Abstract
Dutch’ figures on perinatal mortality and morbidity are poor compared to EU-standards. Considerable within-country differences have been reported too, with decreased perinatal health in deprived urban areas. We investigated associations between perinatal risk factors and adverse perinatal outcomes in 7,359 pregnant women participating in population-based prospective cohort study, to establish the independent role, if any, for living within a deprived urban neighbourhood. Main outcome measures included perinatal death, intrauterine growth restriction (IUGR), prematurity, congenital malformations, Apgar at 5 min < 7, and pre-eclampsia. Information regarding individual risk factors was obtained from questionnaires, physical examinations, ultrasounds, biological samples, and medical records. The dichotomous Dutch deprivation indicator was additionally used to test for unexplained deprived urban area effects. Pregnancies from a deprived neighbourhood had an increased risk for perinatal death (RR 1.8, 95% CI [1.1; 3.1]). IUGR, prematurity, Apgar at 5 min < 7, and pre-eclampsia also showed higher prevalences (P < 0.05). Residing within a deprived neighbourhood was associated with increased prevalence of all measured risk factors. Regression analysis showed that the observed neighbourhood related differences in perinatal outcomes could be attributed to the increased risk factor prevalence only, without a separated role for living within a deprived neighbourhood. Women from a deprived neighbourhood had significantly more ‘possibly avoidable’ risk factors. To conclude, women from a socioeconomically deprived neighbourhood are at an increased risk for adverse pregnancy outcomes. Differences regarding possibly avoidable risk factors imply that preventive strategies may prove effective.
Keywords: Perinatal mortality, Prevention, Residence characteristics, Pregnancy, Risk factors
Introduction
The 2009’ results from EURO-PERISTAT II demonstrated a relatively high and persistent poor perinatal mortality rate in the Netherlands (9.8 per 1,000 total births in 2006) [1]. A broader comparison of maternal and neonatal outcomes revealed that the Dutch figures are among the worst in Europe [1]. Additionally, considerable geographic differences exist with respect to most other perinatal health indicators [2, 3]. Perinatal mortality and morbidity are significantly more prevalent in the four largest cities with particular increased risks for pregnancies from socioeconomically deprived neighbourhoods. In some deprived neighbourhoods perinatal mortality appears to be as high as 17/1,000 [3]. These observed geographic inequalities emphasise the need for improvements. In light of limited opportunities to lower perinatal mortality by therapeutic interventions, increased attention to primary and secondary preventive strategies is warranted. This requires detailed information on modifiable sociodemographic, lifestyle, obstetric, and other health-related risk factors. From comparison of these perinatal risk factors across neighbourhoods novel opportunities may emerge to reduce Dutch perinatal health inequalities. Whilst extensive regarding perinatal outcomes and interventions, the Dutch perinatal registration databases are limited regarding individual- and geographical risk factors [3]. The Generation R Study is a prospective cohort study from early pregnancy onwards in Rotterdam, the second largest city in the Netherlands [4, 5]. Its detailed data collection enables the examination of the presence of neighbourhood’ related effects.
Methods
Design
The study was embedded within The Generation R Study, a population-based cohort study from early pregnancy onwards. [4, 5] The study is conducted in Rotterdam, the second largest city in the Netherlands comprising about 585,000 inhabitants [6]. Participants were pregnant women expected to deliver between 2002 and 2006 [4, 5]. For the present study analyses were restricted to prenatally enrolled women with a singleton pregnancy ≥22 weeks (n = 8,668). With respect to mothers with multiple pregnancies, one of these pregnancies was randomly excluded (n = 460). The study was conducted in accordance with the guidelines as proposed in the World Medical Association Declaration of Helsinki and was approved by the Medical Ethics Committee of the Erasmus MC Rotterdam. Written consent was obtained from all participants [4, 5, 7].
Pregnancy outcomes
Several overlapping sources (obstetric caregivers, ultrasound facilities, Municipal Health Services) provided information about (A) location and mode of delivery (home or hospital delivery as the Dutch obstetric system is characterised by its home delivery policy, emergency or elective caesarean section, instrumental vaginal delivery) and (B) pregnancy outcomes including intrauterine growth restriction (IUGR), pre-eclampsia, intrauterine fetal death, birth weight, gestational age at birth, congenital malformations, Apgar score 5 min after birth, and early neonatal death [4, 5]. IUGR and low birth weight were defined as a SD-score <−1.28, and prematurity as delivery <37.0 weeks. Last, we conveniently defined the compound measure ‘Adverse Outcome (Ao)’ as the presence of one or more of the following perinatal events: perinatal death, congenital malformations, prematurity, low birth weight, and/or Apgar <7.
Individual perinatal risk factors
Sociodemographic risk factors were assessed by a survey in early pregnancy. These included maternal age, marital status, consanguinity, and measures of socioeconomic status and ethnicity. Socioeconomic status was defined by educational level, net household income, and employment status [4, 5, 9]. Maternal occupation was classified as employed (paid or self-employment) or unemployed (job-seeking, social security or disability benefit, housewife, student). The number of weekly working hours was also assessed to make a further distinction. Ethnicity was defined by country of birth of the woman herself and her parents. The following classification was applied: Dutch, Moroccan, Turkish, Cape-Verdean, Antillean, Surinamese, other non-Western, and other Western. Because women of Antillean or Surinamese descent are of mixed ethnic origin we further categorised these women into Surinamese Hindustani or Afro-African, Antillean Afro-African, and Antillean/Surinamese-other [9].
Information regarding obstetric characteristics was obtained from the same questionnaire. This included parity, pregnancy planning, folic acid use, obstetric history, and gestational age at booking [4, 5]. With respect to lifestyle factors, height and weight were measured to calculate body mass index at inclusion (BMI; in kg/m2). Information on smoking, alcohol, and recreational drug use was obtained by questionnaires in early, mid-, and late pregnancy [4, 5]. Psychopathology was assessed using the Brief Symptom Inventory (BSI) in mid-pregnancy [4, 5, 10]. For the present study we used both anxiety (ANX) and depression scales (DEP) with a cut-off score ≥0.67. Other health characteristics included self-reported history of hypertension, diabetes, heart disorders, hypercholesterolaemia, systemic lupus erythomatosus, multiple sclerosis, or thyreoid disease (i.e. comorbidity), as well as sexual transmittable diseases (STD) comprising of chlamydia infection (measured in urine by PCR), hepatitis B (hepatitis B surface antigen measured in blood samples), toxoplasmosis (immunoglobulin G [IgG] and IgM Toxoplasma gonadii antibodies measured in blood samples), and HIV-infection (self-reported) [4, 5].
Neighbourhood deprivation classification
Women were categorised as residing within or outside of a deprived neighbourhood based on the postcode of their place of living which can then be converted using the published Dutch index of deprivation 2007. We acquired participants’ postcodes from the Centre for Research and Statistics Rotterdam [6]. The deprivation index 2007 is national indicator of neighbourhood deprivation designed by the Netherlands’ Department of Housing, Spatial Planning, and the Environment [8]. The index is created from separate scores based on five domains of deprivation: housing, employment, education, integration, and safety. The index assigned the label ‘deprived’ to 83 out of 4,878 Dutch postcodes, of which 23 postcode-areas are located in Rotterdam.
Statistical methods
Of the eligible women 849 had ≥35% missing values. After exclusion of these women the sample available for final analysis was 7,359. With respect to the remaining data missing values were imputed using multiple imputation. In the present study for each missing value five draws were performed providing five substituted data which in turn created five completed data sets. Analyses were performed separately on each completed dataset and thereafter combined into one global result [11].
