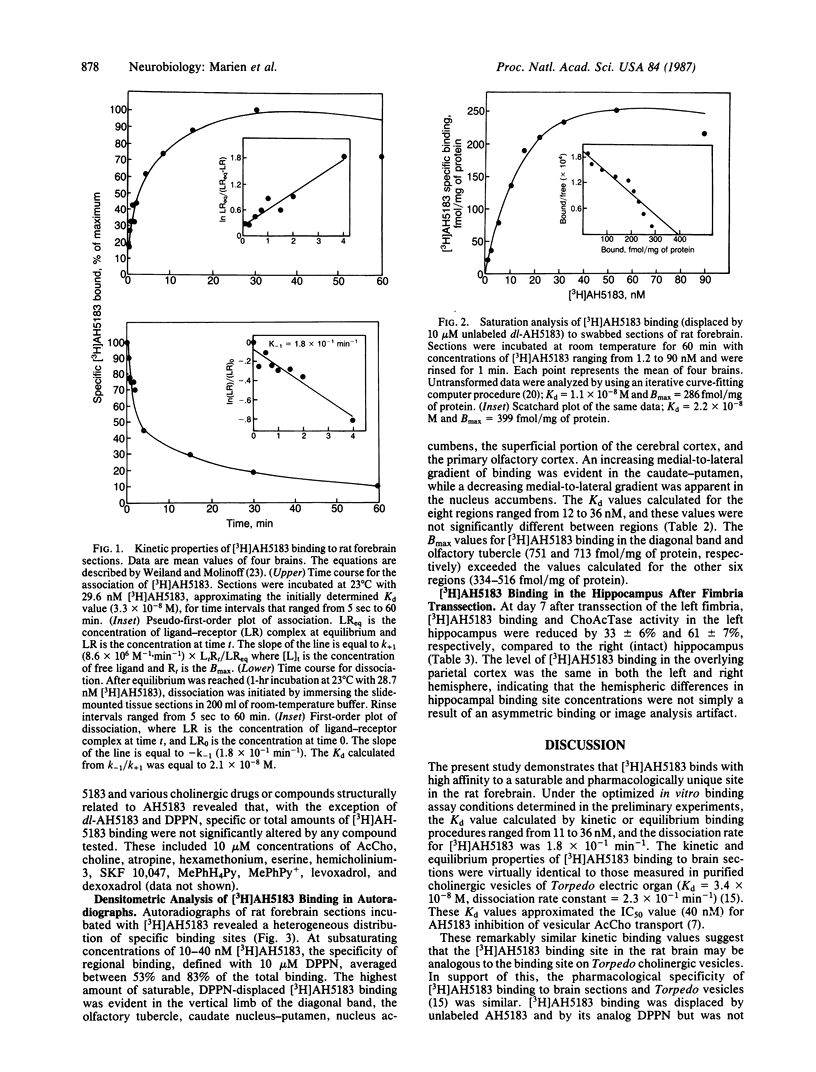
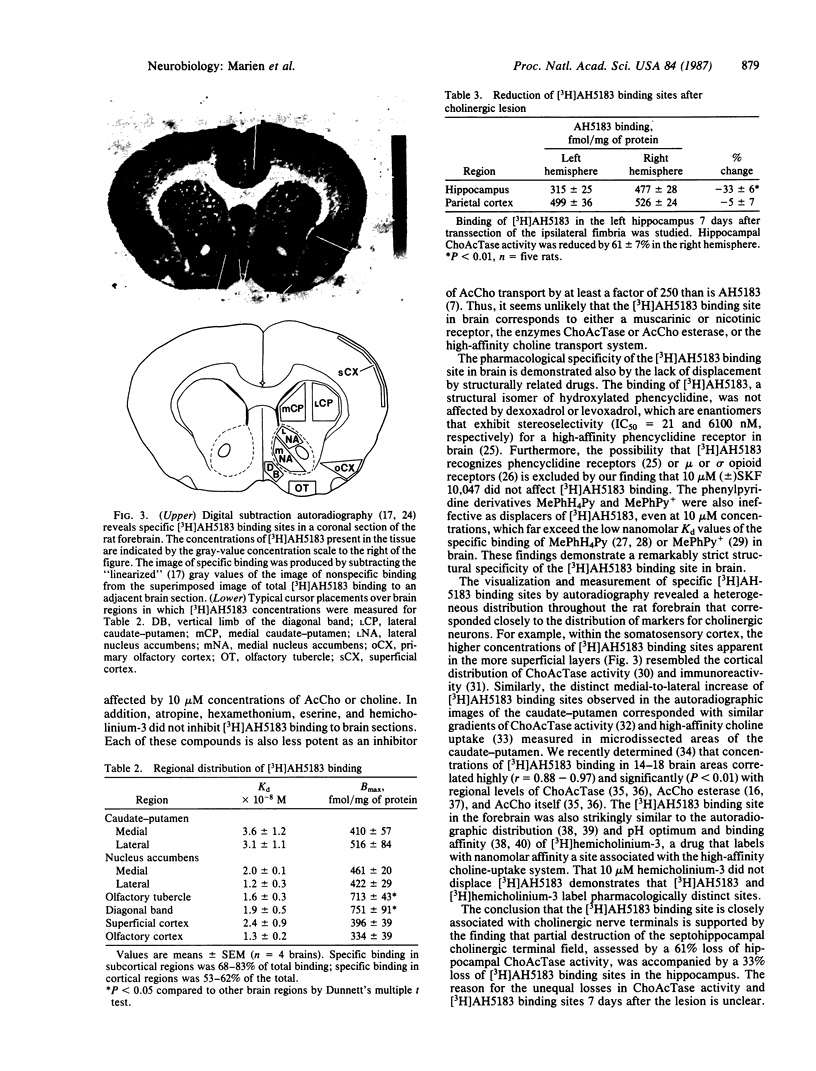
2-(4-Phenylpiperidino)cyclohexanol (AH5183) is a noncompetitive and potent inhibitor of high-affinity acetylcholine transport into cholinergic vesicles. It is reported here that [3H]AH5183 binds specifically and saturably to slide-mounted sections of the rat forebrain (Kd = 1.1 to 2.2 X 10(-8) M; Bmax = 286 to 399 fmol/mg of protein). The association and dissociation rate constants for [3H]AH5183 binding are 8.6 X 10(6) M-1 X min-1 and 0.18 min-1, respectively. Bound [3H]AH5183 can be displaced by nonradioactive AH5183 and by the structural analog (2 alpha,3 beta,4A beta,8A alpha)-decahydro-3-(4-phenyl-1-piperidinyl)-2- naphthalenol but not by 10 microM concentrations of the cholinergic drugs acetylcholine, choline, atropine, hexamethonium, eserine, or hemicholinium-3 or by the structurally related compounds 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, 1-methyl-4-phenylpyridine, (+/-)-N-allylnormetazocine (SKF 10,047), levoxadrol, or dexoxadrol. Quantitative autoradiography reveals that [3H]AH5183 binding sites are distributed heterogenously throughout the rat forebrain and are highly localized to cholinergic nerve terminal regions. At the level of the caudate nucleus-putamen, the highest concentrations of saturable [3H]AH5183 binding (713-751 fmol/mg of protein) are found in the vertical limb of the diagonal band and the olfactory tubercle, with lesser amounts (334-516 fmol/mg of protein) in the caudate-putamen, nucleus accumbens, superficial layers of the cerebral cortex, and the primary olfactory cortex. At day 7 after transsection of the left fimbria, [3H]AH5183 binding and choline acetyltransferase activity in the left hippocampus were reduced by 33 +/- 6% and 61 +/- 7%, respectively. These findings indicate that [3H]AH5183 binds to a unique recognition site in rat brain that is topographically associated with cholinergic nerve terminals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altar C. A., O'Neil S., Walter R. J., Jr, Marshall J. F. Brain dopamine and serotonin receptor sites revealed by digital subtraction autoradiography. Science. 1985 May 3;228(4699):597–600. doi: 10.1126/science.2580352. [DOI] [PubMed] [Google Scholar]
