Abstract
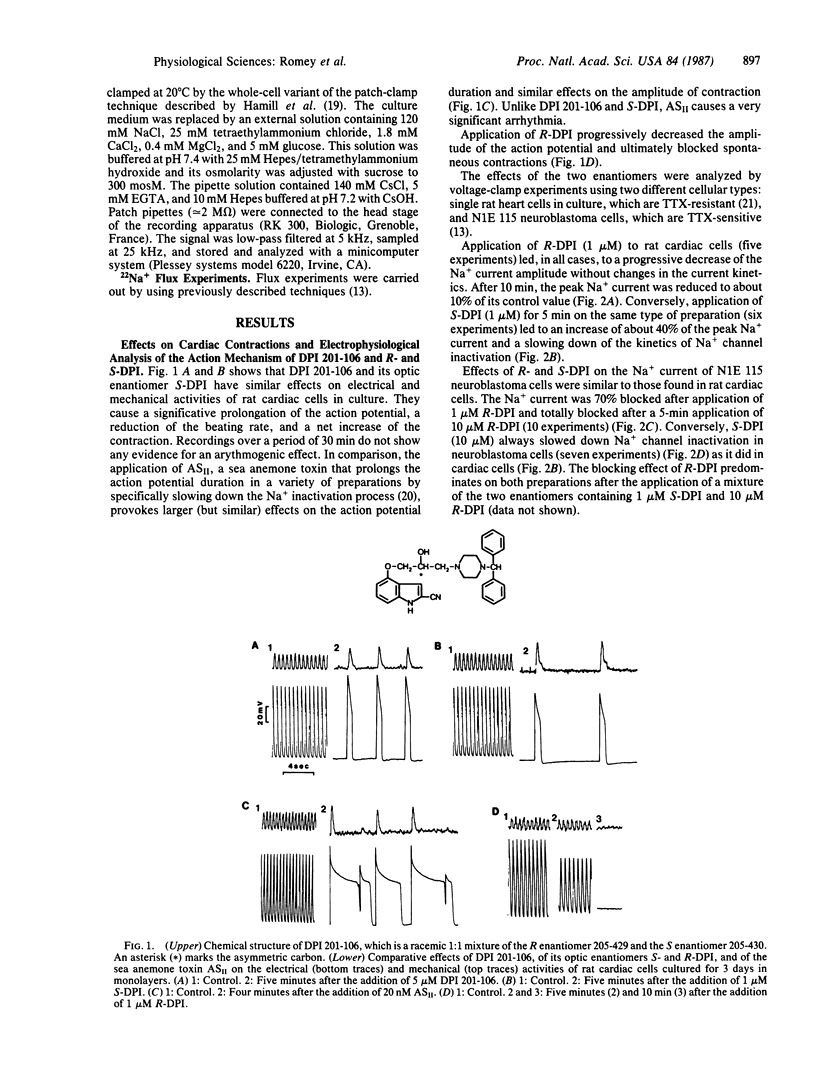
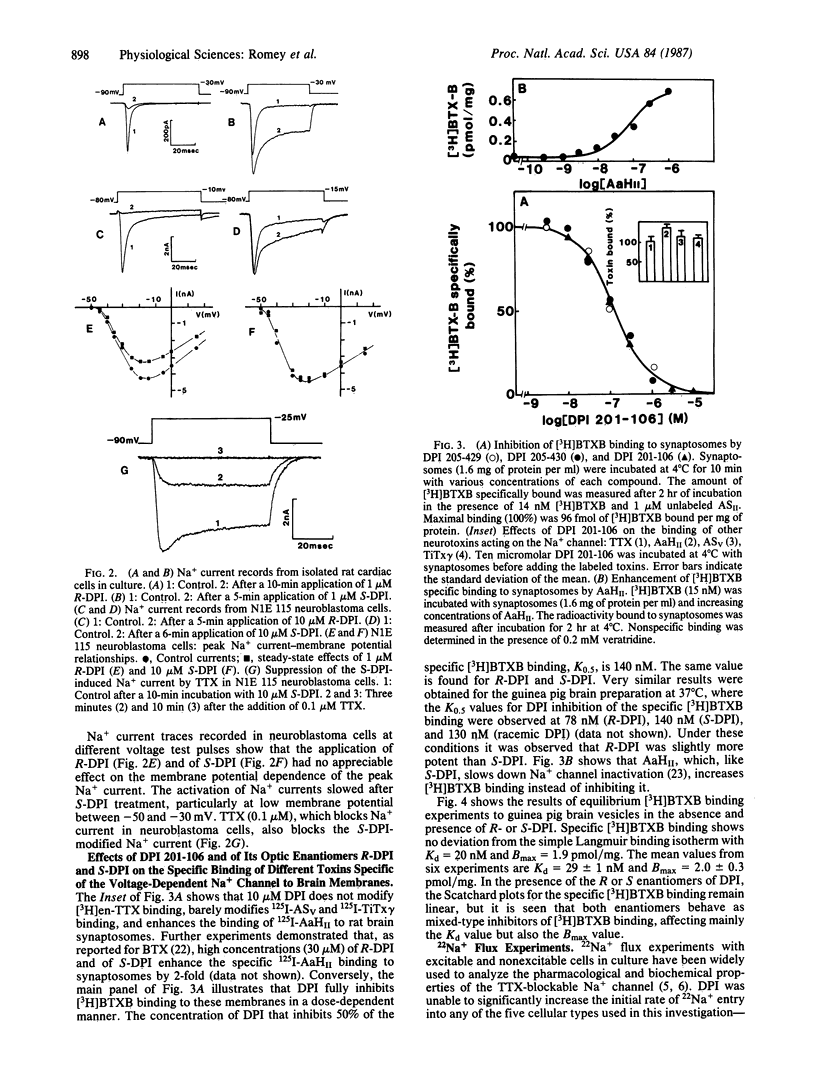
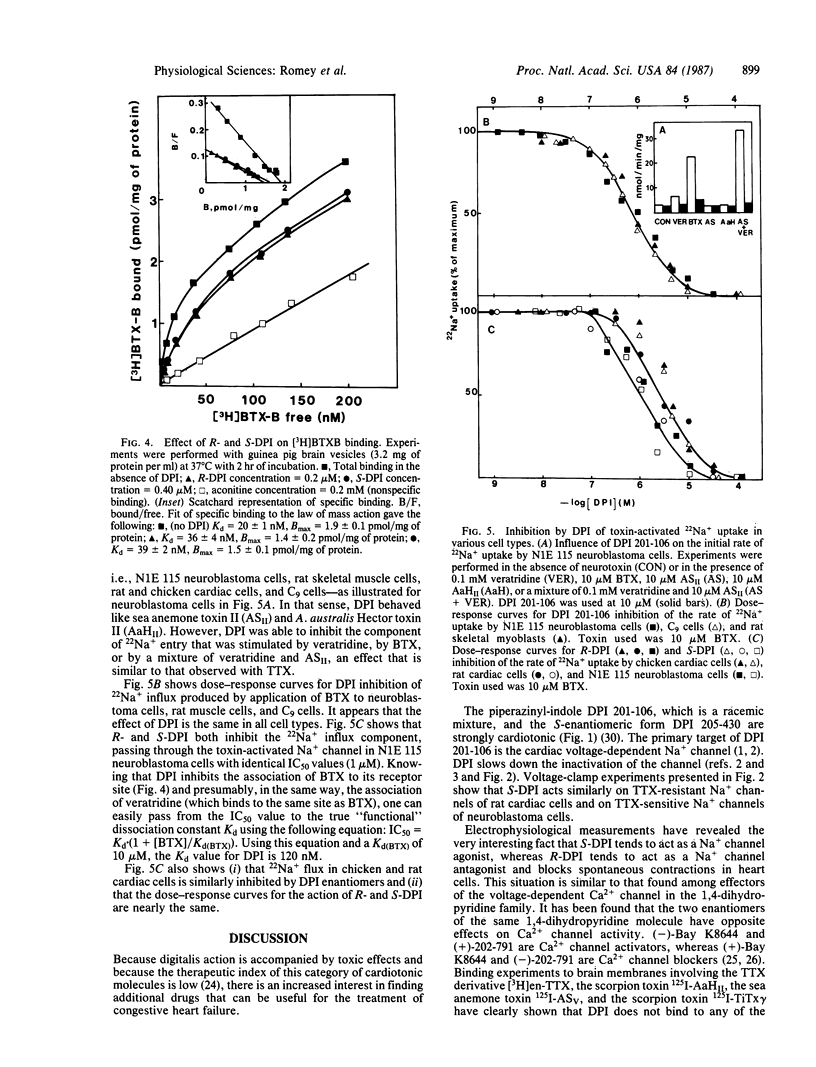
This paper shows the interaction of the cardiotonic agent 4-[3-(4-diphenylmethyl-1-piperazinyl)-2-hydroxypropoxy]-1H-indole- 2-carbonitrile (DPI 201-106) and its optic enantiomers R-DPI (205-429) and S-DPI (205-430) with the Na+ channel of a variety of excitable cells. Voltage-clamp experiments show that DPI 201-106 acts on neuroblastoma cells and rat cardiac cells. S-DPI (205-430) increases the peak Na+ current, slows down the kinetics of Na+ channel inactivation, and is cardiotonic on heart cells. Conversely, R-DPI (205-429) reduces the peak Na+ current and blocks Na+ channel activity and cardiac contractions. Binding experiments using radioactively labeled toxins indicate that DPI 201-106 and its enantiomers do not interact with sites already identified for tetrodotoxin or sea anemone and scorpion toxins. DPI 201-106 and its enantiomers inhibit binding of a 3H-labeled batrachotoxin derivative, [3H]batrachotoxinin A 20-alpha-benzoate, to brain membranes. The dissociation constant of the complex formed between the Na+ channel and both R-DPI and S-DPI is Kd congruent to 100 nM. 22Na+ uptake experiments using different cell types have shown that R and S enantiomers of DPI 201-106 are active on the different Na+ channel subtypes with similar IC50 values. These results are discussed in relation with the cardiotonic properties of DPI 201-106 that are not accompanied by cardiotoxic effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abita J. P., Chicheportiche R., Schweitz H., Lazdunski M. Effects of neurotoxins (veratridine, sea anemone toxin, tetrodotoxin) on transmitter accumulation and release by nerve terminals in vitro. Biochemistry. 1977 May 3;16(9):1838–1844. doi: 10.1021/bi00628a012. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Giglio J. R., Léopold P., Schmid A., Sampaio S. V., Lazdunski M. Tityus serrulatus venom contains two classes of toxins. Tityus gamma toxin is a new tool with a very high affinity for studying the Na+ channel. J Biol Chem. 1982 Nov 10;257(21):12553–12558. [PubMed] [Google Scholar]