The difference of adverse pregnancy outcomes between deprived and non-deprived neighbourhoods was compared using the Independent Student’s t, Mann–Whitney U, and chi-square test. For all defined outcomes, we estimated the crude Relative Risk (RR) of living within a deprived neighbourhood (RR, 95% Confidence Interval [95% CI]).
Similarly, we compared between deprived and non-deprived area’s the prevalence of all separate risk factors, and the prevalence of a combined score (weighted summation of all separate risks into one number representing the overall risk load). To obtain the combined score, we first derived a per risk factor weight from literature (either 0, 1, or 2; for a detailed account see “Appendix 1”). The combined score ranged from 0 to 20, where 0 represents absence of any risk.
As a third step we applied multivariable logistic regression analysis where the occurence of any of the indicators for adverse perinatal outcome was related to all measured individual risk factors plus the deprivation indicator was added. If the individual risk factors in this dataset would explain the prevalence of adverse pregnancy outcomes and no additional role would exist for the variable ‘living within a deprived neighbourhood’, then the excess risk of adverse pregnancy outcome (if present) would be fully explained by the (increased) prevalence of individual risk factors only. In view of collinearity both a forward and backward regression strategy was applied for all five imputed datasets separately (P inclusion = P exclusion = 0.10). If the same association was observed across four or five datasets, this was interpreted as being significant. In that case we report on the median size of that coefficient together with its 95% CI.
Subsequently, we graphically compared the compound risk score distribution, including a comparison of the proportion women with (A) low risk (sum score <3); (B) medium risk (sum score 3–7); or C) high risk (sum score ≥7).
Lastly, to assess possibilities for preventive strategies, we recoded our risk factors into ‘possibly avoidable’ and ‘possibly non-avoidable’, based on the list of Rutstein et al. [13]. Avoidability was judged based on the presence of evidence that the occurrence of an adverse perinatal outcome might be prevented by primary or secondary preventive measures embedded in preconception or prenatal care. This approach classified the following risk factors as ‘possibly avoidable’: number of working hours (occupation), BMI, smoking, alcohol and recreational drug use, pregnancy planning, folic acid use, gestational age at booking, maternal psychopathology, comorbidity, and STDs. Age, ethnicity, education, net income, marital status, consanguinity, parity, complications previous pregnancy, and moving were classified as ‘possibly non-avoidable’.
Statistical analyses were performed using Statistical Package of Social Sciences version 17.0 for Windows (SPSS Inc, Chicago, IL, USA). Multiple imputation was conducted in R version 2.7.2 (2008—6 to 23).
Results
Data were available on 7,359 women belonging to 71 area-postcodes. Of these women 37,8% was resident within a deprived neighbourhood (n = 23 area-postcodes) and 62,2% outside of a deprived neighbourhood (n = 48 area-postcodes). Considerable differences in pregnancy outcomes were apparent across deprived and non-deprived neighbourhoods (Table 1). Overall perinatal mortality encompassing intrauterine and neonatal death was 0.7% (55/7,359). Women residing within a deprived neighbourhood had almost a twofold increase in risk for perinatal death as compared to women residing outside of a deprived neighbourhood (RR 1.8, 95% CI [1.08; 3.11]). This difference was mainly explained by the substantially higher number of intrauterine fetal deaths in deprived neighbourhood pregnancies (20/2,779 versus 17/4,580). Nearly all other adverse pregnancy including IUGR, pre-eclampsia, low birth weight, prematurity, and Apgar <7, showed higher prevalences in pregnancies from deprived neighbourhoods. In contrast, the risks for hospital delivery, elective caesarean section, and instrumental vaginal were similar.
Table 1.
Percentages and relative risks of pregnancy outcomes according to neighbourhood classification
| Total | Deprived neighbourhood | RR (95% CI) | ||
|---|---|---|---|---|
| Yes | No | |||
| N = 7,359 | n = 2,779 | n = 4,580 | ||
| Location of delivery | ||||
| Hospital | 82.3 | 85.6 | 80.2 | 1.07 [1.05; 1.09]a |
| At home | 16.4 | 12.9 | 18.5 | |
| Missing | 1.3 | 1.5 | 1.2 | |
| Start delivery | ||||
| Elective caesarean section | 4.3 | 3.4 | 4.9 | 1.01 [1.00; 1.02]b |
| Induction of labour | 12.7 | 12.7 | 12.7 | |
| Spontaneous | 76.1 | 76.7 | 75.7 | |
| Missing | 6.9 | 7.2 | 6.7 | |
| Method of delivery | ||||
| Instrumental vaginal delivery | 12.6 | 10.9 | 13.7 | 1.06 [1.03; 1.09]c |
| Emergency caesarean section | 6.7 | 6.7 | 6.6 | |
| Elective caesarean section | 4.8 | 3.8 | 5.4 | |
| Breech | 0.1 | 0.1 | 0.2 | |
| Spontaneous | 66.4 | 69.3 | 64.5 | |
| Missing | 9.4 | 9.2 | 9.6 | |
| Pregnancy outcomes | ||||
| IUGR mid-pregnancy | ||||
| Mean EFW mid-pregnancy (grams, SD) | 381.8 (94.3) | 383.2 (99.0) | 381.0 (91.5) | |
| <p10 | 1.7 | 1.9 | 1.6 | 1.25 [0.88; 1.78] |
| Missing | 6.2 | 7.1 | 5.6 | |
| IUGR late pregnancy | ||||
| Mean EFW late pregnancy (grams, SD) | 1,617.4 (262.8) | 1,602.4 (267.5) | 1,626.0 (259.7) | |
| <p10 | 1.6 | 2.2 | 1.2 | 1.78 [1.25; 2.55] |
| Missing | 4.0 | 4.7 | 3.6 | |
| Pre-eclampsia | ||||
| Yes, pre-eclampsia | 2.4 | 3.0 | 2.1 | 1.46 [1.09; 1.95] |
| Missing | 2.8 | 2.7 | 2.8 | |
| Intrauterine fetal death | ||||
| Yes | 0.5 | 0.7 | 0.3 | 1.94 [1.02; 3.69] |
| Birth outcomes | ||||
| Low birth weight | ||||
| Mean birth weight (grams, SD) | 3,416.0 (559.2) | 3,355.8 (569.0) | 3,451.1 (550.5) | |
| <p10 | 11.9 | 14.5 | 10.4 | 1.41 [1.24; 1.59] |
| Missing | 1.2 | 1.7 | 0.9 | |
| Premature delivery | ||||
| Median gestational age delivery (weeks, range) | 40.1 (22.4–43.6) | 40.0 (22.6–43.6) | 40.2 (22.4–43.4) | |
| <37.0 weeks | 5.2 | 5.9 | 4.8 | 1.22 [1.00; 1.48] |
| Missing | 0.5 | 0.7 | 0.4 | |
| Congenital malformations | ||||
| Yes | 4.2 | 3.8 | 4.4 | 1.07 [0.86; 1.35] |
| Missing | 34.8 | 43.9 | 29.3 | |
| Apgar score 5 min after birth | ||||
| <7 | 1.1 | 1.5 | 0.9 | 1.57 [1.02; 2.41] |
| Missing | 4.1 | 4.0 | 4.1 | |
| Neonatal death | ||||
| Yes | 0.2 | 0.3 | 0.2 | 1.65 [0.66; 4.20] |
| Adverse outcome (Ao) | 21.0 | 23.8 | 19.3 | 1.3 [1.2; 1.5] |
Values represent percentage within column or Relative Risk (95% Confidence Interval)
EFW estimated fetal weight
aRR (95% CI) compared to home delivery (reference)
bRR (95% CI) compared to spontaneous start or induction of labour (reference)
cRR (95% CI) compared to spontaneous vaginal delivery (reference)
Maternal characteristics and risk factors are presented in Table 2. Forty percent of the study population was of non-Western descent (n = 2,903). The majority of these women resided within a deprived neighbourhood (1,660/2,779). We observed large differences in the prevalence of sociodemographic, lifestyle, obstetrical, and health-related determinants between women living within and outside of a deprived neighbourhood. Deprived neighbourhood’ women were younger, had lower measures of socioeconomic status, and suffered more often from obesity, psychopathology, and STDs (all P < 0.05). We also observed higher percentages of unplanned pregnancies among these women as well as higher percentages of adverse lifestyle factors including smoking or no folic acid use.
