Abstract
T cell-mediated immunotherapy against malignancies has been shown to be effective for certain types of cancer. However, ex vivo expansion of tumor-reactive T cells has been hindered by the low precursor frequency of such cells, often requiring multiple rounds of stimulation, resulting in full differentiation, loss of homing receptors and potential exhaustion of the expanded T cells. Here, we show that when using highly purified naïve CD8+ T cells, a single stimulation with peptide-pulsed, IFNγ/LPS-matured dendritic cells in combination with the sequential use of IL-21, IL-7 and IL-15 is sufficient for extensive expansion of antigen-specific T cells. Short-term expanded T cells were tumor-reactive, multifunctional and retained a central-memory-like phenotype (CD62L+, CCR7+, CD28+). The procedure is highly reproducible and robust as demonstrated for different healthy donors and for cancer patients. Such short-term tumor-antigen-primed, multifunctional T cells may therefore serve as a platform to target different malignancies accessible to immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0928-8) contains supplementary material, which is available to authorized users.
Keywords: T cell immunotherapy, Antigen-specific T cells, T cell differentiation, Central memory T cells
Introduction
Targeting malignant cells with antigen-specific T cells is an attractive therapeutic strategy due to the high specificity of the T cell receptor [1–3]. Clinical studies of adoptive therapy with transfer of large numbers of tumor-reactive T cells have provided clear evidence of T cell-mediated anti-tumor effects that can lead to objective and sometimes complete responses in cancer patients [4–7]. Adoptive therapy generally requires in vitro expansion of large numbers of tumor-reactive cells for infusion to achieve therapeutic benefit. Such antigen-specific T cells in cancer patients often must be derived from the naïve autologous repertoire, since the immunogenicity of the tumor is insufficient to prime and activate antigen-specific T cells in vivo. After allogeneic stem cell transplantation, donor-derived tumor-reactive T cells can be used to augment the graft-versus-tumor effect. In this setting, donor lymphocyte infusions (DLI) have become a standard procedure for certain hematologic malignancies. Modifications of this principle aim at increasing the specificity of the infused cells by in vitro expansion. As the donor has not been exposed to the tumor antigens, again the T cells need to be derived from the naïve T cell repertoire.
Accessing the naïve T cell repertoire has been hindered by the exceedingly low frequency of precursor T cells, often requiring multiple cycles of in vitro stimulation to even begin detecting specifically reactive T cells. A multitude of relatively low-efficiency protocols to accomplish this have been published, employing different antigen-presenting cells for stimulation, such as dendritic cells, B cells [8] or artificial APC [9], and different cytokines to promote expansion [10]. Initial protocols of adoptive T cell transfer in humans were based entirely on wide ranging doses of supplemental IL-2, but more recent studies have demonstrated advantages of using IL-7, IL-15 and IL-21 for the expansion of antigen-specific T cells [10–12]. Even with efforts to optimize antigen presentation and use of these novel cytokines, repetitive stimulations, the use of enrichment methods or even cloning by limiting dilution remain necessary [10, 13]. These approaches require a prolonged culture time, which has four major disadvantages: (1) current GMP requirements for cells to be transferred to humans make a culture period of 6–8 weeks extremely laborious and costly; (2) feeder cells are generally required to supplement growth, and validation of such feeder cell lines for clinical use adds significantly to costs and labor; (3) poor in vivo persistence is generally observed after transfer due to the repetitive in vitro stimulations that can result in exhaustion and/or reduced proliferative capacity of the generated cells and acquisition of a terminally differentiated phenotype; and (4) repetitive stimulation commonly leads to selection of T cells with an oligoclonal repertoire.
In order to avoid such prolonged culture times, a different approach is being pursued by expanding vaccine-primed T cells by use of OKT3/CD28-stimulation to enhance immunity in lymphopenic patients [14, 15]. Such short-term expanded T cells have the advantage of maintained multifunctionality and high proliferative capacity after transfer, but are limited in the specificity of the transferred cells.
In this study, we describe a method to prime naïve, human CD8+ T cells by a single stimulation with peptide-pulsed dendritic cells (DC). We show that the T cells proliferate vigorously when exposed to IL-21, IL-7 and IL-15 sequentially. Most importantly, the cells remain multifunctional, retain lymph node homing receptors such as CD62L and CCR7, and express receptors that can receive costimulatory signals, thus resembling a central memory phenotype. The method reproducibly yields high numbers of antigen-specific T cells when using the melanosomal antigen Melan-A as model antigen, but is also applicable for other tumor-associated antigens. In addition, we show that naïve antigen-specific T cells from cancer patients after chemotherapy can be successfully expanded. We suggest that such “primed lymphocyte infusions” offer new therapeutic opportunities for immunotherapy in cancer.
Materials and methods
Donors and patients
Peripheral blood mononuclear cells (PBMC) were obtained from HLA-A0201+ healthy donors by leukapheresis after obtaining informed consent (in accordance with the ethical committee, University of Würzburg). Samples from glioblastoma patients were collected by leukapheresis from patients enrolled in an immunotherapy study for relapsed, high-grade glioma, conducted at the University of Leuven, Belgium (PI: Prof. SW. Van Gool). These patients (age 25–45) underwent leukapheresis for generation of a tumor vaccine, consisting of tumor-lysate pulsed dendritic cells as published elsewhere [16]. At the time of leukapheresis, patients had undergone surgery for relapse and were off corticosteroids for at least 1 week. The use of PBMC for scientific analysis from these patients was approved by the ethical committee of the University of Leuven, Belgium.
Media, peptides, pentamers and antibodies
T cells were maintained in Cellgenix DC medium (Cellgenix, Freiburg, Germany) supplemented with 5% human serum off the clot (PAA, Austria) and 0.4% penicillin/streptomycin (Biochrom, Berlin, Germany). All peptides (except for HM1.24) and directly conjugated MHC-multimers (pentamers) were purchased from Proimmune, Oxford, UK. The peptides used were Melan-A26–35, Melan-A26–35A27L, TRP2180–188, gp100209–217M, IL13Rα345–353, MELOE-136–44 and HM1.2422–30 (purchased from JPT, Berlin, Germany). Pentamers were obtained for Melan-A26–35A27L, MELOE-136–44 and gp100209–217M. The following antibodies were used at optimal staining concentrations: CD8-PerCP-Cy5.5, PD1-FITC, CD95L-PE, Perforin-FITC, Granzyme B-PE, CD62L-FITC, IL-2-PE (from eBioscience, San Diego, USA); CD45RO-PE, CD45RA-FITC, IFN-γ-APC, TNFα-FITC (from BD Pharmingen, Heidelberg, Germany); CCR7-PE (from R&D, Wiesbaden, Germany); CD27-FITC and CD28-PE (from BioLegend, San Diego, USA). Cells were analyzed using a FACS-Calibur (BD Pharmingen, Heidelberg, Germany) and FloJo software (Treestar, Ashland, Oregon). Cells were evaluated by gating according to light scatter characteristics first, and then gated on relevant markers (e.g. CD8 and MHC-multimer+ cells).