- Altar C. A., Walter R. J., Jr, Neve K. A., Marshall J. F. Computer-assisted video analysis of [3H]spiroperidol binding autoradiographs. J Neurosci Methods. 1984 Mar;10(3):173–188. doi: 10.1016/0165-0270(84)90054-2. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., King S. C., Parsons S. M. Pharmacological characterization of the acetylcholine transport system in purified Torpedo electric organ synaptic vesicles. Mol Pharmacol. 1983 Jul;24(1):48–54. [PubMed] [Google Scholar]
- Bahr B. A., Parsons S. M. Acetylcholine transport and drug inhibition kinetics in Torpedo synaptic vesicles. J Neurochem. 1986 Apr;46(4):1214–1218. doi: 10.1111/j.1471-4159.1986.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Bahr B. A., Parsons S. M. Demonstration of a receptor in Torpedo synaptic vesicles for the acetylcholine storage blocker L-trans-2-(4-phenyl[3,4-3H]-piperidino) cyclohexanol. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2267–2270. doi: 10.1073/pnas.83.7.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss C. I., James A. T. Fitting the rectangular hyperbola. Biometrics. 1966 Sep;22(3):573–602. [PubMed] [Google Scholar]
- Brittain R. T., Levy G. P., Tyers M. B. The neuromuscular blocking action of 2-(4-phenylpiperidino) cyclohexanol (AH 5183). Eur J Pharmacol. 1969 Oct;8(1):93–99. doi: 10.1016/0014-2999(69)90133-2. [DOI] [PubMed] [Google Scholar]
- Carroll P. T. The effect of the acetylcholine transport blocker 2-(4-phenylpiperidino) cyclohexanol (AH5183) on the subcellular storage and release of acetylcholine in mouse brain. Brain Res. 1985 Dec 9;358(1-2):200–209. doi: 10.1016/0006-8993(85)90964-3. [DOI] [PubMed] [Google Scholar]
- Cheney D. L., LeFevere H. F., Racagni G. Choline acetyltransferase activity and mass fragmentographic measurement of acetylcholine in specific nuclei and tracts of rat brain. Neuropharmacology. 1975 Nov;14(11):801–809. doi: 10.1016/0028-3908(75)90107-0. [DOI] [PubMed] [Google Scholar]
- Collier B., Welner S. A., Rícný J., Araujo D. M. Acetylcholine synthesis and release by a sympathetic ganglion in the presence of 2-(4-phenylpiperidino) cyclohexanol (AH5183). J Neurochem. 1986 Mar;46(3):822–830. doi: 10.1111/j.1471-4159.1986.tb13046.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Gandiha A., Marshall I. G. The effects of 2-(4-phenylpiperidino)-cyclohexanol (AH5183) on the acetylcholine content of, and output from, the chick biventer cervicis muscle preparation. Int J Neurosci. 1973;5(5):191–196. doi: 10.3109/00207457309149474. [DOI] [PubMed] [Google Scholar]
- Guyenet P., Euvrard C., Javoy F., Herbert A., Glowinski J. Regional differences in the sensitivity of cholinergic neurons to dopaminergic drugs and quipazine in the rat striatum. Brain Res. 1977 Nov 18;136(3):487–500. doi: 10.1016/0006-8993(77)90073-7. [DOI] [PubMed] [Google Scholar]
