Abstract
Glomerular thrombotic microangiopathy is a hallmark feature of haemolytic uraemic syndrome, the leading cause of acute renal failure in childhood. This paper is a review of the different mechanistic pathways that lead to this histological picture in the kidney. It will focus on atypical HUS and complement dysregulation, but will also highlight some other recent advances in our understanding of this condition, including the potential role of the molecule vascular endothelial growth factor- A (VEGF-A).
Keywords: Haemolytic uraemic syndrome, Thrombotic microangiopathy, Endothelial cell, Podocyte, VEGF-A
Introduction
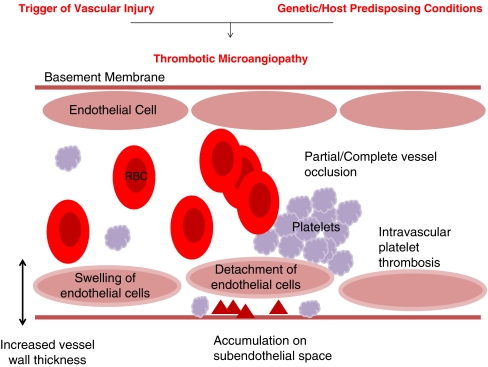
Haemolytic uraemic syndrome (HUS) is the most common cause of paediatric acute renal failure affecting between 0.2 and 4.28 people per 100,000 worldwide [1]. It is categorised as a thrombotic microangiopathy (TMA). TMA is a pathological term used to describe occlusive microvascular thrombus formation [2], which was first described in 1952 by Symmers (cited in Ruggenenti et al. [3]). Pathological features include vessel wall thickening, swelling and detachment of the endothelial cell from the basement membrane, accumulation of material in the subendothelial space, intraluminal platelet thrombosis, partial or complete vessel luminal obstruction and fragmentation of red blood cells (Fig. 1) [3–6].
Fig. 1.
Thrombotic microangiopathy: “the final events”. Thrombotic microangiopathy is the pathological process that is the final common pathway of many disease processes, but is most commonly associated with haemolytic uraemic syndrom (HUS) and thrombotic thrombocytopaenic purpura (TTP). Pathological features include: increased vessel wall thickness, swelling and/or detachment of endothelial cells, platelet aggregation/thrombosis, accumulation of debris/material in subendothelial space and partial or complete vessel occlusion. Clinically: microangiopathic haemolytic anaemia—sheer stress from vessel lumen occlusion produces fragmented red cells, thrombocytopaenia—platelets are consumed by local thrombosis and organ ischaemia—renal failure or neurological symptoms
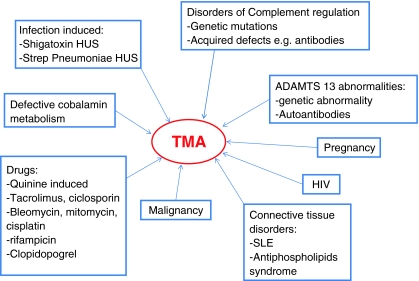
Clinically, TMA is associated with consumptive thrombocytopaenia, microangiopathic haemolytic anaemia and features of organ ischaemia [5]. The symptoms produced depend on the vascular bed and organ affected. TMA is most commonly associated with HUS and thrombotic thrombocytopaenic purpura (TTP) [2]. In TTP neurological endothelial cells are predominantly affected and so clinically neurological features are seen [5]. In HUS the TMA is predominantly seen in the glomeruli and thus results in acute renal insufficiency [5]. TTP and HUS are often considered together because of their similar aetiology and the fact that clinically there can be symptom overlap with neurological features seen in HUS and renal problems seen in TTP. Atypical HUS is more commonly associated with extra-renal effects than typical HUS [3]. Also, HUS is more commonly seen in children and TTP in adults [7]. The underlying reasons for this are unclear at present. TMA has many associations and precipitating factors (Fig. 2) [4, 8, 9].
Fig. 2.
Precipitants of thrombotic microangiopathy (TMA). SLE Systemic lupus erythematosus
Haemolytic uraemic syndrome has a variety of precipitating factors that help to categorise it into the traditional descriptions of typical and atypical [2]. It is more common in children under 5 years with an incidence of 6.1 cases per 100,000 per year [10]. The most common version of HUS is the ‘typical’ variety or diarrhoea-associated HUS (D + HUS). This has recently been termed infection-associated HUS [11]. Ninety percent of HUS cases are associated with infection [12]. Typically, this results from E. coli O157 infection, which produces Shiga toxin and causes a preceding diarrheal illness, but Streptococcus pneumoniae infection can also cause HUS (no diarrhoea). The variation in incidence is thought to reflect the differences in D + HUS caused by Shiga toxin-producing E. coli. In the UK there are higher rates of both E. coli O157 infection and D + HUS in Scotland compared with the rest of the country [13]. This may reflect a more rural population with more private water supplies. Only 10–15% of children who get E. coli O157 go on to develop HUS [10]. It is unclear why some children develop glomerular TMA and others do not. A genetic predisposition is possible, but as yet undefined.
Ten percent of cases of HUS fall into the “atypical” category. European prevalence is estimated to be 7 per million children [11]. Atypical cases have a variety of associated features and triggers, but include familial cases, which are now understood to be disorders of complement activation as a result of loss of normal regulatory factors or by activating mutations [9]. Other factors that can produce atypical HUS include pregnancy, drugs, malignancy, connective tissue disorders and metabolic defects [9]. Atypical HUS can present with an infective trigger and these cases can be difficult to distinguish from typical cases in the early stages of illness. Atypical forms of HUS are rare, but carry a poorer prognosis with significant morbidity and mortality [11]. There has been improved understanding of glomerular TMA and HUS in the past few years; however, there are still many questions yet to be answered.