- Buggisch D., Isenberg G., Ravens U., Scholtysik G. The role of sodium channels in the effects of the cardiotonic compound DPI 201-106 on contractility and membrane potentials in isolated mammalian heart preparations. Eur J Pharmacol. 1985 Dec 3;118(3):303–311. doi: 10.1016/0014-2999(85)90141-4. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S., Daly J. W., Brown G. B. Binding of batrachotoxinin A 20-alpha-benzoate to a receptor site associated with sodium channels in synaptic nerve ending particles. J Biol Chem. 1981 Sep 10;256(17):8922–8927. [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Chicheportiche R., Balerna M., Lombet A., Romey G., Lazdunski M. Synthesis of new, highly radioactive tetrodotoxin derivatives and their binding properties to the sodium channel. Eur J Biochem. 1980 Mar;104(2):617–625. doi: 10.1111/j.1432-1033.1980.tb04466.x. [DOI] [PubMed] [Google Scholar]
- Creveling C. R., McNeal E. T., Daly J. W., Brown G. B. Batrachotoxin-induced depolarization and [3H]batrachotoxinin-a 20 alpha-benzoate binding in a vesicular preparation from guinea pig cerebral cortex. Mol Pharmacol. 1983 Mar;23(2):350–358. [PubMed] [Google Scholar]
- Franckowiak G., Bechem M., Schramm M., Thomas G. The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels. Eur J Pharmacol. 1985 Aug 15;114(2):223–226. doi: 10.1016/0014-2999(85)90631-4. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Schweitz H., Lazdunski M. The interaction of sea anemone and scorpion neurotoxins with tetrodotoxin-resistant Na+ channels in rat myoblasts. A comparison with Na+ channels in other excitable and non-excitable cells. Mol Pharmacol. 1984 Jul;26(1):70–74. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Medzihradsky F., Woods J. H., Dahlstrom P. J. Stereospecific binding of 3H-phencyclidine in brain membranes. Life Sci. 1982 Jun 21;30(25):2147–2154. doi: 10.1016/0024-3205(82)90288-0. [DOI] [PubMed] [Google Scholar]