- Hoover D. B., Muth E. A., Jacobowitz D. M. A mapping of the distribution of acetycholine, choline acetyltransferase and acetylcholinesterase in discrete areas of rat brain. Brain Res. 1978 Sep 22;153(2):295–306. doi: 10.1016/0006-8993(78)90408-0. [DOI] [PubMed] [Google Scholar]
- Houser C. R., Crawford G. D., Salvaterra P. M., Vaughn J. E. Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: a study of cholinergic neurons and synapses. J Comp Neurol. 1985 Apr 1;234(1):17–34. doi: 10.1002/cne.902340103. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., Uhl G. R., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: characterization and localization of receptor binding sites in rat and human brain. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4591–4595. doi: 10.1073/pnas.81.14.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R. S., Johnson G. V. Quinacrine and 2-(4-phenylpiperidino)cyclohexanol (AH5183) inhibit acetylcholine release and synthesis in rat brain slices. Mol Pharmacol. 1986 Jan;29(1):45–51. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshall I. G. A comparison between the blocking actions of 2-(4-phenylpiperidino) cyclohexanol (AH 5183) and its N-methyl quaternary analogue (AH 5954). Br J Pharmacol. 1970 Sep;40(1):68–77. doi: 10.1111/j.1476-5381.1970.tb10611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I. G. Studies on the blocking action of 2-(4-phenyl piperidino) cyclohexanol (AH5183). Br J Pharmacol. 1970 May;38(3):503–516. doi: 10.1111/j.1476-5381.1970.tb10592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega W. P., Howard B. D. Biochemical evidence that vesicles are the source of the acetylcholine released from stimulated PC12 cells. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6535–6538. doi: 10.1073/pnas.81.20.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn L. G., Kerchner G. A., Kalra V., Zimmerman D. M., Leander J. D. Phencyclidine receptors in rat brain cortex. Biochem Pharmacol. 1984 Nov 15;33(22):3529–3535. doi: 10.1016/0006-2952(84)90133-3. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Altar C. A., Wong C. A., Marshall J. F. Quantitative analysis of [3H]spiroperidol binding to rat forebrain sections: plasticity of neostriatal dopamine receptors after nigrostriatal injury. Brain Res. 1984 Jun 4;302(1):9–18. doi: 10.1016/0006-8993(84)91280-0. [DOI] [PubMed] [Google Scholar]