Atypical haemolytic uraemic syndrome
Atypical haemolytic uraemic syndrome (HUS) is an uncommon condition that is now widely accepted to be a disorder of complement over activation. It carries a poorer prognosis than infection-associated HUS with a 25% mortality rate and 50% developing end-stage renal failure [11]. Most of the familial mutations described result in loss of regulation of the complement cascade; however, some activating mutations have also been described, e.g. C3 [14] and factor B [15]. It is members of the alternative complement pathway that are affected, either as a result of genetic mutations or by the presence of antibodies against members of the complement regulatory system [9]. This includes complement factors H and I and membrane co-factor protein (MCP), which are found to be mutated in 50% of atypical HUS patients [2, 5]. These molecules prevent inappropriate complement activation against “self-cells” in the body. Thus, mutations and antibodies that alter their function result in overwhelming complement activation directed at self cells, i.e. glomerular endothelial cells. It has been hypothesised that certain vascular beds are more at risk from this process. The glomerulus is thought to be a target because it is fenestrated and so the subendothelial matrix is continually exposed to circulating proteins [16]. Atypical HUS is commonly recurrent and carries a poor prognosis with significant mortality and can lead quickly to end-stage renal failure. It was first described by Gasser in 1955 (cited in Ruggenenti et al. [3]).
In 1974, it was identified that atypical HUS patients had low C3 levels and normal C4 levels reflecting complement activation and consumption. This was the first link between HUS and complement [16].
Normal complement cascade
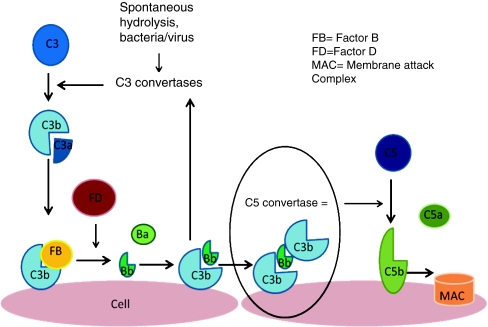
To understand the pathogenesis of atypical HUS we must first understand the normal complement pathway. The complement system is a group of 30 proteins that are part of the innate immune system that protects against invading organisms [16]. They “complement” the antibacterial properties of antibodies. These proteins can be plasma-based (fluid) or membrane-bound (solid) and have either activation or regulatory functions. There are three main branches of the complement pathway: classical, lectin and alternative. The classical pathway is activated by antigen-antibody binding whilst the lectin pathway is activated by serum lectin binding to mannose-containing carbohydrates on bacteria and viruses. The alternative pathway is triggered by complement proteins binding to the surface of pathogens [17]. The end result of each pathway is the production of proteases complexes (C3 convertases and C5 convertases) that cleave C3 and C5, which leads to the activation of the membrane attack complex (MAC), creating pores in membranes. It is the alternative complement pathway that is affected in atypical HUS. This pathway provides an amplification loop that can be triggered alone or complement to the classical pathway. This cascade starts with C3 hydrolysis in plasma and results in C3b deposition onto almost all exposed cell surfaces (see Fig. 3).
Fig. 3.
The alternative pathway is triggered by the covalent binding of C3b to a pathogen or cell surface. Next, factor B binds to surface bond C3b, making it susceptible to plasma factor D cleavage. The result is production of Ba and active protease Bb, which remains bound to C3b creating C3bBb, which is the C3 convertase of the alternative complement pathway. This starts the amplification loop with C3 convertase generating more C3b on the cell surface and the process repeats. Ultimately, there will be C3b saturation on the cell surface with release of C3a, a small inflammatory mediator. Eventually, some of the C3b binds to pre-existing C3 convertase producing C3b2Bb, which is the alternative pathway’s C5 convertase. This cleaves C5 into C5b, which generates the membrane attack complex (MAC), and C5a, a potent pro-inflammatory mediator. Complement-mediated endothelial cell injury creates a prothrombotic state. It exposes subendothelial collagens and releases von Willebrand factor and fibrinogen formation
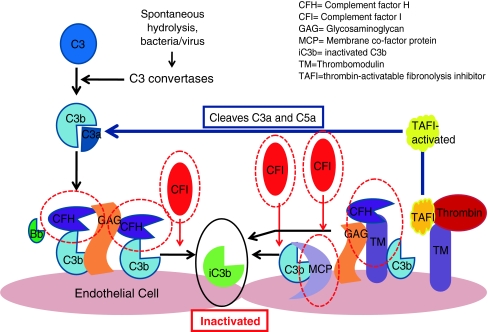
Complement activation is regulated by plasma and membrane-bound regulators that act to cleave C3b to inactive C3b (C3bi; Fig. 4) [16]. Complement factors H and I are crucial for this process. Without this regulation, C3b deposition increases exponentially causing activation of the complement cascade. This continues until components are consumed. If cells do not have membrane-bound regulators or cannot bind soluble receptors, they are attacked by complement. C3b deposits on bacterial cells act as a tags and bind to neutrophil receptors, causing phagocytosis.
Fig. 4.