Table 2.
Prenatal maternal characteristics according to neighbourhood classification
| Total | Deprived neighbourhood | ||
|---|---|---|---|
| Yes | No | ||
| N = 7,359 | n = 2,779 | n = 4,580 | |
| Gestational age enrolment (median, range) | 14.4 (5.1–39.2) | 14.9 (5.0–39.2) | 13.9 (6.4–38.9)* |
| Sociodemographic factors | |||
| Age | |||
| Mean (SD) | 29.7 (5.3) | 28.1 (5.6) | 30.6 (4.9)* |
| <20 | 4.1 | 6.3 | 2.8* |
| 20–35 | 80.9 | 81.6 | 80.4 |
| ≥35 | 15.0 | 12.1 | 16.8 |
| Ethnicity | |||
| Dutch or Western | 60.1 | 39.6 | 72.5* |
| Surinam Hindu | 3.9 | 5.6 | 2.9 |
| Surinam Afro-African | 3.3 | 4.6 | 2.5 |
| Antillean Afro-African | 1.3 | 1.7 | 1.0 |
| Antillean or Surinam other | 2.0 | 2.6 | 1.6 |
| Turkish | 9.3 | 15.8 | 5.4 |
| Moroccan | 6.7 | 11.3 | 3.9 |
| Cape-Verdean | 4.2 | 6.9 | 2.6 |
| Non-Western other | 8.8 | 11.3 | 7.3 |
| Missing | 0.4 | 0.6 | 0.3 |
| Educational level | |||
| Primary | 11.5 | 18.1 | 7.4* |
| Missing | 2.0 | 2.8 | 1.5 |
| Net income | |||
| Euro <1,200 | 15.8 | 25.7 | 9.8* |
| Euro 1,200–2,200 | 19.8 | 23.4 | 17.6 |
| Euro ≥2,200 | 42.4 | 21.2 | 55.2 |
| Missing | 22.0 | 29.7 | 17.4 |
| Occupation | |||
| Non-working | 20.3 | 26.7 | 16.5* |
| <20 h/week | 5.3 | 4.5 | 5.7 |
| 20–36 h/week | 24.7 | 18.0 | 28.8 |
| ≥36 h/week | 23.7 | 17.8 | 27.3 |
| Missing | 26.0 | 33.0 | 21.7 |
| Marital status | |||
| Single | 15.0 | 21.6 | 11.0* |
| Missing | 1.0 | 1.1 | 1.0 |
| Consanguinity | |||
| Yes | 3.9 | 6.9 | 2.1* |
| Missing | 5.6 | 6.4 | 5.1 |
| Moved during pregnancy | |||
| Yes | 12.8 | 14.4 | 12.0* |
| Life style factors | |||
| BMI intake | |||
| Mean (SD) | 24.8 (4.6) | 25.3 (4.9) | 24.5 (4.3)* |
| <20 | 9.0 | 9.1 | 8.9* |
| 20–30 | 78.5 | 75.7 | 80.2 |
| ≥30 | 12.5 | 15.2 | 10.9 |
| Smoking | |||
| Yes, stopped after pregnancy recognition | 8.0 | 7.1 | 8.5* |
| Yes, continued throughout pregnancy | 16.8 | 20.6 | 14.5 |
| Missing | 5.1 | 4.5 | 5,5 |
| Alcohol use | |||
| Yes, but stopped after pregnancy recognition | 12.9 | 10.4 | 14.4* |
| Yes, continued throughout pregnancy | 34.2 | 25.6 | 39.4 |
| Missing | 4.6 | 3.9 | 4.9 |
| Recreational drug use | |||
| Yes, during the periconception period | 2.6 | 3.0 | 2.3* |
| Yes, continued throughout pregnancy | 0.5 | 0.8 | 0.3 |
| Missing | 3.9 | 3.1 | 4.3 |
| Obstetric characteristics | |||
| Parity | |||
| 0 | 57.9 | 57.5 | 58.3* |
| 1–3 | 38.4 | 37.6 | 38.9 |
| ≥3 | 3.7 | 4.9 | 2.8 |
| Pregnancy planning | |||
| No | 28.1 | 36.9 | 22.8* |
| Missing | 5.3 | 5.7 | 5.0 |
| Folic acid use | |||
| No | 24.8 | 36.2 | 17.8* |
| Missing | 5.3 | 5.1 | 5.4 |
| Complications previous pregnancy | |||
| Yes | 7.8 | 7.7 | 7.9 |
| Missing | 0.1 | 0.1 | 0.2 |
| Gestational age at booking | |||
| Median (range) | 12.1 (4.6–33.4) | 12.4 (5.1–32.0) | 11.9 (4.6–33.4)* |
| <14 weeks | 4 | 45.3 | 44.7* |
| ≥14 weeks | 15.0 | 22.1 | 12.1 |
| Missing | 43.3 | 32.6 | 43.2 |
| Health characteristics | |||
| Depressive complaints | |||
| Score DEP median (range) | 0 (0–4.0) | 0.17 (0–4.0) | 0 (0–4.0)* |
| Yes | 8.5 | 11.3 | 6.7* |
| Missing | 20.3 | 25.7 | 16.9 |
| Anxiety complaints | |||
| Score ANX median (range) | 0.17 (0–4.0) | 0.17 (0–4.0) | 0.17 (0–3.7)* |
| Yes | 9.3 | 12.2 | 7.6* |
| Missing | 20.2 | 25.8 | 16.8 |
| Comorbidity | |||
| Yes | 5.9 | 5.5 | 6.2 |
| Missing | 4.2 | 3.4 | 4.7 |
| Sexual transmittable diseases | |||
| Yes | 2.7 | 3.9 | 1.9* |
| Missing | 11.5 | 13.1 | 10.5 |
| Median weighted risk score sum (range) | 4 (0–14) | 5 (0–14) | 3 (0–14)* |
| High risk profile (sum score ≥7) | 17.8 | 35.8 | 15.1* |
Values represent percentage within column, gestational age at inclusion in weeks
* P value < 0.05
Logistic regression (with ‘Adverse Outcome’ as outcome) consistently showed strong effects for the risk factors non-Western and Afro-African ethnicity, low income, BMI < 20, smoking, nulliparity, complications in previous pregnancy, and two interaction-terms (low age × single; and Afro-African ethnicity × single; Table 3). We observed minor differences across the five datasets as well as in the forward/backward approaches (“Appendix 2”). In none of these analyses, living within a deprived neighbourhood emerged as significant independent risk factor. Additional prediction of ‘living within a deprived neighbourhood’ from the same risk set provided the same solution (equal risk factors) as the analysis predicting ‘Adverse Outcome’. Apparently, in the present study the effect of neighbourhood deprivation on perinatal outcome was explained by a differential prevalence of individual risk factors (intermediate factors).
Table 3.