Priming protocol
For dendritic cell generation, monocytes were isolated by plastic adherence and cultured in 6-well plates using 3 ml of Cellgro DC medium supplemented with 1% human serum and penicillin/streptomycin plus GM-CSF (800 IU/ml, Leucomax, Novartis, Basel, Switzerland) and IL-4 (1,000 IU/ml, Peprotech, Hamburg, Germany). After 48 h of culture, 1.5 ml of medium was added (+GM-CSF at 1,600 IU/ml and IL4 at 1,000 IU/ml). 24 h later, nonadherent cells were harvested, and large cells (mostly immature DC) were counted, resuspended in fresh medium containing GM-CSF 800 IU/ml, IL-4 1,000 IU/ml, LPS from E. coli O55:B5 at 10 ng/ml (Sigma, Deisenhofen, Germany) and IFNγ (Peprotech, 100 IU/ml), and plated at ~106 DC per well in 2 ml. Peptide was added for a final concentration of 2.5 μg/ml and pulsed DC were subsequently incubated overnight. The next day nonadherent cells were discarded, and adherent DC were gently removed using cold PBS/1% HS after incubation on ice for 20 min. Large cells consisting of mature DC were counted. The cells were irradiated with 30 Gy to avoid outgrowth of few potentially contaminating NK or memory T cells and were then used for T cell stimulation.
Naïve CD8 T cells were isolated from PBMC by an initial negative selection using a CD8 negative selection kit (Miltenyi, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Antigen-experienced CD8+ T cells were then depleted using CD45RO- and CD57-beads and an LD column. Isolation was performed on the day prior to stimulation (day −1) and the purified naïve T cells were maintained in T cell medium, supplemented with IL-7 (Peprotech, 5 ng/ml). The next day, T cells were harvested, and resuspended in T cell medium supplemented with IL-21 (Peprotech, 30 ng/ml). Irradiated DC were added at a 1:4 DC:T cell ratio with 4 × 105 T cells per well of a 48-well plate. Total volume of each well was 500 μl. 72 h after initiation of the culture, 500 μl T cell medium with IL-7 and IL-15 (Peprotech, 5 ng/ml final concentration) was added and cells were subsequently fed every 2–3 days as outline in the results section. Evaluation was done at day 10 or 11 as indicated.
Pentamer staining, CFSE-labeling
105 T cells were stained for 20 min at room temperature, using a 1:200 dilution of the respective pentamer, followed by staining with additional antibodies for phenotyping. CFSE was obtained from Invitrogen (Karlsruhe, Germany) and T cells were stained according to the manufacturer’s instructions.
Intracellular cytokine staining
The assay was performed as previously described [17]. Briefly, 2 × 105 T cells were stimulated with 5 × 104 antigen-presenting cells (APC) for 5 h in the presence of brefeldin A (Sigma), and subsequently fixed, permeabilized (Fix/Perm and Perm/Wash solution from BD Biosciences, Heidelberg, Germany) and stained. Autologous monocytes isolated with CD14-beads (Miltenyi) were generally used as APC, but in some experiments, as indicated, T2-cells were alternatively used as APC. When using monocytes as APC, the monocytes were pulsed with the respective peptide and cultured overnight with GM-CSF and IL-4.
Chromium release assay
The 4-h assay was performed following standard procedures as previously described [10].
Cell lines
The melanoma cell lines FM55, FM88 and Mel2a were obtained from Prof. Dr. J. Becker, Department of Dermatology, University of Würzburg, Germany. The glioblastoma cell line U373 was obtained from Drs. Vince and Hagemann, Department of Neurosurgery, University of Würzburg, Germany.
Enrichment of pentamer+ T cells
Naïve T cells were isolated as described above. 107 naïve T cells were incubated in 100 μl of a 1:20 solution of APC-labeled pentamer for 20 min, and 40 μl of anti-APC-beads (Miltenyi) were then added and incubated for 15 min at 4°C. The cells were resuspended in wash buffer, spun down and transferred onto an MS column for positive selection and subsequently eluted.
Quantitative PCR/real-time PCR
Total RNA was isolated using the High Pure RNA Isolation Kit (Roche, Germany), and DNA digestion was performed using RNAse-free DNAse (Qiagen). The mRNA from dendritic cell populations was transcribed into cDNA using the Superscript III RNAseH reverse transcriptase (Invitrogen, Carlsbad, USA). Amplification of specific cDNA and quantification of PCR products were performed using Power SYBR® Green Mastermix on a 7500 Real-Time PCR System (Applied Biosystems, Darmstadt, Germany). The efficiency of the PCR reactions was tested for each primer pair and ranged between 1.92 and 2.0. The sequences for the primers will be provided upon request. The expression of genes of specific interest were normalized to the house-keeping gene GAPDH.
Analysis of the TCR repertoire
The diversity of the TCR repertoire of peripheral T cells was analyzed by the immunoscope method as described elsewhere [18].
Results
Induction of Melan-A specific CD8+ T cells by a single stimulation
Overexpression of the melanosomal differentiation antigen Melan-A (also known as MART1) has been described in melanoma, adrenocortical tumors and glioblastoma [19–21]. It has been ranked among the top 20 cancer antigens relevant for cancer immunotherapy [22] and has been targeted in multiple clinical immunotherapy studies for melanoma [2, 5, 7, 19]. It is also often used as a model antigen to study priming of a naïve T cell response, due to the high endogenous frequency of T cell precursors reactive cells in the naïve repertoire (1 in 2 × 104 T cells as opposed to 1 in 105–106 T cells for other antigens) due to positive selection during thymic development [23, 24]. Based on our previous studies evaluating CD8 T cell priming in vitro [10, 17], we used highly purified, naïve T cells as a starting population and stimulated these cells with peptide-pulsed, LPS/IFNγ matured DC (Fig. 1a). Preparation of naïve T cells by initial negative selection for CD8+ cells, followed by depletion of CD45RO+ and CD57+ cells, resulted in a purity of 92–98% CD45RA+CCR7+CD8+ T cells (Fig. 1b). The rationale to exclude memory T cells and isolate highly purified naïve T cells was based on the lower activation threshold of memory T cells, which can result in non-specific, cytokine-driven expansion of the memory T cell fraction (data not shown). Additionally, depletion of memory cells keeps conditions more comparable from donor to donor as the % of naïve cells varies significantly from donor to donor and decreases with age [25]. Initial experiments without prior isolation of naïve T cells resulted in a higher variability of the T cell response, with regard to well-to-well variations (data not shown). In consequence, all data presented in this study were derived using purified naïve T cells as a starting population.
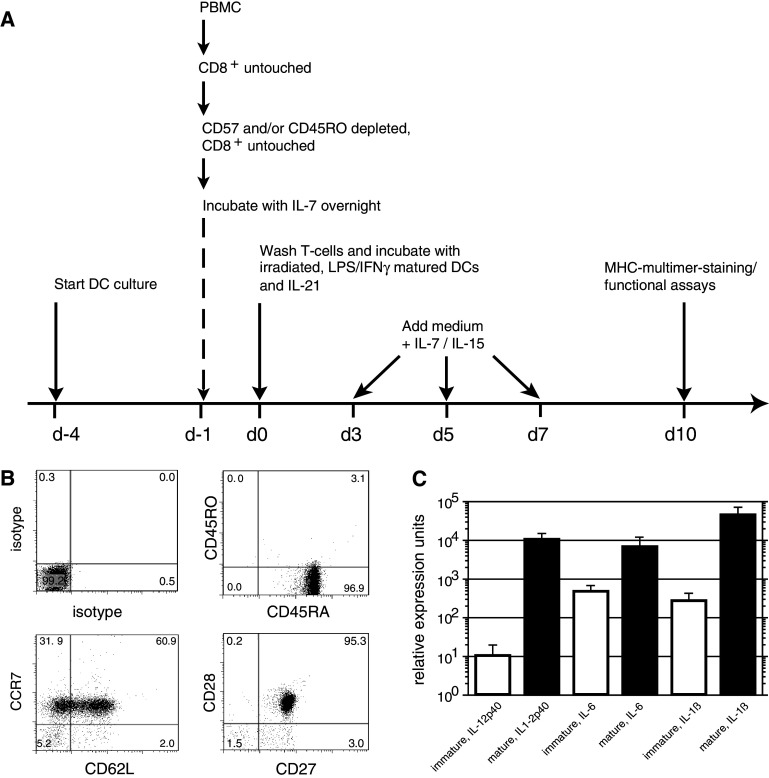
Fig. 1.