- Hof R. P., Rüegg U. T., Hof A., Vogel A. Stereoselectivity at the calcium channel: opposite action of the enantiomers of a 1,4-dihydropyridine. J Cardiovasc Pharmacol. 1985 Jul-Aug;7(4):689–693. doi: 10.1097/00005344-198507000-00012. [DOI] [PubMed] [Google Scholar]
- Honerjäger P. Cardioactive substances that prolong the open state of sodium channels. Rev Physiol Biochem Pharmacol. 1982;92:1–74. doi: 10.1007/BFb0030502. [DOI] [PubMed] [Google Scholar]
- Jacques Y., Fosset M., Lazdunski M. Molecular properties of the action potential Na+ ionophore in neuroblastoma cells. Interactions with neurotoxins. J Biol Chem. 1978 Oct 25;253(20):7383–7392. [PubMed] [Google Scholar]
- Kohlhardt M., Fröbe U., Herzig J. W. Modification of single cardiac Na+ channels by DPI 201-106. J Membr Biol. 1986;89(2):163–172. doi: 10.1007/BF01869712. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Renaud J. F. The action of cardiotoxins on cardiac plasma membranes. Annu Rev Physiol. 1982;44:463–473. doi: 10.1146/annurev.ph.44.030182.002335. [DOI] [PubMed] [Google Scholar]
- Lombet A., Kazazoglou T., Delpont E., Renaud J. F., Lazdunski M. Ontogenic appearance of Na+ channels characterized as high affinity binding sites for tetrodotoxin during development of the rat nervous and skeletal muscle systems. Biochem Biophys Res Commun. 1983 Feb 10;110(3):894–901. doi: 10.1016/0006-291x(83)91046-x. [DOI] [PubMed] [Google Scholar]
- Morrison M., Bayse G. S. Catalysis of iodination by lactoperoxidase. Biochemistry. 1970 Jul 21;9(15):2995–3000. doi: 10.1021/bi00817a010. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Postma S. W., Catterall W. A. Inhibition of binding of [3H]batrachotoxinin A 20-alpha-benzoate to sodium channels by local anesthetics. Mol Pharmacol. 1984 Mar;25(2):219–227. [PubMed] [Google Scholar]
- Ray R., Morrow C. S., Catterall W. A. Binding of scorpion toxin to receptor sites associated with voltage-sensitive sodium channels in synaptic nerve ending particles. J Biol Chem. 1978 Oct 25;253(20):7307–7313. [PubMed] [Google Scholar]