Association between individual risk factors and the occurrence of ‘Adverse Outcome’, results from five imputed datasets and forward and backward regression respectively
| Risk factor | Forward approach | Backward approach | ||
|---|---|---|---|---|
| Significant association | Size (Exp B, 95% CI) | Significant association | Size (Exp B, 95% CI) | |
| Neighbourhood deprivation | – | – | ||
| Age <20 years | – | + | 1.34 (1.00; 1.79) | |
| Ethnicity Afro-African | – | + | 1.36 (1.11; 1.65) | |
| Ethnicity non-Western other | + | 1.20 (1.05; 1.37) | + | 1.25 (1.08; 1.44) |
| Net income Euro <1,200–, | + | 1.22 (1.05; 1.42) | + | 1.20 (1.02; 1.40) |
| Occupation non-working | – | – | ||
| Occupation ≥36 h/week | – | – | ||
| Marital status single | – | – | ||
| BMI intake <20 | + | 1.34 (1.11; 1.61) | + | 1.36 (1.13; 1.63) |
| Smoking during pregnancy | + | 1.17 (1.03; 1.33) | + | 1.21 (1.06; 1.37) |
| Recreational drug use | – | – | ||
| Nullipara | + | 1.84 (1.61; 2.10) | + | 1.85 (1.62; 2.11) |
| Unplanned pregnancy | – | – | ||
| No folic acid use | ± | ± | ||
| Complications previous pregnancy | + | 1.33 (1.04; 1.68) | + | 1.32 (1.04; 1.67) |
| Gestational age booking ≥14 weeks | ± | ± | ||
| Depressive or anxiety complaints | – | – | ||
| Comorbidity | – | ± | ||
| Sexual transmittable diseases | – | – | ||
| Interaction: single marital status × ethnicity Afro-African | + | 1.39 (1.19; 1.62) | – | |
| Interaction: single marital status × age <20 years | – | + | 1.35 (1.12; 1.61) | |
| Interaction: single marital status × unplanned pregnancy | – | – | ||
| Interaction: net income Euro <1,200 × folic acid | – | – | ||
Results from logistic regression analysis relating individual risk factors to the occurrence of ‘Adverse Outcome’. Both forward and backward approaches are shown (in-/exclusion cut-off P value <0.10). The analyses were performed separately on each multiple imputed dataset (5N = 7,359). Here, we show those covariables that significantly contributed to the outcome measure ‘Adverse Outcome’ in at least 4 (out of 5) multiple imputed datasets (+). In that case the median size of that coefficient (exp B) together with its 95% CI is presented
–, not included in model at all, or only in 1 multiple imputed dataset; ± significant contributor in 2/3 multiple imputed datasets; +, significant contributor in 4/5 multiple imputed datasets
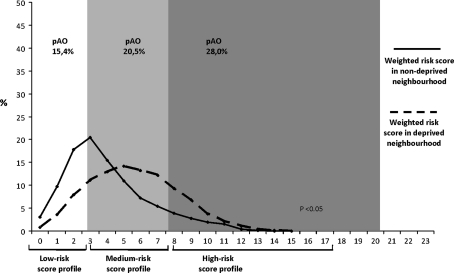
In Fig. 1 the summated risk scores for women from a deprived neighbourhood are shown (35.8% high-risk score for women from a deprived neighbourhood versus 15.1% high-risk score for women from a non-deprived neighbourhood). The figure graphically supports the key role of individual risk excess in deprived neighbourhood areas.
Fig. 1.
Distribution of individual weighted risk scores for women residing within and outside of a deprived neighbourhood. The graph represents the distribution of individual weighted risk scores between deprived and non-deprived neighbourhoods. Differences in weighted risk score profiles were tested using Mann–Whitney U test. The risk for the compound measure ‘Adverse Outcome’ is represented by ‘pAo’ given as percentage per risk stratum. Analyses are based on multiple imputed dataset, N = 7,359
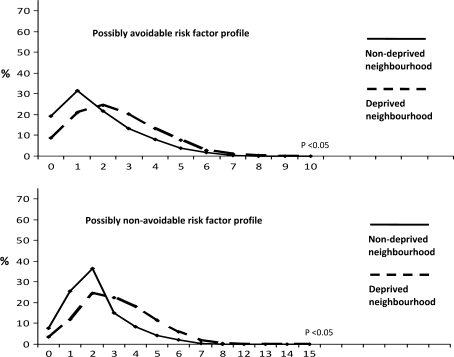
Lastly, Fig. 2 shows that women residing within a deprived neighbourhood have significantly more ‘possibly avoidable’ risk factors as compared to women from of a non-deprived neighbourhood.
Fig. 2.
‘Possibly avoidable’ and ‘possibly non-avoidable’ weighted risk scores according to neighbourhood classification. The graphs represent the distributions of ‘possibly avoidable’ and ‘possibly non-avoidable’ risk factors between deprived and non-deprived neighbourhoods. Differences in risk factors were tested using Mann–Whitney U test. Analyses are based on multiple imputed dataset, N = 7,359
Discussion
To our knowledge, this is the first study to examine associations between modifiable perinatal risk factors and perinatal outcomes on the neighbourhood level to enable the narrowing of perinatal inequalities in disadvantaged urban areas. Women from a deprived neighbourhood are at a substantially increased risk for adverse pregnancy outcomes, with a twofold increased risk for perinatal death. This excess risk can be attributed to an accumulation of sociodemographic, lifestyle, obstetric, and health-related risk factors present within deprived neighbourhoods. The higher proportion of modifiable risk factors in disadvantaged areas provides opportunities for primary and secondary prevention.
Key findings
Our findings on perinatal outcome inequalities within deprived neighbourhoods strengthen previous studies from Europe and the United States [2, 3, 14–18]. The majority of these studies focussed only on preterm birth and low birth weight, as important risk factors for infant mortality and morbidity [15–18]. We added pre-eclampsia, congenital anomalies, and suboptimal start at birth, since the latter two contribute to perinatal mortality, whereas pre-eclampsia is a major driver for both perinatal and maternal mortality. To our knowledge to date no study on neighbourhood deprivation and perinatal outcome has been conducted in such detail. An important finding that has not been reported before was the 50% increased risk of pre-eclampsia for women from a deprived neighbourhood, despite the inevitably higher prevalence of ‘protective’ smoking. Neighbourhood deprivation and cardiovascular disease are known to be strongly linked [19]. Since pre-eclampsia has been shown to be related to cardiovascular disease, a higher prevalence of the latter may have contributed to these results [20]. Interestingly, the risk for congenital malformations for women from a deprived neighbourhood was not increased. Previously, de Graaf et al. [3]. reported similar results. They suggested that the higher uptake of prenatal screening in urban areas, as compared to rural areas, might be causative [3]. Future studies are needed to investigate this finding in closer detail.
In our study accumulation of risk factors largely explained perinatal inequalities according to place of living. Although the exact underlying mechanisms through which neighbourhood deprivation influences both perinatal risk factors and outcomes are unknown, several pathways have been proposed which share that geographical and individual characteristics are closely linked [21]. One model proposes that neighbourhood-characteristics affect reproductive outcome by modelling of a wide range of individual-level economic opportunities and risk behaviours [21–23]. In this model access to education and training programs is determined by place of living. Better education yields higher socioeconomic status which in turn benefits perinatal outcomes [22]. On the other hand, locally shared social characteristics may influence unhealthy risk behaviours through common cultural norms and beliefs, which in turn are associated with perinatal outcomes (i.e. smoking habits, sexual behaviour) [21, 23]. Essentially all perinatal risk factors investigated in our study have been previously reported to be associated with both adverse neighbourhood conditions and reproductive health [21–23].
During recent years increasing attention has focused on preconception care as a means of optimising women’s health and knowledge before planning and conceiving pregnancy in order to reduce the risk of adverse health effects for the woman, fetus, or neonate [24, 25]. Several countries have succesfully introduced structured preconception health care programmes [24–30]. One of the most effective programmes, The Hungarian Periconceptional Service, reported reductions in congenital defects and ectopic pregnancy rates, and higher birth weights. Moreover, urogenital infections were detected and treated, and screening for diabetes and cardiovascular disease led to referral of 3% of women to special clinics [30].