Culture protocol and characterization of dendritic cells and T cells for stimulation. a Protocol outline. b Phenotype of naïve CD8 T cells used for expansion, following negative selection for CD8+ T cells and subsequent depletion of CD45RO+ and/or CD57+ T cells. The depleted T cells were used for T cell culture subsequently. c Relative expression values for mRNA expression in dendritic cells used for the stimulation of the naïve T cells. Values for IL12p40, IL6 and IL-1β are shown as a mean and SD of 3 independent experiments
We utilized a maturation stimulus for DC employing LPS in combination with IFNγ that has been demonstrated to be effective [17, 26]. DC matured with this regimen upregulated CD80, CD86 and HLA-DR and displayed increased levels of CD83 (data not shown). In comparison to immature DC, the mature DC expressed high mRNA levels of IL12p40, IL-6 and IL1β, thus appearing to be fully functional APC (Fig. 1c).
DC and naïve T cells were coincubated in the presence of IL-21; IL-7 and IL-15 were added after 72 h. To accommodate ongoing expansion the cells were transferred from 48-h-well plates to 12-well plates on day 5 and to 6-well plates on day 7, each time refreshing the medium and cytokines. On day 10, pMHC-multimer-staining was performed. Regardless of whether testing the same donor multiple times (A1–A7) or different donors (A–J), a high % of Melan-A-specific T cells was reproducibly detected in each T cell line. Numbers 1–12 represent the individual experiments (Fig. 2a). The median % of Melan-A-specific T cells from 156 individual wells was 46.4% (25th/75th quartile: 39.3/54.1%, mean over all T cell lines: 46.9%, STD 10.1%). The coefficient of variation calculated for each individual donor and experiment ranged between 5 and 21% and therefore below the generally accepted cut-off of 30%.
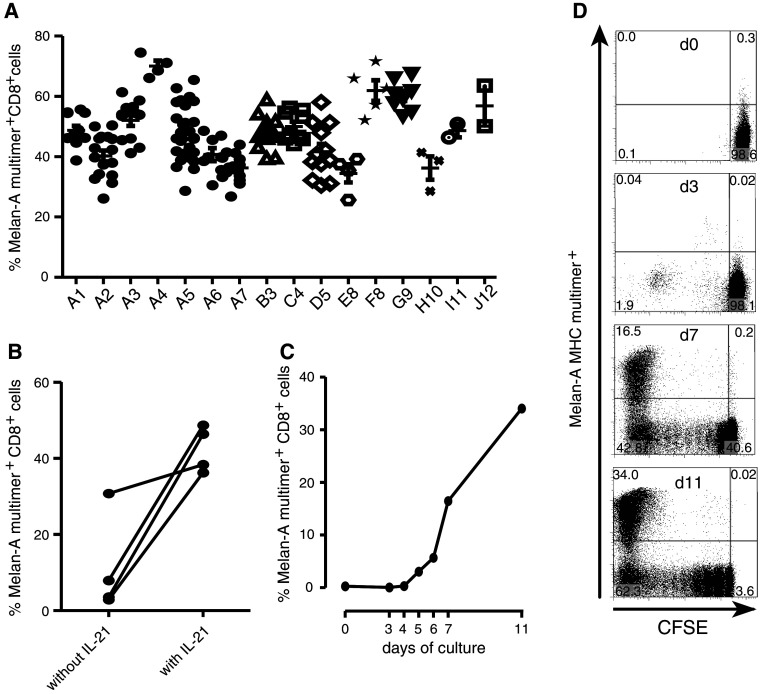
Fig. 2.
Robust and reproducible expansion of Melan-A-specific T cells from healthy donors. a The expansion protocol was applied in independent experiments (1–12) to either the same donor (A1–A7) or to different donors (A–J). For each experiment multiple wells were set up (depending on the T cells available) and each well was evaluated by MHC-multimer-staining separately by day 10 of culture. b IL-21 was required for reproducible expansion: T cells were stimulated either with or without the addition of IL21 and MHC-multimer-staining was performed on day 10. Each data point represents the mean of Melan-A MHC-multimer positive fraction of several wells (3–8), depending on how many T cells were initially available. c and d Selective expansion of Melan-A-specific T cells over time. Naïve T cells were CFSE-labeled on day −1, rested overnight in T cell medium containing IL-7 and stimulated the next day according to protocol. On the time points indicated, cells from individual wells were stained with Melan-A-MHC-multimer and CD8. c shows all data points for a representative experiment (out of 2 experiments), d shows data for selected time points. Plots represent CD8+ T cells. For all staining the Melan-A26–35A27L -MHC-multimer was used
In our previous work, CD8 T cell maintenance/expansion was supported by the addition of exogenous IL-7 and IL-15 [13]. However, another γc-cytokine, IL-21, has been described to be beneficial for in vitro T cell expansion [12, 27]. Therefore, we assessed if adding IL-21 at the beginning of the culture enhanced the expansion of Melan-A-specific T cells. In the absence of IL-21, the mean multimer-binding CD8 T cells was 11.3%, whereas the addition of IL-21 resulted in achieving a mean of 42.4% (4 experiments, p = 0.03, paired t test, Fig. 2b).
To better visualize antigen-specific expansion of the CD8 T cells through the culture period, naïve T cells were labeled with CFSE on day −1, incubated with IL-7 overnight, and then washed and stimulated with irradiated peptide-pulsed DC. Little proliferation of Melan-A-specific T cells was seen during the first 4 days of culture. However, by day 6, expansion of Melan-A-multimer+ T cells was readily detectable, and then increased significantly over the next 6 days (Fig. 2c, d). The absolute cell number did not change significantly during the first 3 days of culture. However, by day 11 each well had expanded to an average of 106 T cells per well from starting population of 4 × 105 T cells/well. As the precursor frequency of Melan-A-specific T cells on day 0 was ~0.4% of the naïve CD8+ T cells, this translates into a calculated expansion of at least 200-fold of the initial antigen-specific population.
The induced antigen-specific T cells represent a broad repertoire of functional T cells with a less differentiated phenotype
Despite the extensive expansion, the induced Melan-A specific T cells displayed many markers associated with an early effector memory or central memory phenotype: most MHC-multimer-binding T cells maintained expression of the homing receptor CD62L, with many also expressing CCR7. Moreover, the cells maintained CD28 and CD27 expression, molecules that can deliver costimulatory signals to the T cell and are closely associated with the capacity to produce autocrine IL-2 [28, 29]. Molecules associated with further differentiation or functional exhaustion such as PD1 [30] and CD95L were not detected [31]. The chemokine receptor pattern of MHC-multimer+ T cells was most consistent with that found on naïve and central memory T cells, expressing CD184/CXCR4, which is associated with homing to the bone marrow, and not expressing CCR5 or CD181/CXCR1, which is found on differentiated effector T cells (Fig. 3a; Suppl. Fig. 3) [32]. In accordance with this central-memory-like and less differentiated phenotype, expression of granzyme B and perforin was intermediate in comparison to freshly isolated CD45RA+ naïve and CD45RO+ memory T cells (data not shown).