Complement regulators are shown circled in red. These include factors H and I and membrane co-factor protein. Each acts to promote the inactivation of C3b and prevent further progression of the complement cascade. Factor H binds Cb3 and works with factor I to inactivate it. Both complement factors H and I are serum-based. Membrane co-factor protein is cell-bound. It also binds to C3b, which has become attached to cells and works with factor I to inactivate it. Thrombomodulin is also shown as mutations and has been associated with atypical HUS. It regulates complement by acting to inactivate the proinflammatory mediators C3a and C5a and accelerating factor I-mediated C3b inactivation. It also plays a role in local coagulation regulation through its interactions with thrombin
Serum-based complement factors
This review will focus on the two factors that have been most intensely studied, factors H and I.
Complement factor H
Complement factor H is a serum glycoprotein synthesised in the liver and a regulatory member of the alternative complement pathway [18]. Mutations in the gene were first described in 1998 [19]. Further studies went on to show that reduced complement factor H levels can be found in patients with atypical HUS, but also in their asymptomatic family members. Most mutations are inherited in an autosomal dominant fashion with variable penetrance. Furthermore, normal levels of complement factor H do not rule out functional problems. There have been reports of antibodies directed against this factor, which reduces its functional capacity [20]. Factor H mutations can be seen from the neonatal period to adulthood. Antibodies against factor H are seen predominantly in the pre-adolescent period. Mutations account for 6–11% of cases of atypical HUS [20]. The factor H gene is found on 1q32 where many other complement regulatory genes are also found [19, 21, 22].
Factor H is a fluid phase complement regulator [20]. It inhibits the formation of the alternative C3-convertase and accelerates its decay (see Fig. 4). The complement regulatory section of factor H is found in its N-terminal whilst the C-terminal harbours the membrane-binding section [20, 23, 24]. Sixty to eighty percent of aHUS mutations have been identified in the C-terminal [25]. This terminal binds to glycosaminoglycans on endothelial cells and basement membrane [24]. Factor H binds to cell surface-bound C3b (Fig. 4). Factor H is a serum-based factor that can bind to self cells and protects them from being attacked by the complement system. It is thought that dysfunction or reduced levels of factor H causes problems with cell recognition during an inflammatory insult [16]. The result is endothelial cell damage causing exposure of the subendothelial matrix. The result is a full complement attack and thrombus formation. This will produce platelet consumption and red cell damage. Thus, TMA is produced (Fig. 5) [25]. Interestingly, there is now also good evidence that factor H is also produced by platelets and can modify their function [26, 27].
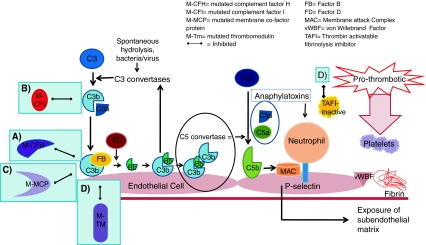
Fig. 5.
Development of thrombotic microangiopathy with loss of normal complement regulatory factor function. Loss of alternative complement pathway regulation caused by loss of function of factors H and I, membrane co-factor protein or thrombomodulin results in complement activation directed against endothelial cells. a Mutated complement factor H cannot bind to cell surface C3b to stop alternative complement pathway attack. b Mutated complement factor I cannot cleave C3b. c Mutated membrane co-factor protein cannot work with factor I to degrade C3b. d Mutated thrombomodulin cannot bind C3b and accelerate factor I-medicated inactivation of C3b. It cannot activate thrombin activatable fibrinolysis inhibitor (TAFI), which inactivates anaphylatoxins C3a and C5a. It also cannot bind thrombin. With the loss of these regulatory functions of each of these factors C3b is not inactivated and progression of the complement cascade ensues. C3b then binds with Bb and then further C3b bind to this complex, which forms C5 convertase. This cleaves C5 to C5b and the resulting cascade results in formation of the membrane attack complex (MAC). C3a and C5a act as anaphylatoxins, which attract neutrophils to the cells under complement attack. Activating mutations of factor B and C3b also result in progression of the complement pathway. The result of any of these mutations is endothelial cell damage, causing exposure of the subendothelial matrix. This results in a prothrombotic state with fibrin deposition and release of von Willebrand factor, which attracts platelets to the site. Platelets will aggregate. The result is a full complement attack and thrombus formation. Partial or complete vessel occlusion will result. This will produce platelet consumption and red cell damage and TMA is the result
Linkage studies helped identify mutations in factor H in cases of atypical HUS [19]. Naturally, attention then turned to the creation of mouse models to better understand the pathogenesis and cellular interactions. Interestingly, the factor H null mouse [28] does not get HUS, but develops severe membranoproliferative glomerulonephritis (MPGN), which can also occur in humans with factor H mutations. This mouse developed uncontrolled alternative complement pathway activation and has very low C3 levels. A similar scenario occurred in a pig model [29]. Other models have been developed, in particular a mouse that lacks the C-terminal function of factor H [30]. This mouse had higher C3 levels and did develop HUS. This proved that factor H mutations cause HUS by impairing cell surface recognition, resulting in local complement dysregulation. It was only the homozygous mice that developed HUS, in contrast to humans, where one defective allele is enough to predispose to HUS.
Complement factor I
Factor I is another serum-based member (fluid component) of the complement regulation system. It is also predominantly produced in the liver. It is a serine protease that cleaves C3b and thus plays a key inhibitory role in preventing alternative pathway amplification (prevents formation of C3 convertase [C3bBb] from C3b; Fig. 4) [16]. The function of factor I depends on many co-factors, including factor H and C4-binding protein. There are also membrane-bound molecules, which also play a role in C3b cleavage. These include CD35 and membrane co-factor protein (CD46) [31].