The observed difference in modifiable perinatal risk factors between deprived and non-deprived neighbourhood’ women provides opportunities for improvement. In this respect we consider the active provision of information as vital to reduce perinatal inequalities since most women appear to be largely ignorant on the consequences of an adverse lifestyle or of the presence of a medical condition during pregnancy, and in particular during the periconception period [24, 31].
A precondition for success of intensified care is free access to, and due start of preconception and prenatal care. Most women from a deprived neighbourhood entered antenatal care at such a late stage that it can be concluded that opportunities to improve the health of these women and their offspring were completely missed [32]. Immigrant women and women of very low socioeconomic class require special attention because they are often not reached in the provision of information about the consequences of an adverse lifestyle or medical condition due to language, cultural, and/or social barriers [33]. Without extra attention, intensification of prenatal care will have a differential effect according to the place of living [21].
Strengths and weaknesses
This was a survey embedded in a large population-based prospective cohort with an extensive data collection. The use of structural variables to characterise neighbourhood deprivation rather than using aggregates of individual-level variables adds to its strength [8, 21]. Nevertheless, some limitations need to be discussed. First, selective participation has been demonstrated for deprived areas [34]. Though participation rates for the Generation R Study are relatively high and the ethnic distribution differs only moderately from that of the eligible population, the Generation R Study is characterised by a rather highly educated and healthy study population compared to available city data [4, 5]. This selective non-response towards higher socioeconomic status may have influenced the associations [35]. Moreover, we had to exclude ten percent of the study population because of high levels of missing data with subsequent considerable multiple imputations. Since these participants were predominantly lower educated and of non-western ethnicity this may have biased our results. Lastly, we obtained most information on perinatal risk factors by questionnaires in Dutch and English. If required individual support in Arabic, French, Portuguese, or Turkish was available. Despite these efforts, misclassification through language proficiency should always be considered.
Future research
The inequalities in the prevalence of aetiological factors urge for detailed analysis of the provision of care, in particular the presence of substandard care relative to risk exposure. In the Netherlands, recently concerns were raised on the quality of delivered perinatal care [36–39]. Substandard factors in maternity care were more likely to occur in immigrant than in indigeneous women [38, 39]. The similar prevalence of instrumental vaginal delivery and caesarean sections across deprived neighbourhoods may suggest under-treatment in view of the much higher prevalence of conditions like IUGR and pre-eclampsia. Such research should apply multiple methods: epidemiological analysis of national data, qualitative in-depth research into professional mechanisms, and perinatal audit methods such as the one currently being implemented [37, 40].
Conclusions
Women from a deprived neighbourhood are at almost double risk for adverse pregnancy outcomes, which can be largely attributed to accumulation of individual risk factors present within underprivileged urban areas. Targeted preconception healthcare programmes should be implemented in order to lower the geographic inequalities.
Acknowledgments
We gratefully acknowledge the contribution of general practitioners, hospitals, midwives, and pharmacies in Rotterdam. The work was supported by the Erasmus Medical Centre Rotterdam, the Erasmus University Rotterdam, and the Netherlands Organisation for Health Research and Development (ZonMw). Vincent WV Jaddoe also acknowledges the personal funding by the Netherlands Organization for Health Research (ZonMw 90700303).
Conflicts of interest
None to declare.
Ethical approval
The study was approved by the Medical Ethics Committee of the Erasmus MC Rotterdam, the Netherlands.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- IUGR
Intrauterine growth restriction
- BMI
Body mass index
- BSI
Brief symptom inventory
- ANX
Anxiety
- DEP
Depression
- STD
Sexual transmittable diseases
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- Ao
Adverse outcome
- CI
Confidence interval
- RR
Relative risk
- EFW
Estimated fetal weight
Appendix 1
See Table 4.
Table 4.
Whole number weight that has been assigned to the adverse level of each separate risk factor for the construction of individual weighted risk scores
| Risk factor | Whole number weight |
|---|---|
| Age | |
| <20 years | 1 |
| 20–35 years | 0 |
| ≥35 years | 1 |
| Ethnicity | |
| Dutch or western | 0 |
| Moroccan | 0 |
| Non-western Afro-African | 2 |
| Antillean or Surinam Afro-African single | 1 |
| Antillean or Surinam Afro-African other | 2 |
| Non-western other | 1 |
| Educational level | |
| Primary | 1 |
| Secondary or higher | 0 |
| Net income | |
| Euro <1,200 | 1 |
| Euro 1,200–2,200 | 0 |
| Euro ≥2,200 | 0 |
| Occupation | |
| Non-working | 1 |
| <20 h/week | 0 |
| 20–36 h/week | 0 |
| ≥36 h/week | 1 |
| Marital status | |
| Single | 1 |
| Married/living together | 0 |
| Consanguinity | |
| Yes | 0 |
| No | 0 |
| Moved during pregnancy | |
| Yes | 1 |
| No | 0 |
| BMI intake | |
| <20 | 1 |
| 20–30 | 0 |
| ≥30 | 1 |
| Smoking habits | |
| Yes, stopped after pregnancy recognition | 1 |
| Yes, continued throughout pregnancy | 2 |
| No | 0 |
| Alcohol consumption | |
| Yes but stopped after pregnancy recognition | 0 |
| Yes, continued throughout pregnancy | 0 |
| No | 0 |
| Recreational drug use | |
| Yes, during the periconception period | 1 |
| Yes, continued throughout pregnancy | 2 |
| No | 0 |
| Parity | |
| 0 | 1 |
| 1–3 | 0 |
| ≥3 | 1 |
| Pregnancy planning | |
| Yes | 0 |
| No | 1 |
| Folic acid use | |
| Yes | 0 |
| No | 1 |
| Complications previous pregnancy | |
| Yes | 1 |
| No | 0 |
| Missing | 0 |
| Gestational age at booking | |
| <14 weeks | 0 |
| ≥14 weeks | 0 |
| Psychopathology | |
| Yes | 1 |
| No | 0 |
| Comorbidity | |
| Yes | 1 |
| No | 0 |
| Sexual transmittable diseases | |
| Yes | 1 |
| No | 0 |
Appendix 2
See Table 5.
Table 5.