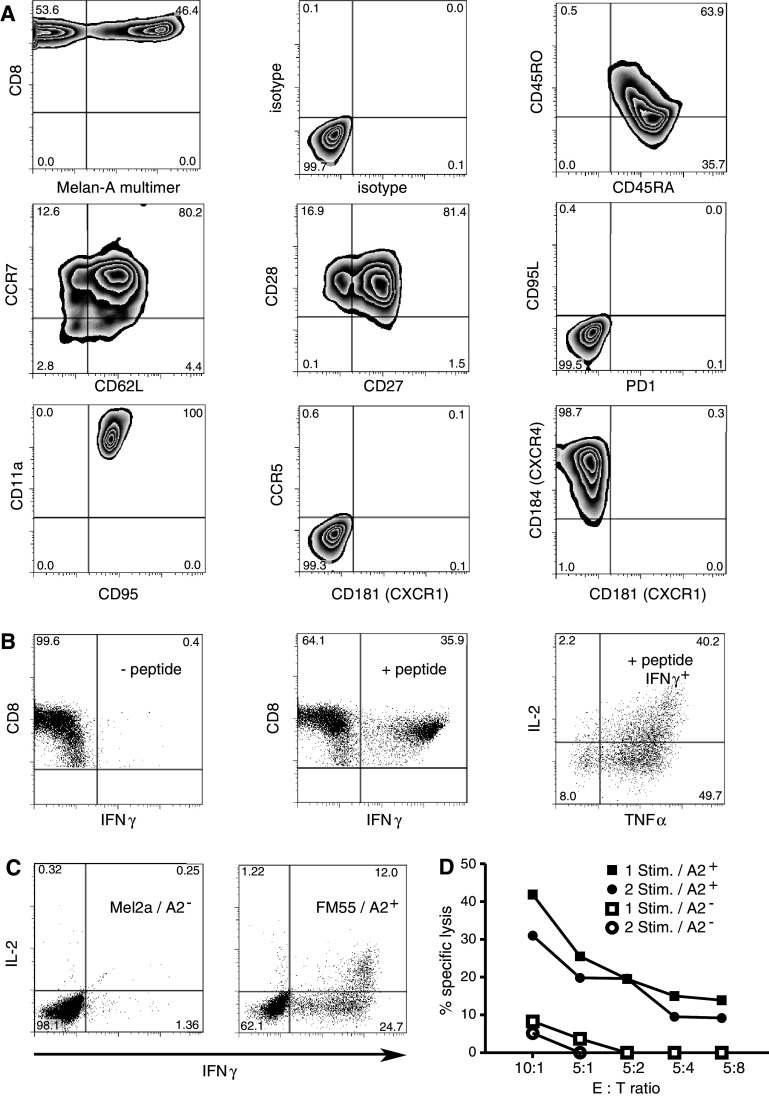
Fig. 3.
Phenotypic and functional characterization of short-term-expanded T cells. a T cells on day 10 of culture were harvested, stained with MHC-multimer and CD8-antibody and then stained for immunephenotyping. Plots showing MHC-multimer staining display CD8+ T cells. Plots not showing MHC-multimer staining are gated on CD8+ MHC multimer+ T cells. b 10-day cultured T cells are polyfunctional. 10-day cultured Melan-A-specific T cells were restimulated using peptide-pulsed T2 cells pulsed either without peptide (left) or with cognate peptide (middle and right). The middle plot shows INFγ production in response to peptide. The right plot shows the same experimental group, but gated on the IFNγ+ fraction (double and triple producers). c Recognition of endogenously processed and presented antigen on tumor cells. 105 Melan-A-specific T cells were co-cultured with the respective tumor cell lines (2 × 104) in the presence of brefeldin A for 5 h and then fixed, permeabilized and stained for IFNγ, TNFα and IL2. Displayed are CD8+ events. Data is representative for five independent experiments. d 4 h standard chromium release assay using the HLA-A2+ melanoma cell line FM55 (closed symbols) as targets in comparison to the HLA-A2− melanoma cell line Mel2a (open symbols). Two different T cell lines derived from the same donor were tested: either T cells after a single stimulation tested on day 13 of culture (squares) or T cells after restimulation on day 11 and testing on day 20 of culture (circles). e T ratios are calculated for MHC-multimer+ T cells and cell numbers were adjusted to correct for differing percentages of antigen-specific T cells in the T cell lines. For all staining the Melan-A26–35A27L -MHC-multimer was used. For stimulation the heteroclitic peptide Melan-A26–35A27L was used
Fully differentiated CD8+ effector T cells generally produce large amounts of IFNγ upon antigen encounter, but have lost the ability to produce IL-2, which may serve as an autocrine or paracrine survival factor. By contrast, long-lived memory cells generally exhibit polyfunctionality, as defined by the simultaneous production of multiple cytokines including not only IFNγ or TNFα but also IL-2 [33]. Analysis of the Melan-A-specific CD8 T cells expanded by the short-term culture described here revealed that, following restimulation with peptide-pulsed T2 cells, nearly all of the specific T cells produced IFNγ [Melan-A-multimer+ (not shown): 37.8%, IFNγ+: 35.9%]. Of the IFNγ+ T cells, 90% also produced TNFα and 40% of the IFNγ+ T cells produced TNFα and IL-2 (Fig. 3b), thus demonstrating multifunctionality.
We next wanted to assess whether the affinity of the ex vivo expanded T cells is sufficient to recognize endogenously presented antigen on tumor cells. When T cells were stimulated with an HLA-A2+ Melan-A+ melanoma cell line, a large fraction of the MHC-multimer+ T cells produced IFN-γ [36.7% IFN-γ+ vs. 53.6% MHC-multimer+ (not shown)]. Moreover, one-third of IFNγ+ T cells produced IL-2 in response to endogenously presented antigen (Fig. 3c). Melan-A-specific T cells expanded with this stimulation protocol also lysed the HLA-A2+ melanoma cell line FM55, but not the HLA-A2− control melanoma cell line Mel2a in a standard chromium release assay (Fig. 3d). One additional round of restimulation with peptide-loaded monocytes to potentially further differentiate the T cells did not increase the lytic capacity of the T cells.
We also performed, TCR immunoscope analysis on the MHC-multimer+ fraction and compared to the MHC-multimer− fraction on day 10 of culture. Expansion following a single stimulation maintained a relatively broad TCR repertoire within the antigen-specific population and only little skewing towards a more oligoclonal repertoire when compared to the non-specific internal control (Supplemental Fig. 1). In summary, the results suggest that a single stimulation gives rise to a population of polyfunctional T cells with a broad repertoire and maintenance of surface marker expression associated with improved survival and proliferation characteristics of these T cells.
Expansion of antigen-specific T cells against the wild-type epitope Melan-A26–35
It has been suggested that stimulation with the naturally occurring sequence of Melan-A26–35 (EAAGIGILTV) and not the heteroclitic sequence Melan-A26–35A27L (ELAGIGILTV), bearing a leucine substitution in position 2 to increase HLA-binding, may induce T cells with a higher avidity and enhanced tumor recognition [34]. Side-by-side testing of both peptides resulted in a robust expansion of antigen-specific T cells in both groups, with a mean percentage of 34% of Melan-A26–35A27L-multimer binding T cells in the groups induced with the heteroclitic epitope compared to 7.1% in the group previously stimulated with the wild-type peptide (Fig. 4a). Functionally, T cells derived against wild-type epitope recognized the heteroclitic epitope slightly better [EC50(M) ELA-specific T cells: 1.8 × 10−10 EAA-specific T cells 7.6 × 10−11]; however, there was no difference when the wild-type peptide was pulsed onto monocytes [EC50(M): ELA-specific T cells: 4.9 × 10−9 EAA-specific T cells 3.6 × 10−9]. More importantly, both T cell populations recognized endogenous peptide on the HLA-A0201+ melanoma cell line FM88 (Fig. 4b), and the response was comparable to stimulation with monocytes pulsed with 5 μg/ml of the respective peptide. Of note, there are several reports on cross-recognition of a variety of peptides by Melan-A-specific T cells [35–37]. We tested cross-recognition by Melan-A26–35A27L-specific T cells against a myeloma-associated epitope derived from HM1.24 (CD317), as was described recently [38]. This epitope, HM1.2422–30, consists of the amino acid sequence LLLGIGILV, resembling the Melan-A26–35A27L epitope (ELAGIGILTV). Surprisingly, only a small percentage of Melan-A26–35A27L-specific T cells were reactive when stimulated with HM1.2422–30 (Supplemental Fig. 2). Further studies are needed to evaluate crossreactivity of these short-time-cultured T cells against other epitopes in multiple donors, as has been described for Melan-A-specific T cells expanded under different culture conditions. We conclude that T cells against both epitopes, the wild-type and the heteroclitic form of Melan-A26–35 can be reproducibly generated and may be useful for adoptive immunotherapy.