Factor I mutations causing HUS were first described in 2004 [31]. The factor I gene is found on chromosome 4. Mutations were found in sporadic cases rather than familial, suggesting a low penetrance of mutations. The abnormal gene encodes truncated factor I protein, which lacks the C-terminal including the serine proteinase region. The serine proteinases are a large group of biologically important enzymes that includes trypsin, other complement proteins, C2, C1r, C1s, factor D, and factor B, and proteins of the fibrinolytic and coagulation cascades. Factor I contains the catalytic triad of amino acids aspartic acid, histidine and serine, a triad common to all serine proteinases.
In factor I deficiency, the alternative pathway is not regulated (Fig. 5) and in a similar way to factor H deficiency, the final result is TMA. This leads to a consumptive depletion of C3 and factor B. Complete hereditary deficiency of factor I has been reported in at least 30 different pedigrees and is associated with severe pyogenic infections [31].
Patients with mutations or antibodies against these serum-based complement regulators have a poorer prognosis especially with regard to transplantation. They have been shown to develop recurrence in their grafts and so transplantation cannot be recommended in this group for this reason at present [11]. However, as complement factors H and I are synthesised by the liver, either an isolated liver transplant or a combined liver and kidney transplant can be considered, but only in centres with previous experience of these cases [11]. Factor I knockout mice show uncontrolled alternative pathway activation, but, similar to factor H knockouts, do not develop HUS, instead they develop mesangial C3 deposits [29].
Membrane-bound complement factors
Membrane co-factor protein (MCP or CD46)
Membrane co-factor protein is a widely expressed transmembrane complement regulator. It is expressed on almost every cell except erythrocytes. It works with factor I to degrade C3b and C4b, which are bound to the host cell surface (see Fig. 4). The MCP gene is also found on chromosome 1q32 [21].
Membrane co-factor protein mutations cause the protein to be incorrectly processed and so remains intracellular or affects C3b binding. Both autosomal dominant inheritance with variable penetrance and autosomal recessive patterns have been seen. In some pedigrees subjects carry mutations, but do not develop HUS suggesting that the mutation alone is not always enough to develop the disease. There does seem to be an endothelial insult that triggers the initiation of HUS. It is after this that overwhelming complement activation results and TMA develops (Fig. 5). As MCP is membrane-bound and not serum-bound, renal transplantation is successful in this patient group [21].
There is no way of developing a good mouse model to investigate this membrane-bound protein because of inherent differences between human and mouse complement systems. Mice lack MCP expression in their glomeruli; it is only expressed in testes [29]. Mice instead express complement regulatory protein (crry), which has co-factor activity and decay-accelerating factor activity. Crry knockout is embryologically lethal. A mouse that has crry knockout and C5 knockout does exist. Kidneys from this mouse have been transplanted into mice with fully functioning complement. These kidneys fail due to uncontrolled complement activation.
Thrombomodulin
Recently, it has been discovered that mutations in thrombomodulin can trigger atypical HUS. Thrombomodulin is a ubiquitous transmembrane endothelial cell glycoprotein with anticoagulant, anti-inflammatory and cytoprotective properties [32]. It is anchored to the cell by a short cytoplasmic tail and single transmembrane domain. In vitro it binds C3b and complement factor H. It negatively regulates complement by accelerating factor I-mediated inactivation of C3b in the presence of co-factors (factor H and C4b binding protein). It also promotes activation of plasma procarboxypeptidase B (or thrombin activatable fibrinolysis inhibitor = TAFI) and accelerates inactivation of anaphylatoxins C3a and C5a (Fig. 4). It also accelerates thrombin-mediated activation of protein C, which down-regulates further thrombin generation and suppresses clot formation.
Thrombomodulin interferes with inflammation by suppressing leukocyte trafficking and dampening complement activation via its lectin-like domain. Mutations in this domain alter factor H and C3b binding and thus complement regulation. Mutations in the serine–threonine-rich region alter factor I-mediated Cb3 inactivation. Interestingly, in the landmark paper by Delvaeye et al. [32], one patient had recurrence of atypical HUS post-renal transplantation despite thrombomodulin being a solid phase protein. It will be of interest to see what subsequent clinical studies reveal with regard to the post-transplant course of this genetic mutation.
Thrombotic thrombocytopaenic purpura
Thrombotic thrombocytopaenic purpura (TTP) was first described in 1924 [33]. It is more common in female subjects between the ages of 10 and 39 years. The highest incidence is seen in the fourth decade. The annual incidence is 3.7 cases per 1,000,000 [7]. Clinically, it presents as a pentad of symptoms: microangiopathic haemolytic anaemia, thrombocytopaenia, neurological symptoms, renal damage and fever. It used to be a diagnosis of exclusion; however, there is now an ADAMTS13 activity assay that clinches the diagnosis [33]. Plasma exchange was shown to improve symptoms and is now standard treatment for TTP [2]. It took 20 years before the reason why plasma exchange is a useful treatment in TTP was understood [34]. In 1982, it was noted that TTP patients had ultra-large multimers of von Willebrand factor circulating in their blood during periods of remission; thus, it was hypothesised that, as these were not seen in healthy people, that TTP patients lack a protease that normally cleaves these ultra-large multimers [33]
Thrombotic thrombocytopaenic purpura is now known to be a disorder of von Willebrand factor (vWF) regulation [5]. vWF is a glycoprotein produced by endothelial cells that regulates platelet aggregation and adhesion. When vascular injury occurs, vWF is released from endothelial cells as ultra-large multimers (UL-vWF). Some of these stay associated with the endothelial cell surface providing platelet-binding sites. They may bind other blood components too, e.g. leukocytes. Platelet binding to UL-vWF is regulated by the metalloprotease ADAMTS13 (a-disintegrin-like and metalloprotease and thrombospondin repeats). It is a deficiency of ADAMTS 13 that accounts for the majority of patients with congenital TTP. The majority of acquired cases occur due to antibody formation against this molecule [33].