Contribution of the individual risk factors to the occurrence of ‘Adverse Outcome’
| A Forward approach | |||||
|---|---|---|---|---|---|
| Multiple imputed dataset 1 | Multiple imputed dataset 2 | Multiple imputed dataset 3 | |||
| Risk factor | Exp (B) 95% CI | Risk factor | Exp (B) 95% CI | Risk factor | Exp (B) 95% CI |
| Neighbourhood deprivation | NI | Neighbourhood deprivation | NI | Neighbourhood deprivation | NI |
| Age <20 years | NI | Age <20 years | NI | Age <20 years | NI |
| Ethnicity Afro-African | NI | Ethnicity Afro-African | NI | Ethnicity Afro-African | NI |
| Ethnicity non-Western other | 1.20 (1.05; 1.37) | Ethnicity non-Western other | 1.20 (1.05; 1.38) | Ethnicity non-Western other | 1.19 (1.03; 1.36) |
| Net income Euro <1,200 | 1.39 (1.20; 1.62) | Net income Euro <1,200 | 1.19 (1.02; 1.40) | Net income Euro <1,200 | 1.23 (1.05; 1.44) |
| Occupation non-working | NI | Occupation non-working | NI | Occupation non-working | NI |
| Occupation ≥36 h/week | NI | Occupation ≥36 h/week | NI | Occupation ≥36 h/week | NI |
| Marital status single | NI | Marital status single | NI | Marital status single | NI |
| BMI intake <20 | 1.34 (1.11; 1.61) | BMI intake <20 | 1.34 (1.11; 1.61) | BMI intake <20 | 1.34 (1.11; 1.61) |
| Smoking during pregnancy | 1.21 (1.06. 1.37) | Smoking during pregnancy | 1.22 (1.08; 1.39) | Smoking during pregnancy | 1.17 (1.03; 1.33) |
| Recreational drug use | NI | Recreational drug use | NI | Recreational drug use | NI |
| Nullipara | 1.81 (1.59; 2.07) | Nullipara | 1.83 (1.61; 2.09) | Nullipara | 1.84 (1.61; 2.10) |
| Unplanned pregnancy | NI | Unplanned pregnancy | NI | Unplanned pregnancy | NI |
| No folic acid use | NI | No folic acid use | 1.18 (1.02; 1.35) | No folic acid use | NI |
| Complications previous pregnancy | 1.33 (1.05; 1.69) | Complications previous pregnancy | 1.33 (1.05; 1.69) | Complications previous pregnancy | 1.33 (1.04; 1.68) |
| Gestational age booking ≥14 weeks | NI | Gestational age booking ≥14 weeks | NI | Gestational age booking ≥14 weeks | NI |
| Depressive or Anxiety complaints | NI | Depressive or Anxiety complaints | NI | Depressive or Anxiety complaints | NI |
| Comorbidity | NI | Comorbidity | NI | Comorbidity | NI |
| Sexual transmittable diseases | NI | Sexual transmittable diseases | NI | Sexual transmittable diseases | NI |
| Interaction: single marital status × ethnicity | Interaction: single marital status × ethnicity | Interaction: single marital status × ethnicity | |||
| Afro-African | 1.37 (1.17; 1.59) | Afro-African | 1.40 (1.20; 1.63) | Afro-African | 1.39 (1.19; 1.62) |
| Interaction: single marital status × age <20 years | NI | Interaction: single marital status × age <20 years | NI | Interaction: single marital status × age <20 years | NI |
| Interaction: single marital status × unplanned pregnancy | NI | Interaction: single marital status × unplanned pregnancy | NI | Interaction: single marital status × unplanned pregnancy | NI |
| Interaction: net income Euro <1,200 × folic acid | NI | Interaction: net income Euro <1,200 × folic acid | NI | Interaction: net income Euro <1,200 × folic acid | NI |
| A Forward approach | |||||
|---|---|---|---|---|---|
| Multiple imputed dataset 4 | Multiple imputed dataset 5 | Combined estimate/Median | |||
| Risk factor | Exp (B) 95% CI | Risk factor | Exp (B) 95% CI | Risk factor | Exp (B) 95% CI |
| Neighbourhood deprivation | NI | Neighbourhood deprivation | 1.13 (1.00; 1.28) | Neighbourhood deprivation | NI |
| Age <20 years | NI | Age <20 years | NI | Age <20 years | NI |
| Ethnicity Afro-African | NI | Ethnicity Afro-African | NI | Ethnicity Afro-African | NI |
| Ethnicity non-Western other | 1.24 (1.08; 1.41) | Ethnicity non-Western other | 1.22 (1.07; 1.39) | Ethnicity non-Western other | 1.20 (1.05; 1.37) |
| Net income Euro <1,200 | 1.22 (1.05; 1.42) | Net income Euro <1,200 | NI | Net income Euro <1,200 | 1.22 (1.05; 1.42) |
| Occupation non-working | NI | Occupation non-working | NI | Occupation non-working | NI |
| Occupation ≥36 h/week | NI | Occupation ≥36 h/week | NI | Occupation ≥36 h/week | NI |
| Marital status single | NI | Marital status single | NI | Marital status single | NI |
| BMI intake <20 | 1.35 (1.12; 1.62) | BMI intake <20 | 1.34 (1.12; 1.62) | BMI intake <20 | 1.34 (1.11; 1.61) |
| Smoking during pregnancy | 1.19 (1.05; 1.35) | Smoking during pregnancy | 1.20 (1.06; 1.36) | Smoking during pregnancy | 1.20 (1.06; 1.36) |
| Recreational drug use | NI | Recreational drug use | NI | Recreational drug use | NI |
| Nullipara | 1.81 (1.58; 2.06) | Nullipara | 1.82 (1.60; 2.08) | Nullipara | 1.82 (1.60; 2.08) |
| Unplanned pregnancy | NI | Unplanned pregnancy | NI | Unplanned pregnancy | NI |
| No folic acid use | NI | No folic acid use | 1.21 (1.05; 1.39) | No folic acid use | NI |
| Complications previous pregnancy | 1.31 (1.03; 1.66) | Complications previous pregnancy | 1.32 (1.04; 1.68) | Complications previous pregnancy | 1.33 (1.04; 1.68) |
| Gestational age booking ≥14 weeks | 1.14 (1.00; 1.30) | Gestational age booking ≥14 weeks | NI | Gestational age booking ≥14 weeks | NI |
| Depressive or Anxiety complaints | NI | Depressive or Anxiety complaints | NI | Depressive or Anxiety complaints | NI |
| Comorbidity | NI | Comorbidity | NI | Comorbidity | NI |
| Sexual transmittable diseases | NI | Sexual transmittable diseases | NI | Sexual transmittable diseases | NI |
| Interaction: single marital status × ethnicity | Interaction: single marital status × ethnicity | Interaction: single marital status × ethnicity | |||
| Afro-African | 1.41 (1.21; 1.65) | Afro-African | 1.49 (1.30; 1.71) | Afro-African | 1.40 (1.20; 1.63) |
| Interaction: single marital status × age <20 years | NI | Interaction: single marital status × age <20 years | NI | Interaction: single marital status × age <20 years | NI |
| Interaction: single marital status × unplanned pregnancy | NI | Interaction: single marital status × unplanned pregnancy | NI | Interaction: single marital status × unplanned pregnancy | NI |
| Interaction: net income Euro <1,200 × folic acid | NI | Interaction: net income Euro <1,200 × folic acid | NI | Interaction: net income Euro <1,200 × folic acid | NI |
| B Backward approach | |||||
|---|---|---|---|---|---|
| Multiple imputed dataset 1 | Multiple imputed dataset 2 | Multiple imputed dataset 3 | |||
| Risk factor | Exp (B) 95% CI | Risk factor | Exp (B) 95% CI | Risk factor | Exp (B) 95% CI |
| Neighbourhood deprivation | NI | Neighbourhood deprivation | NI | Neighbourhood deprivation | NI |
| Age <20 years | 1.37 (1.02; 1.84) | Age <20 years | 1.34 (1.00; 1.79) | Age <20 years | 1.37 (1.02; 1.83) |
| Ethnicity Afro-African | 1.34 (1.11; 1.63) | Ethnicity Afro-African | 1.36 (1.11; 1.65) | Ethnicity Afro-African | 1.33 (1.09; 1.62) |
| Ethnicity non-Western other | 1.23 (1.07; 1.42) | Ethnicity non-Western other | 1.25 (1.08; 1.44) | Ethnicity non-Western other | 1.22 (1.06; 1.41) |
| Net income Euro <1,200 | 1.35 (1.15; 1.58) | Net income Euro <1,200 | 1.17 (0.99; 1.37) | Net income Euro <1,200 | 1.22 (1.04; 1.43) |
| Occupation non-working | NI | Occupation non-working | NI | Occupation non-working | NI |