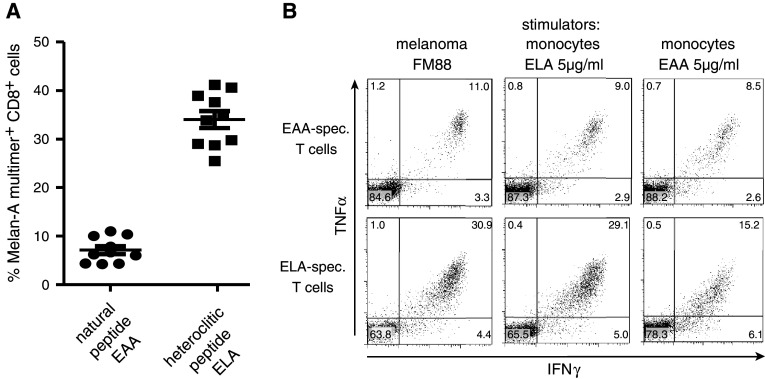
Fig. 4.
Expansion of T cells specific for the natural epitope Melan-A(27–25). A. Freshly isolated, naïve T cells were stimulated with dendritic cells pulsed either with the heteroclitic Melan-A peptide26–35A27L (ELA) or the natural peptide26–35 (EAA) (2.5 μg/ml). A. The percentage of peptide26–35A27L -multimer+ T cells in each of the 10 individual wells is displayed. b Similar recognition of the melanoma cell line FM88 by T cells raised against the heteroclitic or natural peptide. Upper panels T cells raised against the natural peptide (EAA)or lower panels T cells raised against the heteroclitic peptide (ELA) were stimulated with the HLA-A2+ Melan-A+ melanoma cell line FM88 and cytokine production within 5 h of culture was assessed (left panels). The number of multimer(ELA)+ T cells in the T cell line raised against the heteroclitic peptide (ELA) was 29% s opposed to 10% in the T cell line raised against the natural peptide (EAA) (not shown). For comparison, responses to autologous monocytes pulsed with 5 μg/ml of the respective peptide are shown in the middle and right panels
Expansion of T cells against other cancer-associated antigens
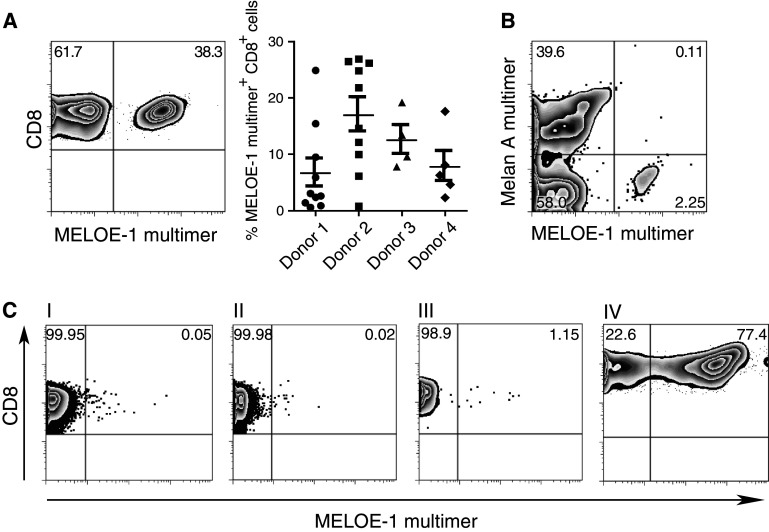
We next wanted to determine if this approach could be used to efficiently expand T cells specific for other antigens that are present in lower frequency in the naïve repertoire. Recently, a new antigen, MELOE-1, has been described for melanoma [39]. Presence of MELOE-136–44-specific T cells has been suggested to be linked to reduced relapse rates in HLA-A0201+ patients infused with ex vivo expanded tumor infiltrating lymphocytes. We therefore tried to expand MELOE-136–44-specific T cells from healthy donors. In 4 out of 4 donors tested, strong responses could be induced by a single stimulation (Fig. 5a). A slightly higher well-to-well variation was observed, with few wells remaining negative, suggesting that the precursor frequency is likely lower than that observed for Melan-A. Similar to the Melan-A-specific T cells, the phenotype of the expanded MELOE-1-specific T cells resembled a central memory phenotype (CD27+CD28+, 87%; CD62L+, 95%; CD62L+CCR7+, 41%; Supplemental Fig. 3). Using dendritic cells pulsed separately with either the Melan-A peptide or the MELOE-136–44-peptide, we asked whether specific T cells could be expanded in the same culture, as this approach may be relevant for later immunotherapeutic trials targeting multiple antigens. As demonstrated in Fig. 5b, expansion of T cells against both epitopes was achieved, suggesting that priming for multiple epitopes in one culture is feasible. Other peptides associated with different cancer-associated antigens such as gp100209–217, TRP2180–188, and IL13Rα2345–353 were also tested. Primary stimulation gave rise to specific T cell responses in the range of 0.2-7%, which increased up to 22% after one additional round of expansion (Table 1; Supplemental Fig. 4). Of note, continued culture and/or repetitive stimulations along with the use of IL-2 leads to stronger differentiation, with a gradual downregulation of costimulatory and homing receptors.
Fig. 5.
Expansion of antigen-specific T cells MELOE-136–44 after a single stimulation. a Naïve T cells from 4 different healthy HLA-A0201+ donors were stimulated with semimature DC loaded with the MELOE-1 peptide. Cultures were evaluated on day 10 using an MHC-multimer. One exemplary staining is shown (left) as well as the summary of the different parallel wells for each donor. b Expansion of T cells with different specificities in the same culture. Dendritic cells were either pulsed with the Melan-A26–35A27L peptide or the MELOE-136–44 peptide separately, washed, mixed and incubated with naïve T cells. Evaluation was performed on day 10 of the culture. Plots show live CD8+ T cells. c Enrichment of antigen-specific T cells prior to stimulation results in robust expansion similar to the kinetics seen for Melan-A-specific T cells. Naïve T cells were stained with the MELOE-1-multimer-APC (I) and subsequently coincubated with anti-APC-beads. The cells were then separated via a magnetic column into the negative fraction (II) or the positive fraction (III). The positive fraction was cultured overnight in T cell medium containing IL-7 and stimulated with MELOE-136–44-pulsed dendritic cells the next day. Expansion of the MELOE-1-specific T cell line on day 10 is shown in plot (IV)
Table 1.