Banno et al. [35] has developed an ADAMTS13 knockout mouse on a pure genetic background SV129. These mice do not normally develop TMA, but do show a UL-vWF multimers pattern similar to that seen in TTP. However, when challenged with platelet and endothelial agonists, they develop severe thrombocytopaenia. This suggests that ADAMTS13 deficiency alone is not enough to cause TMA. A further environmental or genetic hit is required to develop TTP.
Infection-associated HUS
Diarrhoea-associated HUS
This is the most common cause of HUS [10]. It is predominantly a disease of childhood, but can also affect adults, particularly the elderly. Children present with a history of diarrhoea, which is often bloody in nature. Two to five days later they present with pallor, weakness and oligo-anuria. There are approximately 100 cases per year in the UK [36]. It is fatal in 3–5% of cases. Two-thirds of diarrhoea-associated HUS (D + HUS) cases require dialysis therapy in the acute phase [10]. The diarrhoeal illness is most commonly caused by E. coli O157: H7, although there are many other serotypes of E. coli that can also cause HUS. Each of these serotypes produces Shiga toxin (stx), which is thought to produce HUS. Other bacteria can also cause HUS, e.g. Shigella, Campylobacter. Interestingly, only 10–15% of children who are infected with enterohaemorrhagic E. coli develop HUS. It is not clear why these children are susceptible. However, it has been hypothesised that they may have an underlying genetic pre-disposition or some other environmental factor that puts them more at risk. The renal pathogenesis of D + HUS is not yet fully understood.
At the cellular level the target from Shiga toxin (stx) is Gb3 [37]. This glycosphingolipid receptor is found on human endothelial cells, podocytes and tubular cells. It is unclear which cell is the main target for stx binding to cause HUS. The glomerular endothelial cell has been the main focus of study to date. Podocytes and human proximal tubule cells are also sensitive to stx cytotoxicity.
There are species differences in Gb3 expression [37]. Mice have Gb3 on their tubular cells, but lack Gb3 in their glomeruli (both endothelial cells and podocytes). This has limited the ability to use small animal models to study this disease [29]. Interestingly, Gb3 synthase knockout mice are fully protected from stx injections suggesting that this is the only receptor that stx binds and exerts its deleterious effects [38].
It was previously hypothesised that differences in Gb3 expression between children and adults explained why HUS was seen more frequently in children, especially younger children. It was proposed that glomerular Gb3 expression reduced with age, although total renal Gb3 expression increased with age. One study looked at stx 1 and 2 staining of kidneys from fatal adult and paediatric cases of E. coli-related HUS. They found that infant cases showed stx binding in the glomeruli and tubules, whilst the geriatric cases had stx binding in the proximal and distal tubules [39]. However, a later study showed identical patterns of Gb3 expression and stx 1 binding in both adult and paediatric kidneys, both at glomerular and tubular levels [40]. This study did only look at stx 1 binding and it is known that stx2 is linked with more severe disease and produces HUS [41]. Older studies looked specifically at Stx2.
Animal models have been created to investigate this disease process. Greyhounds [42] fed raw meat containing E. coli developed bloody diarrhoea, skin ulcers and renal failure. Pathologically, they showed glomerular TMA. Baboons [43] also developed typical features of HUS after stx (1 or 2) injections including glomerular TMA. Large animal models are expensive and impractical to study and so attention turned to smaller animal models where transgenic technology is also available [29]. Rodents (mice and rats) develop tubular disease with no glomerular effects in response to stx (oral, IV or IP administration). This is thought to relate to differences in Gb3 receptor expression among species. Cows are the reservoir of infection with E. coli O157. They are asymptomatic carriers and this may aid the spread of E. coli O157. Their Gb3 expression also varies from that of humans. Cattle kidneys express Gb3 in their tubules and collecting ducts, but not in their glomeruli. This may explain why they do not get glomerular microangiopathy, but a complete explanation of why this occurs is lacking [44].
A mouse model was created in 2006 that developed some features of HUS [45]. This model was given stx2 and lipopolysaccharide (LPS). Mice developed thrombocytopaenia, haemolytic anaemia and renal failure. Pathologically, they still showed evidence of tubular disease, but did show glomerular fibrin thrombi. It is unclear how the glomerular effects were produced or whether they followed the primary tubular damage. Further study is required to help understand the pathogenesis of D + HUS and TMA.
Streptococcal pneumoniae
Streptococcal pneumoniae-related HUS follows invasive pneumococcal disease and is linked to having a high bacterial load. It accounts for 5% of childhood cases of HUS [46]. The incidence of HUS following pneumococcal infection is estimated to be 0.4–0.6%. Children under 2 years are most commonly affected. HUS usually develops 3–13 days after infection starts. It is associated with a longer period of oligo-anuria and acute dialysis period than stx HUS. Ten percent of patients progress to end-stage renal failure and there is 12% mortality. The highest mortality is related to Streptococcal pneumoniae meningitis complicated by HUS.