| Occupation ≥36 h/week | NI | Occupation ≥36 h/week | NI | Occupation ≥36 h/week | NI |
| Marital status single | NI | Marital status single | NI | Marital status single | NI |
| BMI intake <20 | 1.37 (1.13; 1.64) | BMI intake <20 | 1.36 (1.13; 1.63) | BMI intake <20 | 1.36 (1.13; 1.63) |
| Smoking during pregnancy | 1.23 (1.08; 1.39) | Smoking during pregnancy | 1.24 (1.09; 1.41) | Smoking during pregnancy | 1.18 (1.04; 1.34) |
| Recreational drug use | NI | Recreational drug use | NI | Recreational drug use | NI |
| Nullipara | 1.85 (1.62; 2.11) | Nullipara | 1.85 (1.62; 2.11) | Nullipara | 1.86 (1.63; 2.12) |
| Unplanned pregnancy | NI | Unplanned pregnancy | NI | Unplanned pregnancy | NI |
| No folic acid use | NI | No folic acid use | 1.17 (1.01; 1.34) | No folic acid use | 1.16 (1.01; 1.34) |
| Complications previous pregnancy | 1.33 (1.05; 1.69) | Complications previous pregnancy | 1.31 (1.03; 1.66) | Complications previous pregnancy | 1.31 (1.03; 1.66) |
| Gestational age booking ≥14 weeks | 1.14 (0.99; 1.30) | Gestational age booking ≥14 weeks | NI | Gestational age booking ≥14 weeks | NI |
| Depressive or Anxiety complaints | NI | Depressive or Anxiety complaints | NI | Depressive or Anxiety complaints | NI |
| Comorbidity | NI | Comorbidity | 1.23 (0.98; 1.55) | Comorbidity | 1.23 (0.98; 1.54) |
| Sexual transmittable diseases | NI | Sexual transmittable diseases | NI | Sexual transmittable diseases | NI |
| Interaction: single marital status × ethnicity | Interaction: single marital status × ethnicity | Interaction: single marital status × ethnicity | |||
| Afro-African | NI | Afro-African | NI | Afro-African | NI |
| Interaction: single marital status × age <20 years | 1.29 (1.08; 1.55) | Interaction: single marital status × age <20 years | 1.35 (1.12; 1.62) | Interaction: single marital status × age <20 years | 1.36 (1.13; 1.63) |
| Interaction: single marital status × unplanned pregnancy | NI | Interaction: single marital status × unplanned pregnancy | NI | Interaction: single marital status × unplanned pregnancy | NI |
| Interaction: net income Euro <1,200, × folic acid | NI | Interaction: net income Euro <1,200, × folic acid | NI | Interaction: net income Euro <1,200, × folic acid | NI |
| B Backward approach | |||||
|---|---|---|---|---|---|
| Multiple imputed dataset 4 | Multiple imputed dataset 5 | Combined estimate/Median | |||
| Risk factor | Exp (B) 95% CI | Risk factor | Exp (B) 95% CI | Risk factor | Exp (B) 95% CI |
| Neighbourhood deprivation | NI | Neighbourhood deprivation | 1.11 (0.98; 1.26) | Neighbourhood deprivation | NI |
| Age <20 years | 1.33 (1.00; 1.79) | Age <20 years | 1.34 (1.00; 1.79) | Age <20 years | 1.34 (1.00; 1.79) |
| Ethnicity Afro-African | 1.37 (1.13; 1.67) | Ethnicity Afro-African | 1.37 (1.12; 1.66) | Ethnicity Afro-African | 1.36 (1.11; 1.65) |
| Ethnicity non-Western other | 1.28 (1.12; 1.48) | Ethnicity non-Western other | 1.26 (1.09; 1.45) | Ethnicity non-Western other | 1.25 (1.08; 1.44) |
| Net income Euro <1,200 | 1.20 (1.02; 1.40) | Net income Euro <1,200 | NI | Net income Euro <1,200 | 1.20 (1.02; 1.40) |
| Occupation non-working | NI | Occupation non-working | NI | Occupation non-working | NI |
| Occupation ≥36 h/week | NI | Occupation ≥36 h/week | NI | Occupation ≥36 h/week | NI |
| Marital status single | NI | Marital status single | NI | Marital status single | NI |
| BMI intake <20 | 1.36 (1.13; 1.64) | BMI intake <20 | 1.36 (1.13; 1.63) | BMI intake <20 | 1.36 (1.13; 1.63) |
| Smoking during pregnancy | 1.20 (1.06; 1.37) | Smoking during pregnancy | 1.21 (1.06; 1.37) | Smoking during pregnancy | 1.21 (1.06; 1.37) |
| Recreational drug use | NI | Recreational drug use | NI | Recreational drug use | NI |
| Nullipara | 1.83 (1.60; 2.09) | Nullipara | 1.83 (1.61; 2.10) | Nullipara | 1.85 (1.62; 2.11) |
| Unplanned pregnancy | NI | Unplanned pregnancy | NI | Unplanned pregnancy | NI |
| No folic acid use | NI | No folic acid use | 1.19 (1.04; 1.37) | No folic acid use | NI |
| Complications previous pregnancy | 1.32 (1.04; 1.67) | Complications previous pregnancy | 1.33 (1.05; 1.68) | Complications previous pregnancy | 1.32 (1.04; 1.67) |
| Gestational age booking ≥14 weeks | 1.14 (1.00; 1.31) | Gestational age booking ≥14 weeks | NI | Gestational age booking ≥14 weeks | NI |
| Depressive or Anxiety complaints | NI | Depressive or Anxiety complaints | NI | Depressive or Anxiety complaints | NI |
| Comorbidity | NI | Comorbidity | NI | Comorbidity | NI |
| Sexual transmittable diseases | NI | Sexual transmittable diseases | NI | Sexual transmittable diseases | NI |
| Interaction: single marital status × ethnicity | Interaction: single marital status × ethnicity | Interaction: single marital status × ethnicity | |||
| Afro-African | NI | Afro-African | NI | Afro-African | NI |
| Interaction: single marital status × age <20 years | 1.35 (1.12; 1.61) | Interaction: single marital status × age <20 years | 1.44 (1.22; 1.70) | Interaction: single marital status × age <20 years | 1.35 (1.12; 1.61) |
| Interaction: single marital status × unplanned pregnancy | NI | Interaction: single marital status × unplanned pregnancy | NI | Interaction: single marital status × unplanned pregnancy | NI |
| Interaction: net income Euro <1,200 × folic acid | NI | Interaction: net income Euro <1,200 × folic acid | NI | Interaction: net income Euro <1,200 × folic acid | NI |
Results from logistic regression analysis with forward and backward approach (P inclusion = P exclusion = 0.10) for all 5 imputed datasets separately. If the same association was observed across 4 or 5 datasets, this was interpreted as a significant association. In that case we report on the combined estimate given as the median size of that coefficient together with its 95% CI. NI not included in final step
References
- 1.EURO-PERISTAT project with SCPE, EUROCAT and EURONEONET. European Perinatal Health Report 2008. www.europeristat.com.
- 2.Agyemang C, Vrijkotte TG, Droomers M, van der Wal MF, Bonsel GJ, Stronks K. The effect of neighbourhood income and deprivation on pregnancy outcomes in Amsterdam, The Netherlands. J Epidemiol Community Health. 2009;63:755–760. doi: 10.1136/jech.2008.080408. [DOI] [PubMed] [Google Scholar]
- 3.de Graaf JP, Ravelli AC, Wildschut HI, Denktas S, Voorham AJ, Bonsel GJ, et al. Perinatal outcomes in the four largest cities and in deprived neighbourhoods in The Netherlands. Nederlands tijdschrift voor geneeskunde. 2008;152:2734–2740. [PubMed] [Google Scholar]
- 4.Jaddoe VW, Bakker R, van Duijn CM, van der Heijden AJ, Lindemans J, Mackenbach JP, et al. The Generation R Study Biobank: a resource for epidemiological studies in children and their parents. Eur J Epidemiol. 2007;22:917–923. doi: 10.1007/s10654-007-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaddoe VW, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EA, et al. The Generation R Study: design and cohort update until the age of 4 years. Eur J Epidemiol. 2008;23:801–811. doi: 10.1007/s10654-008-9309-4. [DOI] [PubMed] [Google Scholar]
- 6.Central Bureau of Research and Statistics Rotterdam (COS). Core data Rotterdam 2008. www.cos.rotterdam.nl.