Expansion of T cells against with different epitope-specificities as assessed in % IFNγ+ T cells after one or 2 stimulations
| Antigen | No peptide control | 1 stimulation | 2 stimulations |
|---|---|---|---|
| gp100(209–217M) | 0 | 7.25 | 22.0 |
| TRP2(180–188) | 0.25 | 0.7 | 11.2 |
| IL13Rα2(345–354) | 0.04 | 0.23 | 1.07 |
For Melan-A, the precursor frequency is reported to be higher than for other self-/tumor-antigens, and we asked if artificially increasing the number of antigen-specific T cells would lead to similar proliferation as observed for Melan-A-specific T cells. We therefore tried to enrich for antigen-specific T cells directly ex vivo from the naïve T cell repertoire using an enrichment technique based on MHC-multimers and magnetic beads as described previously for murine CD4+ T cells [40]. Naïve T cells were stained with the APC-labeled pMHC-multimer and incubated with anti-APC-beads, followed by positive selection using the Miltenyi system. Two different peptide antigens and multimers were tested: gp100209–217M and MELOE-136–44. The yield of cells remaining on the column was approximately 1 × 105 from an initial 2 × 108 PBMC and the percentage of MHC-pentamer+ cells within this fraction was 0.74% for gp100 and 1.15% for MELOE-1, representing a 7- and 23-fold enrichment of antigen-specific T cells, respectively. This relatively poor enrichment efficacy is due to the low precursor frequency, with similar results being reported for CD4+ T cells [40]. Stimulation of these cells with dendritic cells with the respective peptide according to protocol resulted in a population with 34.6% functional gp100209–217M-specific T cells (data not shown) and 77.4% of MELOE-136–44-specific T cells at 10 days of culture (Fig. 5c, IV). Thus, despite differences in precursor frequencies among different antigens, the results with Melan-A as a model antigen are translatable to other antigen-specific responses arising from the naïve repertoire.
Expansion of antigen-specific T cells from tumor patients
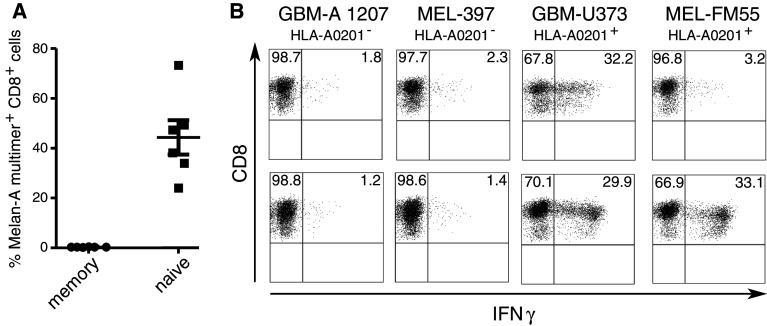
The expansion of tumor/self-antigen-specific T cells from healthy individuals serves as proof-of-principle that this strategy induces responses from the naïve T cell repertoire. However, for individual tumor patients the generation and ex vivo expansion of antigen-specific T cells may be hindered by factors related to the disease or as pre-treatment with chemotherapy that includes lymphotoxic drugs. One example of tumor-induced systemic immunosuppression is in patients with glioblastoma, a highly aggressive malignant brain cancer. Early work in the 1970s and 1980s revealed that glioma patients often suffer from impaired cell-mediated immunity (for references see review [37]) [41]. Defects in the T cell compartment are well documented, as peripheral blood leukocytes from glioma patients proliferate poorly in response to T cell mitogens, anti-CD3 and T-dependent B cell mitogens. We therefore wanted to know, whether a specific CD8 T cell response could be induced when using patient-derived dendritic cells and naïve CD8 T cells. Starting with naïve T cells from three glioblastoma patients, a strong Melan-A-response similar to healthy individuals was induced in each case. As it was possible, that in patient-derived T cells the Melan-A-specific T cells of the naïve population might be contaminated by a few responding in vivo primed, antigen-experienced cells, we also tested the memory fraction. A specific T cell response could only be induced from the purified naïve fraction (Fig. 6a). The T cells were fully functional with regard to cytokine production in response to HLA-A2+ melanoma and glioblastoma cell lines (Fig. 6b). Furthermore, using the same protocol, it was possible to expand T cells specific for IL13Rα2345–353, which is reported to be homogenously expressed on glioblastoma cells [42]. IL13Rα2345–353-specific T cells recognized the HLA-A2+ glioblastoma cell line U373, whereas the HLA-A2+ melanoma cell line FM55 was only recognized by the Melan-A-specific T cells. Further studies are under way, to validate this protocol for patients with different kinds of cancer and also to further assess potential limitations in priming naïve T cells, e.g. due to tumor-mediated immunosuppression or a reduced repertoire of naïve T cells in the elderly. However, in the context of this culture system and the limited number of patients tested so far, there were no signs for altered T cell priming ex vivo.
Fig. 6.
Expansion of cancer-associated antigen-specific T cells from the naïve repertoire of glioblastoma patients. Naïve T cells from glioblastoma patients shortly after relapse surgery were stimulated with autologous dendritic cells as described. For comparison the memory T cell fraction (as described in Fig. 1b, right panel) was also stimulated in parallel. A. On day 10 of culture, Melan-A26–35A27L -MHC-multimer -staining was performed. Shown are data from the patient for whom most parallel wells could be performed. Due to the limited T cell number available, only a single well could be set up for each of the other two patients. b Intracellular cytokine staining in response to melanoma and glioblastoma cell lines. Two different T cell lines were assessed: upper panel: patient-derived T cell line against the HLA-A0201-restricted peptide IL-13Rα345–353. Lower panel patient-derived T cell line against the heteroclitic Melan-A peptide26–35A27L. For primary stimulation either the IL-13Rα345–353 or the Melan-A peptide26–35A27L was used
Discussion
Adoptive T cell therapy has become a viable and promising treatment strategy for individualized therapy against cancer [2]. In combination with other treatments strategies such as non-myeloablative chemotherapy or irradiation to promote homeostatic proliferation of adoptively transferred T cells, a survival benefit for patients with otherwise refractory cancer has recently been demonstrated [6]. Technical advances in the methods for generating and expanding tumor-reactive T cells in vitro would make this immunotherapy a more effective and broadly applicable form of therapy for cancer patients.
Our results describe a reproducible protocol for generating antigen-specific T cells that are multifunctional and express relevant homing and costimulatory molecules, resembling T cells with an early effector memory phenotype or central memory phenotype. The differentiation state as reflected by the phenotype of the T cells is closely linked to multifunctionality in terms of cytokine production, as well as persistence and proliferation after transfer in vivo [33]. Multifunctionality is therefore critical for effectively targeting viral diseases or cancer by adoptive T cell transfer. For T cell therapy obtaining enough specific T cells without inducing full differentiation and maintaining multifunctionality has been a technical conundrum.