Streptococcal pneumoniae-related HUS is caused by exposure of the Thomsen–Friedenreich (TF) crypt antigen. This antigen is found on the surface of erythrocytes, platelets and glomerular endothelial cells, but is normally masked by neuraminic acid. All serotypes of S. pneumoniae produced neuraminidase, which cleaves the n-acetyl neuraminic acid from the cell surface and exposes the TF antigen. The host then produces IgM antibodies, which bind to the TF antigen. This initiates an immune response that culminates in the development of HUS with red cells, platelets and glomerular endothelial cell damage. The TF antigen can also be found on hepatocytes and this explains why some patients can develop hepatic dysfunction.
Vascular endothelial growth factor
Recently, it has been shown that the glomerular podocyte might be important in the pathogenesis of HUS through vascular endothelial growth factor A (VEGF-A).
Vascular endothelial growth factor is the most important endothelial growth factor and is vital in maintaining healthy, normally functioning endothelial cells [47]. In the kidney it is the podocyte that produces VEGF. VEGF is a complex protein that is present in various spice variants. Recently, there has been much interest in this growth factor. In adults, monoclonal antibodies against VEGF-A (bevacizumab) have been used in some cancer therapy regimens, as VEGF is found to be unregulated in many human tumours [48]. Some of these adults develop renal side effects whilst receiving this treatment. Initially, proteinuria and hypertension were seen, but some patients developed glomerular TMA and acute renal insufficiency. This sparked interest in investigating this further using VEGF-A transgenic mice models. Whole-body VEGF-A null mice are embryonically lethal [47]. Homozygous podocyte-specific VEGF-A null mice [49] who had VEGF knocked out early in development developed grossly abnormal glomeruli whose endothelial cells were immature. Heterozygous podocyte-specific VEGF-A mice developed renal lesions like those seen in pre-eclampsia (endotheliosis and bloodless glomeruli). This progressed to nephrotic syndrome and then renal failure. Thus, VEGF is essential for the development of normal glomeruli, but did not explain why the cancer patients developed TMA. A conditional mouse model in which VEGF-A could be knocked out in the fully developed podocyte was therefore developed to selectively knock down VEGF-A in podocytes using tetracycline-responsive technology [50]. Fascinatingly, this resulted in a phenotype that closely resembled HUS with glomerular thrombotic microangiopathy.
At the cellular level VEGF has been investigated in the context of Shiga-toxin-induced HUS. VEGF-A is predominantly produced by the podocyte in the kidney and there is evidence that stx modulates its production of human, but not murine origin. This is interesting as mice do not develop a glomerular phenotype when challenged with stx, unlike man. In vitro it has been found that human podocytes exposed to stx produce 60% less VEGF-A compared with controls. However, there is no effect of Shiga toxin on murine podocytes that do not express the gb3 receptor even when exposing these cells to 10 times the amount of stx given to human cells [37].
Vascular endothelial growth factor A is a molecule of great interest and requires further study to fully understand its role in other disease models that pathologically exhibit TMA.
Conclusions
It is now clear that there are multiple initiators of glomerular microangiopathy. Our understanding of atypical HUS has greatly progressed over recent decades, as illustrated by the plethora of alternative complement regulatory abnormalities that result in this condition. However, there are still a number of interesting and fundamental questions to be answered.
Why does HUS predominantly affect the kidney and TTP the brain?
Why does HUS mostly affect children, but TTP adults?
What is the renal pathogenesis of D + HUS? Does it have a link to complement or VEGF-A?
Why does E. coli O157 lead to HUS in only 10–15% of children? Is there a genetic component?
Could we manipulate the complement system in patients with atypical HUS to switch off therelentless complement activation and thus have a new way to treat HUS?
Although the endothelial cell is a major site of pathological damage there is now exciting evidence that the podocyte may play a role in microangiopathy through modulation of VEGF-A. It will be of interest to see whether a unifying pathological mechanism can be found for typical and atypical HUS in years to come.
Acknowledgement
This work is supported by the British Medical Research Council (grant number G0501901).
Multiple choice questions
Answers appear following the reference list.
- A major cellular receptor in human Shiga toxin HUS is called
- crry
- Gb3
- CD42
- MCP
- Factor I
- The predominant pathway that is affected by atypical HUS is the
- VEGF-A pathway
- Lectin complement pathway
- Alternative complement pathway
- Classical complement pathway
- Coagulation pathway
- Which of the following are solid phase complement components?