- Otero D. H., Wilbekin F., Meyer E. M. Effects of 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (AH5183) on rat cortical synaptosome choline uptake, acetylcholine storage and release. Brain Res. 1985 Dec 16;359(1-2):208–214. doi: 10.1016/0006-8993(85)91430-1. [DOI] [PubMed] [Google Scholar]
- Rainbow T. C., Parsons B., Wieczorek C. M. Quantitative autoradiography of [3H]hemicholinium-3 binding sites in rat brain. Eur J Pharmacol. 1984 Jun 15;102(1):195–196. doi: 10.1016/0014-2999(84)90360-1. [DOI] [PubMed] [Google Scholar]
- Rea M. A., Simon J. R. Regional distribution of cholinergic parameters within the rat striatum. Brain Res. 1981 Aug 31;219(2):317–326. doi: 10.1016/0006-8993(81)90294-8. [DOI] [PubMed] [Google Scholar]
- Sandberg K., Coyle J. T. Characterization of [3H]hemicholinium-3 binding associated with neuronal choline uptake sites in rat brain membranes. Brain Res. 1985 Dec 2;348(2):321–330. doi: 10.1016/0006-8993(85)90451-2. [DOI] [PubMed] [Google Scholar]
- Satoh K., Armstrong D. M., Fibiger H. C. A comparison of the distribution of central cholinergic neurons as demonstrated by acetylcholinesterase pharmacohistochemistry and choline acetyltransferase immunohistochemistry. Brain Res Bull. 1983 Dec;11(6):693–720. doi: 10.1016/0361-9230(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Su T. P. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther. 1982 Nov;223(2):284–290. [PubMed] [Google Scholar]
- Toll L., Howard B. D. Evidence that an ATPase and a protonmotive force function in the transport of acetylcholine into storage vesicles. J Biol Chem. 1980 Mar 10;255(5):1787–1789. [PubMed] [Google Scholar]
- Weiland G. A., Molinoff P. B. Quantitative analysis of drug-receptor interactions: I. Determination of kinetic and equilibrium properties. Life Sci. 1981 Jul 27;29(4):313–330. doi: 10.1016/0024-3205(81)90324-6. [DOI] [PubMed] [Google Scholar]
- Wieczorek C. M., Parsons B., Rainbow T. C. Quantitative autoradiography of [3H]MPTP binding sites in rat brain. Eur J Pharmacol. 1984 Mar 2;98(3-4):453–454. doi: 10.1016/0014-2999(84)90299-1. [DOI] [PubMed] [Google Scholar]
- Wood P. L., Cheney D. L., Costa E. Modulation of the turnover rate of hippocampal acetylcholine by neuropeptides: possible site of action of alpha-melanocyte-stimulating hormone, adrenocorticotrophic hormone and somatostatin. J Pharmacol Exp Ther. 1979 Apr;209(1):97–103. [PubMed] [Google Scholar]