Clinical studies targeting cancer-associated antigens by adoptive T cell therapy so far required multiple rounds of stimulation, sometimes followed by single cell cloning and subsequent expansion to high numbers, requiring a prolonged culture time, and therefore bear the risk of enhanced differentiation of the T cells. For non-viral antigens, the shortest stimulation period in a clinical protocol reported so far has been in melanoma patients with Melan-A-specific T cells [2.1 × 108 antigen-specific T cells per infusion (mean)] expanded for approximately 6 weeks and by 4 rounds of stimulation [7]. However, the infused T cell lines lacked CD62L, a receptor closely associated with homing to the lymph nodes. In the murine model CD62L expression has been shown to be relevant for homeostatic proliferation in the lymphopenic host [43]. However, no clinical study has been performed using tumor/self-antigen-specific CD62L+, CD27+ T cells to target cancer cells, in large part because the technology to efficiently expand such T cells was not available. However, if available, such T cells are potentially more effective as they retain their homing capacity and their capacity to proliferate after transfer. In consequence, much lower numbers may be required than anticipated from previous trials using more differentiated effector cells. So far, we generated approx. 1 × 107 antigen-specific T cells in a medium-size scale and additional studies are necessary to up-scale our protocol to generate T cells for the clinical setting, but our results indicate that clinically relevant T cell numbers can be achieved at least for certain epitopes. The protocol as described here, gives rise to less differentiated cells, which may be classified as central-memory-like—on the basis of CCR7 and CD62L-expression—or early effector memory cells, as some have lost CCR7 expression by day 11. Besides preserving multifunctionality of the T cells, short-term culture also significantly increases feasibility of this form of T cell therapy in the clinical setting. Short-term cultured, ex vivo expanded T cells have been applied clinically using OKT3 and CD28-antibody as a non-specific stimulus for expansion in the setting of autologous transplantation, showing improved immune recovery [14, 15]. Using T cells primed in vitro against leukemia antigens, it was recently demonstrated in a mouse model, that such cells can exert strong anti-leukaemic effects [44]. Our priming protocol for human T cells combines the advantages of a short expansion period with antigen-specific priming. We suggest, that especially in the autologous setting, e.g. when combined with autologous hematopoietic stem cell transplantation, it may serve as a platform for T cell immunotherapy, which we tentatively term “primed lymphocyte infusions (PLI)” in analogy to “donor lymphocyte infusions” used in the allogeneic transplant setting.
Other expansion protocols for antigen-specific T cells from the naïve repertoire have been published by us [10, 13] and others [9, 12, 45–47]. Good expansion with three rounds of stimulation of naïve Melan-A and effector memory CMVpp65-specific CD8 T cells has been reported using bead-bound peptide-loaded HLA dimers in combination with a stimulating CD28-specific antibody [9]. Such artificial APC serve as a robust reagent for stimulation, but dendritic cells may still provide a more physiologic and complete stimulus including signaling through multiple costimulatory pathways as well as secretion of various cytokines. Expansion of Melan-A-specific T cells after a single stimulation to percentages similar to our results has been reported, using purified CD8 T cells in a 96-well microculture format, treated either with IL-2 alone or in combination with IL-7 or IL-15 [46]. However, the efficiency to expand antigen-specific T cells varied substantially between donors, regardless of the initial frequency of antigen-specific precursors. A similar approach has been recently published, but responses seem to be lower as to what we observed using our protocol [48]. Such differences may be explained by the use of total CD8+ T cells, which contains a variable number of memory T cells, as well as priming without the use of IL-21. IL-21 has been shown to act synergistically with IL-15, when used to induce activation, proliferation and maintenance of CD28 expression [27]. For priming of antigen-specific T cell responses, the addition of IL-21 has been shown to provide a proliferative advantage, with an increased percentage of Melan-A-specific T cells to about 20% after two rounds of stimulation [12]. Depletion of regulatory T cells prior to in vitro priming led to enhanced T cell generation, an effect that may also play a role in our protocol since no CD4 T cells are present [47]. Our protocol adds three key aspects to the aforementioned protocols: (1) the culture time and number of restimulations is greatly reduced, (2) reduced culture time and use of a combination of IL-7, IL-15 and IL-21 preserves polyfunctionality and a favorable phenotype in the T cells and (3) the protocol is very robust. Due to this robustness, the described culture conditions may serve as internal positive control for the induction of a T cell response in vitro. In comparison to other protocols, there are probably multiple, sometimes small differences that may add to this robustness, including the choice of dendritic cell maturation cocktail, use of highly purified naïve T cells, timing and dosage of the cytokines as well as DC/TC ratio and the ratio of cells per volume or cells per cm2. We are currently in the process of translating this protocol into clinical practice, which requires processing of the cells according to good manufacturing practice. Of note all of the cytokines used with this method, with the exception of IL-21, are available in GMP-grade. Furthermore, clinical grade monophosphoryl lipid A is available and can replace LPS for maturation of the dendritic cells [49, 50].
In conclusion we have demonstrated that it is possible to reproducibly expand antigen-specific T cells rapidly from the naïve repertoire either from healthy donors or cancer patients. We believe that these findings may be technologically useful for both translation of adoptive T cell therapy into clinical practice and for studying the biology of the events leading to effective or ineffective priming of human CD8 T cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank B. Bünting for help with the Immunoscope analysis and Prof. J. Becker (Department of Dermatology, Würzburg) and PD Dr. Vince and Dr. Hagemann (Department of Neurosurgery, Würzburg) for providing tumor cell lines. M.W. is the recipient of a post-doctoral stipend from the Child-Philipp-Foundation (T/237/16586/2007), Germany. This work was supported by the Parent’s Initiative Group for Children with Leukemia and Solid Tumors Würzburg and Main-Tauber e.V. as well as by a program project grant from BayImmuNet (to MW and PGS)(F2-F5121.7.1.1/13/1/2009). S.W.V.G. is supported by the Olivia Hendrickx Research Fund, the TBM program of the IWT-Flanders and a grant from the Belgian federation against cancer. P.D.G. was supported by grants from the National Institutes of Health (CA18029 and CA33084), the Leukemia and Lymphoma Society (LLS 7040–03), and the Bill and Melinda Gates Foundation.
References
- 1.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373(9664):673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Invest. 2002;110(10):1415–1417. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, Kenyon K, Davis MM, Riddell SR, Greenberg PD. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J Exp Med. 2000;192(11):1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24(31):5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 8.Schultze JL, Grabbe S, von Bergwelt-Baildon MS. Dcs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004;25(12):659–664. doi: 10.1016/j.it.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9(5):619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 10.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310(1–2):40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Alves NL, Arosa FA, van Lier RA. Common gamma chain cytokines: dissidence in the details. Immunol Lett. 2007;108(2):113–120. doi: 10.1016/j.imlet.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175(4):2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 13.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110(1):201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapoport AP, Stadtmauer EA, Aqui N, Vogl D, Chew A, Fang HB, Janofsky S, Yager K, Veloso E, Zheng Z, Milliron T, Westphal S, Cotte J, Huynh H, Cannon A, Yanovich S, Akpek G, Tan M, Virts K, Ruehle K, Harris C, Philip S, Vonderheide RH, Levine BL, June CH. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of costimulated autologous T cells. Clin Cancer Res. 2009;15(13):4499–4507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rapoport AP, Stadtmauer EA, Aqui N, Badros A, Cotte J, Chrisley L, Veloso E, Zheng Z, Westphal S, Mair R, Chi N, Ratterree B, Pochran MF, Natt S, Hinkle J, Sickles C, Sohal A, Ruehle K, Lynch C, Zhang L, Porter DL, Luger S, Guo C, Fang HB, Blackwelder W, Hankey K, Mann D, Edelman R, Frasch C, Levine BL, Cross A, June CH. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11(11):1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 16.De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M, Soerensen N, Wolff JE, Wagner S, Kaempgen E, Van Gool SW. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14(10):3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 17.Wolfl M, Rutebemberwa A, Mosbruger T, Mao Q, Li HM, Netski D, Ray SC, Pardoll D, Sidney J, Sette A, Allen T, Kuntzen T, Kavanagh DG, Kuball J, Greenberg PD, Cox AL. Hepatitis c virus immune escape via exploitation of a hole in the T cell repertoire. J Immunol. 2008;181(9):6435–6446. doi: 10.4049/jimmunol.181.9.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDr3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90(9):4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero P, Valmori D, Pittet MJ, Zippelius A, Rimoldi D, Levy F, Dutoit V, Ayyoub M, Rubio-Godoy V, Michielin O, Guillaume P, Batard P, Luescher IF, Lejeune F, Lienard D, Rufer N, Dietrich PY, Speiser DE, Cerottini JC. Antigenicity and immunogenicity of Melan-A/Mart-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol Rev. 2002;188:81–96. doi: 10.1034/j.1600-065X.2002.18808.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JG, Eguchi J, Kruse CA, Gomez GG, Fakhrai H, Schroter S, Ma W, Hoa N, Minev B, Delgado C, Wepsic HT, Okada H, Jadus MR. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res. 2007;13(2 Pt 1):566–575. doi: 10.1158/1078-0432.CCR-06-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busam KJ, Iversen K, Coplan KA, Old LJ, Stockert E, Chen YT, McGregor D, Jungbluth A. Immunoreactivity for A103, an antibody to Melan-A (Mart-1), in adrenocortical and other steroid tumors. Am J Surg Pathol. 1998;22(1):57–63. doi: 10.1097/00000478-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zippelius A, Pittet MJ, Batard P, Rufer N, de Smedt M, Guillaume P, Ellefsen K, Valmori D, Lienard D, Plum J, MacDonald HR, Speiser DE, Cerottini JC, Romero P. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J Exp Med. 2002;195(4):485–494. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, Van Den Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065X.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 25.Ginaldi L, De Martinis M, D’Ostilio A, Marini L, Loreto MF, Martorelli V, Quaglino D. The immune system in the elderly: II. Specific cellular immunity. Immunol Res. 1999;20(2):109–115. doi: 10.1007/BF02786467. [DOI] [PubMed] [Google Scholar]
- 26.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164(9):4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 27.Alves NL, Arosa FA, van Lier RA. IL-21 sustains CD28 expression on IL-15-activated human naive CD8+ T cells. J Immunol. 2005;175(2):755–762. doi: 10.4049/jimmunol.175.2.755. [DOI] [PubMed] [Google Scholar]
- 28.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topp MS, Riddell SR, Akatsuka Y, Jensen MC, Blattman JN, Greenberg PD. Restoration of CD28 expression in CD28− CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med. 2003;198(6):947–955. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54(4):307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunner T, Wasem C, Torgler R, Cima I, Jakob S, Corazza N. Fas (CD95/apo-1) ligand regulation in T cell homeostasis, cell-mediated cytotoxicity and immune pathology. Semin Immunol. 2003;15(3):167–176. doi: 10.1016/S1044-5323(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi N, Takata H, Yokota S, Takiguchi M. Down-regulation of CXCR4 expression on human CD8+ T cells during peripheral differentiation. Eur J Immunol. 2004;34(12):3370–3378. doi: 10.1002/eji.200425587. [DOI] [PubMed] [Google Scholar]
- 33.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14(6):623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 34.Speiser DE, Baumgaertner P, Voelter V, Devevre E, Barbey C, Rufer N, Romero P. Unmodified self antigen triggers human CD8 T cells with stronger tumor reactivity than altered antigen. Proc Natl Acad Sci USA. 2008;105(10):3849–3854. doi: 10.1073/pnas.0800080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loftus DJ, Castelli C, Clay TM, Squarcina P, Marincola FM, Nishimura MI, Parmiani G, Appella E, Rivoltini L. Identification of epitope mimics recognized by CTL reactive to the melanoma/melanocyte-derived peptide MART-1(27–35) J Exp Med. 1996;184(2):647–657. doi: 10.1084/jem.184.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutoit V, Rubio-Godoy V, Pittet MJ, Zippelius A, Dietrich PY, Legal FA, Guillaume P, Romero P, Cerottini JC, Houghten RA, Pinilla C, Valmori D. Degeneracy of antigen recognition as the molecular basis for the high frequency of naive A2/Melan-A peptide multimer(+) CD8(+) T cells in humans. J Exp Med. 2002;196(2):207–216. doi: 10.1084/jem.20020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voelter V, Rufer N, Reynard S, Greub G, Brookes R, Guillaume P, Grosjean F, Fagerberg T, Michelin O, Rowland-Jones S, Pinilla C, Leyvraz S, Romero P, Appay V. Characterization of Melan-A reactive memory CD8+ T cells in a healthy donor. Int Immunol. 2008;20(8):1087–1096. doi: 10.1093/intimm/dxn066. [DOI] [PubMed] [Google Scholar]
- 38.Christensen O, Lupu A, Schmidt S, Condomines M, Belle S, Maier A, Hose D, Neuber B, Moos M, Kleist C, Terness P, Ho AD, Goldschmidt H, Klein B, Hundemer M. Melan-A/Mart1 analog peptide triggers anti-myeloma T-cells through crossreactivity with HM1.24. J Immunother. 2009;32(6):613–621. doi: 10.1097/CJI.0b013e3181a95198. [DOI] [PubMed] [Google Scholar]
- 39.Godet Y, Moreau-Aubry A, Guilloux Y, Vignard V, Khammari A, Dreno B, Jotereau F, Labarriere N. Meloe-1 is a new antigen overexpressed in melanomas and involved in adoptive T cell transfer efficiency. J Exp Med. 2008;205(11):2673–2682. doi: 10.1084/jem.20081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27(2):203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008;222:70–100. doi: 10.1111/j.1600-065X.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saikali S, Avril T, Collet B, Hamlat A, Bansard JY, Drenou B, Guegan Y, Quillien V. Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRviii, IL-13ralpha2, gp100 and TRP-2 for immunotherapy. J Neurooncol. 2007;81(2):139–148. doi: 10.1007/s11060-006-9220-3. [DOI] [PubMed] [Google Scholar]
- 43.Schuster K, Gadiot J, Andreesen R, Mackensen A, Gajewski TF, Blank C. Homeostatic proliferation of naive CD8+ T cells depends on CD62L/L-selectin-mediated homing to peripheral LN. Eur J Immunol. 2009;39(11):2981–2990. doi: 10.1002/eji.200939330. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh A, Koestner W, Hapke M, Schlaphoff V, Langer F, Baumann R, Koenecke C, Cornberg M, Welte K, Blazar BR, Sauer MG. Donor T cells primed on leukemia lysate-pulsed recipient APCs mediate strong graft-versus-leukemia effects across MHC barriers in full chimeras. Blood. 2009;113(18):4440–4448. doi: 10.1182/blood-2008-09-181677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tawab A, Fan Y, Read EJ, Kurlander RJ. Effect of ex vivo culture duration on phenotype and cytokine production by mature dendritic cells derived from peripheral blood monocytes. Transfusion. 2009;49(3):536–547. doi: 10.1111/j.1537-2995.2008.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montes M, Rufer N, Appay V, Reynard S, Pittet MJ, Speiser DE, Guillaume P, Cerottini JC, Romero P, Leyvraz S. Optimum in vitro expansion of human antigen-specific CD8 T cells for adoptive transfer therapy. Clin Exp Immunol. 2005;142(2):292–302. doi: 10.1111/j.1365-2249.2005.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111(1):229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y, Gallardo HF, Ku GY, Li H, Manukian G, Rasalan TS, Xu Y, Terzulli SL, Old LJ, Allison JP, Houghton AN, Wolchok JD, Yuan J. Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses. Cytotherapy. 2009;11(7):912–922. doi: 10.3109/14653240903136987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ten Brinke A, van Schijndel G, Visser R, de Gruijl TD, Zwaginga JJ, van Ham SM. Monophosphoryl lipid a plus IFNgamma maturation of dendritic cells induces antigen-specific CD8(+) cytotoxic T cells with high cytolytic potential. Cancer Immunol Immunother. 2010;59(8):1185–1195. doi: 10.1007/s00262-010-0843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid a plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25(41):7145–7152. doi: 10.1016/j.vaccine.2007.07.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.