- Factor H
- Factor I
- MCP
- C3
- C4
- After renal transplantation, in which of the following is there least likely to be a recurrence of HUS in the transplanted kidney
- Factor H mutations
- Factor I mutations
- C3 mutations
- MCP mutations
- Thrombomodulin mutations
- The cause of pneumococcal-induced HUS is thought to be
- Alterations in podocyte-derived VEGF-A
- Abnormalities in the classical complement pathway
- Thromboxane hyper-stimulation
- Exposure of neuraminidase on the cell surfaces
- Production of neuraminidase by the bacteria exposing the TF antigen
Footnotes
Answers
1. b
2. c
3. c
4. d
5. e
References
- 1.Serna A, Boedeker EC. Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr Opin Gastroenterol. 2008;24:38–47. doi: 10.1097/MOG.0b013e3282f2dfb8. [DOI] [PubMed] [Google Scholar]
- 2.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008;3:249–277. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001;60:831–846. doi: 10.1046/j.1523-1755.2001.060003831.x. [DOI] [PubMed] [Google Scholar]
- 4.Copelovitch L, Kaplan BS. The thrombotic microangiopathies. Pediatr Nephrol. 2008;23:1761–1767. doi: 10.1007/s00467-007-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai HM. The molecular biology of thrombotic microangiopathy. Kidney Int. 2006;70:16–23. doi: 10.1038/sj.ki.5001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benz K, Amann K. Pathological aspects of membranoproliferative glomerulonephritis (MPGN) and haemolytic uraemic syndrome (HUS)/thrombocytic thrombopenic purpura (TTP) Thromb Haemost. 2009;101:265–270. [PubMed] [Google Scholar]
- 7.Michael M, Elliott EJ, Ridley GF, Hodson EM, Craig JC (2009) Interventions for haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Cochrane Database Syst Rev CD003595 [DOI] [PMC free article] [PubMed]
- 8.Franchini M. Thrombotic microangiopathies: an update. Hematology. 2006;11:139–146. doi: 10.1080/10245330600667583. [DOI] [PubMed] [Google Scholar]
- 9.Ariceta G, Besbas N, Johnson S, Karpman D, Landau D, Licht C, Loirat C, Pecoraro C, Taylor CM, Kar N, Vandewalle J, Zimmerhackl LB. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol. 2009;24:687–696. doi: 10.1007/s00467-008-0964-1. [DOI] [PubMed] [Google Scholar]
- 10.Scheiring J, Andreoli SP, Zimmerhackl LB. Treatment and outcome of Shiga-toxin-associated hemolytic uremic syndrome (HUS) Pediatr Nephrol. 2008;23:1749–1760. doi: 10.1007/s00467-008-0935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor CM, Machin S, Wigmore SJ, Goodship TH. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol. 2010;148:37–47. doi: 10.1111/j.1365-2141.2009.07916.x. [DOI] [PubMed] [Google Scholar]
- 12.Desch K, Motto D. Is there a shared pathophysiology for thrombotic thrombocytopenic purpura and hemolytic-uremic syndrome? J Am Soc Nephrol. 2007;18:2457–2460. doi: 10.1681/ASN.2007010062. [DOI] [PubMed] [Google Scholar]
- 13.Pearce MC, Chase-Topping ME, McKendrick IJ, Mellor DJ, Locking ME, Allison L, Ternent HE, Matthews L, Knight HI, Smith AW, Synge BA, Reilly W, Low JC, Reid SW, Gunn GJ, Woolhouse ME. Temporal and spatial patterns of bovine Escherichia coli O157 prevalence and comparison of temporal changes in the patterns of phage types associated with bovine shedding and human E. coli O157 cases in Scotland between 1998–2000 and 2002–2004. BMC Microbiol. 2009;9:276. doi: 10.1186/1471-2180-9-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault de Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJ, Goodship TH, Atkinson JP. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, Lopez-Trascasa M, Sanchez-Corral P, Morgan BP, Rodriguez de Cordoba S. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 17.Jokiranta TS, Zipfel PF, Fremeaux-Bacchi V, Taylor CM, Goodship TJ, Noris M. Where next with atypical hemolytic uremic syndrome? Mol Immunol. 2007;44:3889–3900. doi: 10.1016/j.molimm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Estaller C, Schwaeble W, Dierich M, Weiss EH. Human complement factor H: two factor H proteins are derived from alternatively spliced transcripts. Eur J Immunol. 1991;21:799–802. doi: 10.1002/eji.1830210337. [DOI] [PubMed] [Google Scholar]
- 19.Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, Turnpenny P, Goodship JA. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998;53:836–844. doi: 10.1111/j.1523-1755.1998.00824.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee BH, Kwak SH, Shin JI, Lee SH, Choi HJ, Kang HG, Ha IS, Lee JS, Dragon-Durey MA, Choi Y, Cheong HI. Atypical hemolytic uremic syndrome associated with complement factor H autoantibodies and CFHR1/CFHR3 deficiency. Pediatr Res. 2009;66:336–340. doi: 10.1203/PDR.0b013e3181b1bd4a. [DOI] [PubMed] [Google Scholar]
- 21.Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, Muslumanoglu MH, Kavukcu S, Filler G, Pirson Y, Wen LS, Atkinson JP, Goodship TH. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2003;100:12966–12971. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41:355–367. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira VP, Herbert AP, Cortes C, McKee KA, Blaum BS, Esswein ST, Uhrin D, Barlow PN, Pangburn MK, Kavanagh D. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J Immunol. 2009;182:7009–7018. doi: 10.4049/jimmunol.0804031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt CQ, Herbert AP, Kavanagh D, Gandy C, Fenton CJ, Blaum BS, Lyon M, Uhrin D, Barlow PN. A new map of glycosaminoglycan and C3b binding sites on factor H. J Immunol. 2008;181:2610–2619. doi: 10.4049/jimmunol.181.4.2610. [DOI] [PubMed] [Google Scholar]