- 7.The International Response to Helsinki VI—The WMA’s Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, as adopted by the 52nd WMA General Assembly, Edinburgh. 2000.
- 8.The Netherlands’ Ministry of Housing, Spatial Planning and the Environment/Directorate General for Housing, Communities and Integration. http://international.vrom.nl/pagina.html?id=37564.
- 9.Statistics Netherlands. Standard classification people with a foreign background [in Dutch]. Voorburg/Heerlen; 2004.
- 10.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. doi: 10.1017/S0033291700048017. [DOI] [PubMed] [Google Scholar]
- 11.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed]
- 12.Berkenfield J, Schwartz JB. Nutrition intervention in the community—the “WIC” program. N Engl J Med. 1980;302:579–581. doi: 10.1056/NEJM198003063021013. [DOI] [PubMed] [Google Scholar]
- 13.Rutstein DD, Berenberg W, Chalmers TC, Child CG III, Fishman AP, Perrin EB. “Measuring the quality of medical care”: revision of tables of indexes. New Eng J Med. 1977;297:508. [PubMed]
- 14.Elo IT, Culhane JF, Kohler IV, O’Campo P, Burke JG, Messer LC, et al. Neighbourhood deprivation and small-for-gestational-age term births in the United States. Paediatr Perinat Epidemiol. 2009;23:87–96. doi: 10.1111/j.1365-3016.2008.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray R, Bonellie SR, Chalmers J, Greer I, Jarvis S, Williams C. Social inequalities in preterm birth in Scotland 1980–2003: findings from an area-based measure of deprivation. BJOG. 2008;115:82–90. doi: 10.1111/j.1471-0528.2007.01582.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Campo P, Burke JG, Culhane J, Elo IT, Eyster J, Holzman C, et al. Neighborhood deprivation and preterm birth among non-Hispanic Black and White women in eight geographic areas in the United States. Am J Epidemiol. 2008;167:155–163. doi: 10.1093/aje/kwm277. [DOI] [PubMed] [Google Scholar]
- 17.Sellstrom E, Arnoldsson G, Bremberg S, Hjern A. Are there differences in birth weight between neighbourhoods in a Nordic welfare state? BMC public health. 2007;7:267. doi: 10.1186/1471-2458-7-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farley TA, Mason K, Rice J, Habel JD, Scribner R, Cohen DA. The relationship between the neighbourhood environment and adverse birth outcomes. Paediatr Perinat Epidemiol. 2006;20:188–200. doi: 10.1111/j.1365-3016.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 19.Eames M, Ben-Shlomo Y, Marmot MG. Social deprivation and premature mortality: regional comparison across England. BMJ. 1993;307:1097–1102. doi: 10.1136/bmj.307.6912.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culhane JF, Elo IT. Neighborhood context and reproductive health. Am J Obstet Gynecol. 2005;192:22–29. doi: 10.1016/j.ajog.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 22.AVDR. The examination of neighbourhood effects on health: conceptual and methodological issues related to the presence of multiple levels of organisation. In: Neighbourhoods and health. Oxford, UK: Oxford University Press. 2003. pp. 45–64.
- 23.Kleinschmidt I, Hills M, Elliott P. Smoking behaviour can be predicted by neighbourhood deprivation measures. J Epidemiol Community Health. 1995;49(Suppl 2):72–77. doi: 10.1136/jech.49.Suppl_2.S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ACOG Committee Opinion number 313, September 2005. The importance of preconception care in the continuum of women’s health care. Obstet Gynecol. 2005;106:665-66. [DOI] [PubMed]
- 25.Sheldon T. Netherlands considers introducing preconception care. BMJ. 2007;335:686–687. doi: 10.1136/bmj.39353.518067.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wildschut HI, van Vliet-Lachotzki EH, Boon BM, Lie Fong S, Landkroon AP, Steegers EA. Preconception care: an essential part of the care for mother and child. Nederlands tijdschrift voor geneeskunde. 2006;150:1326–1330. [PubMed] [Google Scholar]
- 27.Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, et al. Recommendations to improve preconception health and health care–United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006 A;55:1–23. [PubMed]
- 28.Boulet SL, Parker C, Atrash H. Preconception care in international settings. Matern Child Health J. 2006;10:29–35. doi: 10.1007/s10995-006-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebrahim SH, Lo SS, Zhuo J, Han JY, Delvoye P, Zhu L. Models of preconception care implementation in selected countries. Matern Child Health J. 2006;10:S37–S42. doi: 10.1007/s10995-006-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czeizel AE. Ten years of experience in periconceptional care. Eur J Obstet Gynecol Reprod Biol. 1999;84:43–49. doi: 10.1016/S0301-2115(98)00246-2. [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth DR, Jones OW, Resnik R. Expanded care in obstetrics for the 1980 s: preconception and early postconception counseling. Am J Obstet Gynecol. 1984;149:811–814. doi: 10.1016/0002-9378(84)90596-9. [DOI] [PubMed] [Google Scholar]
- 32.Alderliesten ME, Vrijkotte TG, van der Wal MF, Bonsel GJ. Late start of antenatal care among ethnic minorities in a large cohort of pregnant women. BJOG. 2007;114:1232–1239. doi: 10.1111/j.1471-0528.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruhl C, Moran B. The clinical content of preconception care: preconception care for special populations. Am J Obstet Gynecol. 2008;199:384–388. doi: 10.1016/j.ajog.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Goodman A, Gatward R. Who are we missing? Area deprivation and survey participation. Eur J Epidemiol. 2008;23:379–387. doi: 10.1007/s10654-008-9248-0. [DOI] [PubMed] [Google Scholar]
- 35.Tromp M, van Eijsden M, Ravelli AC, Bonsel GJ. Anonymous non-response analysis in the ABCD cohort study enabled by probabilistic record linkage. Paediatr Perinat Epidemiol. 2009;23:264–272. doi: 10.1111/j.1365-3016.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 36.Sheldon T. Obstetric care must change if Netherlands is to regain reputation for safe childbirth. BMJ. 2008;336:239. doi: 10.1136/bmj.39472.657384.DB. [DOI] [Google Scholar]
- 37.Merkus JM. Perinatal mortality in The Netherlands: an audit is now more necessary than ever. Nederlands tijdschrift voor geneeskunde. 2008;152:603–605. [PubMed] [Google Scholar]
- 38.Alderliesten ME, Stronks K, van Lith JM, Smit BJ, van der Wal MF, Bonsel GJ, et al. Ethnic differences in perinatal mortality. A perinatal audit on the role of substandard care. Eur J Obstet Gynecol Reprod Biol. 2008;138:164–170. doi: 10.1016/j.ejogrb.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 39.van Roosmalen J, Schuitemaker NW, Brand R, van Dongen PW, Bennebroek Gravenhorst J. Substandard care in immigrant versus indigenous maternal deaths in The Netherlands. BJOG. 2002;109:212–213. [PubMed] [Google Scholar]
- 40.Ravelli AC, Tromp M, van Huis M, Steegers EA, Tamminga P, Eskes M, et al. Decreasing perinatal mortality in The Netherlands, 2000–2006: a record linkage study. J Epidemiol Community Health. 2009;63:761–765. doi: 10.1136/jech.2008.080440. [DOI] [PubMed] [Google Scholar]
- 41.http://www.sandovalhealth.org/docs/FSP_WIC_Prenatal_Risk_Factor_Score_Card.pdf.