- Renaud J. F., Kazazoglou T., Lombet A., Chicheportiche R., Jaimovich E., Romey G., Lazdunski M. The Na+ channel in mammalian cardiac cells. Two kinds of tetrodotoxin receptors in rat heart membranes. J Biol Chem. 1983 Jul 25;258(14):8799–8805. [PubMed] [Google Scholar]
- Renaud J. F., Scanu A. M., Kazazoglou T., Lombet A., Romey G., Lazdunski M. Normal serum and lipoprotein-deficient serum give different expressions of excitability, corresponding to different stages of differentiation, in chicken cardiac cells in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7768–7772. doi: 10.1073/pnas.79.24.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Renaud J. F., Fosset M., Lazdunski M. Pharmacological properties of the interaction of a sea anemone polypeptide toxin with cardiac cells in culture. J Pharmacol Exp Ther. 1980 Jun;213(3):607–615. [PubMed] [Google Scholar]
- Scholtysik G., Salzmann R., Berthold R., Herzig J. W., Quast U., Markstein R. DPI 201-106, a novel cardioactive agent. Combination of cAMP-independent positive inotropic, negative chronotropic, action potential prolonging and coronary dilatory properties. Naunyn Schmiedebergs Arch Pharmacol. 1985 May;329(3):316–325. doi: 10.1007/BF00501887. [DOI] [PubMed] [Google Scholar]
- Thormann J., Kramer W., Kindler M., Kremer F. P., Schlepper M. Comparative efficacy of the new cardiotonic agent DPI 201-106 versus dobutamine in dilated cardiomyopathy: analysis by serial pressure/volume relations and "on-line" MVO2 assessment. J Cardiovasc Pharmacol. 1986 Jul-Aug;8(4):749–757. [PubMed] [Google Scholar]
- Vincent J. P., Balerna M., Barhanin J., Fosset M., Lazdunski M. Binding of sea anemone toxin to receptor sites associated with gating system of sodium channel in synaptic nerve endings in vitro. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1646–1650. doi: 10.1073/pnas.77.3.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]