- 25.Jozsi M, Heinen S, Hartmann A, Ostrowicz CW, Halbich S, Richter H, Kunert A, Licht C, Saunders RE, Perkins SJ, Zipfel PF, Skerka C. Factor H and atypical hemolytic uremic syndrome: mutations in the C-terminus cause structural changes and defective recognition functions. J Am Soc Nephrol. 2006;17:170–177. doi: 10.1681/ASN.2005080868. [DOI] [PubMed] [Google Scholar]
- 26.Licht C, Pluthero FG, Li L, Christensen H, Habbig S, Hoppe B, Geary DF, Zipfel PF, Kahr WH. Platelet-associated complement factor H in healthy persons and patients with atypical HUS. Blood. 2009;114:4538–4545. doi: 10.1182/blood-2009-03-205096. [DOI] [PubMed] [Google Scholar]
- 27.Alexander JJ, Quigg RJ. The simple design of complement factor H: looks can be deceiving. Mol Immunol. 2007;44:123–132. doi: 10.1016/j.molimm.2006.07.287. [DOI] [PubMed] [Google Scholar]
- 28.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 29.Richards A, Kavanagh D. Pathogenesis of thrombotic microangiopathy: insights from animal models. Nephron Exp Nephrol. 2009;113:e97–e103. doi: 10.1159/000235253. [DOI] [PubMed] [Google Scholar]
- 30.Pickering MC, Jorge EG, Martinez-Barricarte R, Recalde S, Garcia-Layana A, Rose KL, Moss J, Walport MJ, Cook HT, Cordoba SR, Botto M. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 2007;204:1249–1256. doi: 10.1084/jem.20070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, Vigneau C, Kuypers D, Boudailliez B, Loirat C, Rondeau E, Fridman WH. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet. 2004;41:e84. doi: 10.1136/jmg.2004.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delvaeye M, Noris M, Vriese A, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, Remuzzi G, Conway EM. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112:11–18. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moake J. Thrombotic thrombocytopenia purpura (TTP) and other thrombotic microangiopathies. Best Pract Res Clin Haematol. 2009;22:567–576. doi: 10.1016/j.beha.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Banno F, Kokame K, Okuda T, Honda S, Miyata S, Kato H, Tomiyama Y, Miyata T. Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood. 2006;107:3161–3166. doi: 10.1182/blood-2005-07-2765. [DOI] [PubMed] [Google Scholar]
- 36.Lynn RM, O'Brien SJ, Taylor CM, Adak GK, Chart H, Cheasty T, Coia JE, Gillespie IA, Locking ME, Reilly WJ, Smith HR, Waters A, Willshaw GA. Childhood hemolytic uremic syndrome, United Kingdom and Ireland. Emerg Infect Dis. 2005;11:590–596. doi: 10.3201/eid1104.040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Psotka MA, Obata F, Kolling GL, Gross LK, Saleem MA, Satchell SC, Mathieson PW, Obrig TG. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infect Immun. 2009;77:959–969. doi: 10.1128/IAI.00679-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okuda T, Tokuda N, Numata S, Ito M, Ohta M, Kawamura K, Wiels J, Urano T, Tajima O, Furukawa K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J Biol Chem. 2006;281:10230–10235. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 39.Chaisri U, Nagata M, Kurazono H, Horie H, Tongtawe P, Hayashi H, Watanabe T, Tapchaisri P, Chongsa-nguan M, Chaicumpa W. Localization of Shiga toxins of enterohaemorrhagic Escherichia coli in kidneys of paediatric and geriatric patients with fatal haemolytic uraemic syndrome. Microb Pathog. 2001;31:59–67. doi: 10.1006/mpat.2001.0447. [DOI] [PubMed] [Google Scholar]
- 40.Ergonul Z, Clayton F, Fogo AB, Kohan DE. Shigatoxin-1 binding and receptor expression in human kidneys do not change with age. Pediatr Nephrol. 2003;18:246–253. doi: 10.1007/s00467-002-1025-9. [DOI] [PubMed] [Google Scholar]
- 41.Johannes L, Romer W. Shiga toxins—from cell biology to biomedical applications. Nat Rev Microbiol. 2010;8:105–116. doi: 10.1038/nrmicro2279. [DOI] [PubMed] [Google Scholar]
- 42.Hertzke DM, Cowan LA, Schoning P, Fenwick BW. Glomerular ultrastructural lesions of idiopathic cutaneous and renal glomerular vasculopathy of greyhounds. Vet Pathol. 1995;32:451–459. doi: 10.1177/030098589503200501. [DOI] [PubMed] [Google Scholar]
- 43.Siegler RL, Obrig TG, Pysher TJ, Tesh VL, Denkers ND, Taylor FB. Response to Shiga toxin 1 and 2 in a baboon model of hemolytic uremic syndrome. Pediatr Nephrol. 2003;18:92–96. doi: 10.1007/s00467-002-1035-7. [DOI] [PubMed] [Google Scholar]
- 44.Hoey DE, Currie C, Else RW, Nutikka A, Lingwood CA, Gally DL, Smith DG. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J Med Microbiol. 2002;51:143–149. doi: 10.1099/0022-1317-51-2-143. [DOI] [PubMed] [Google Scholar]
- 45.Keepers TR, Psotka MA, Gross LK, Obrig TG. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J Am Soc Nephrol. 2006;17:3404–3414. doi: 10.1681/ASN.2006050419. [DOI] [PubMed] [Google Scholar]
- 46.Copelovitch L, Kaplan BS. Streptococcus pneumoniae-associated hemolytic uremic syndrome. Pediatr Nephrol. 2008;23:1951–1956. doi: 10.1007/s00467-007-0518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF—a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106:p32–p37. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 48.Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC. VEGF signalling inhibition-induced proteinuria: mechanisms, significance and management. Eur J Cancer. 2010;46:439–448. doi: 10.1016/j.ejca.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]