Abstract
Brucellosis is a chronic infectious disease caused by Brucella spp., a Gram-negative facultative intracellular pathogen that affects humans and animals, leading to significant impact on public health and animal industry. Human brucellosis is considered the most prevalent bacterial zoonosis in the world and is characterized by fever, weight loss, depression, hepato/splenomegaly, osteoarticular, and genital infections. Relevant aspects of Brucella pathogenesis have been intensively investigated in culture cells and animal models. The mouse is the animal model more commonly used to study chronic infection caused by Brucella. This model is most frequently used to investigate specific pathogenic factors of Brucella spp., to characterize the host immune response, and to evaluate therapeutics and vaccines. Other animal species have been used as models for brucellosis including rats, guinea pigs, and monkeys. This paper discusses the murine and other laboratory animal models for human and animal brucellosis.
1. Introduction
Brucellosis is an infectious disease caused by bacteria of the genus Brucella that affects humans as well as domestic and wild animals, leading to significant impact on public health and animal industry. Brucella spp. is a Gram-negative, facultative intracellular bacterium that is able to survive and replicate in phagocytic and nonphagocytic cells, establishing a chronic infection in both humans and animals [1]. Human brucellosis is considered the most prevalent bacterial zoonosis in the world, with more than 500,000 new reported cases in humans each year, mainly in Mediterranean countries, Central Asia, Arabic Peninsula, India, and Latin America [2]. The disease is characterized by nonspecific symptoms, including undulant fever, weight loss, depression, hepatomegaly, and splenomegaly. Arthritis, spondylitis, osteomyelitis, epididymitis, and orchitis, as well as other more severe complications as neurobrucellosis, liver abscesses, and endocarditis, are also commonly described in patients [1, 2].
There are currently 8 recognized species of Brucella, of which six are known to be capable of infecting humans. Brucella melitensis, B. abortus, B. suis, and B. canis are considered important zoonotic agents, and each one has a domestic animal as preferential host: small ruminants, bovines, swine, and dogs, respectively. Humans also can be infected by two Brucella species recently isolated from marine mammals, B. ceti and B. pinnipedialis, and by B. inopinata, the new species isolated in breast implant and lung biopsy from human [3–5]. In domestic animals, Brucella colonize the reticuloendothelial system and genital organs causing chronic infection and reproductive disease characterized by abortion, stillbirth, orchitis, epididymitis, and infertility, resulting in significant economic losses [3, 6].
Relevant aspects of Brucella pathogenesis have been intensively investigated in both cellular and animal models. The mouse is the animal model most extensively used to study chronic infection caused by Brucella spp. Moreover, a few other animal species have been used as models for brucellosis. This paper discusses well-characterized murine models of brucellosis as well as other laboratory animal models that have been used to study infection and disease caused by Brucella spp.
2. Murine Models for Human and Animal Brucellosis
The mouse is often used as an animal model to investigate the pathogenesis of human and animal brucellosis [7–9]. In addition, the murine models are widely employed to test antimicrobial drugs for treating the disease in humans [10–12]. Availability of new molecular tools allowed the use of murine models for identification of specific pathogenic factors of Brucella spp. and the characterization of host immune response. As a result, control methods are being improved and new vaccine candidates are being developed [8, 9, 13].
2.1. Mouse Strain-Specific Differences in Brucella Infection
Several early studies using the mouse model demonstrated that all of the mouse strains tested could be infected by B. abortus, suggesting a lack of genetic loci in mice that determine complete resistance to B. abortus infection. A comparison of susceptibility of different strains of mice to B. abortus strain 19 demonstrated susceptibility of CBA/H, BALB/c, or C57BL/10 to B. abortus infection [14]. A subsequent study comparing B. abortus infection in CD-1, BALB/cByJ, CBA/NJ (containing the X-linked immunodeficiency trait), C3H/HeJ (deficient in TLR4), and C3H/HeN mice found similar colonization levels between all mouse strains, with a trend for higher colonization of BALB/cByJ mice over a 12-week time course [15]. A higher level of B. abortus colonization in the BALB/cByJ strain was demonstrated definitively by comparison with C57BL/10 mice, a mouse strain that is closely related with the commonly used C57BL/6 strain [16, 17]. More detailed studies have demonstrated that an increased Th1 polarization of the immune response in the C57BL strains is responsible for their more resistant phenotype (see below for a more detailed discussion). However, it should be kept in mind that most of these comparative studies were performed using B. abortus, so that it is possible that the susceptibility toward infection or the infection kinetics may differ for other Brucella species.
Several mouse strains have been used to characterize suitable murine models to study Brucella sp. infection. Four- to 9-week-old BALB/c female mice are often used to evaluate systemic distribution of Brucella sp. during the course of infection [7, 17, 18]. Additionally, this animal model has been used to study gene expression during Brucella sp. infection, leading to the identification of several host genes associated with innate and adaptive immune responses that are activated during the course of infection [8, 19, 20]. Previous studies have shown that a high production of interferon-gamma (IFN-γ) and interleukin 12 (IL-12) may lead to efficient control of Brucella spp. infection in the mouse model, due to activation of macrophages and induction of natural killer cells and Th1 cellular response [8, 21, 22]. High serum levels of both IFN-γ and IL-12 are also described in humans during Brucella sp. infection, which is associated with the induction of a Th1 response at early stages of infection [23–25]. Additionally, natural killer (NK) cells play an important role in controlling intracellular bacterial infections, due to their ability to kill infected cells and secrete IFN-γ. NK cells have a deficient cytotoxic activity in patients with acute brucellosis, although these cells show normal activity in treated patients [26]. However, it seems that NK cells are not required to control B. abortus early infection in the mouse, since mice with nonfunctional NK cells have similar bacterial load when compared to immunocompetent mice [27]. Moreover, previous studies have shown that CD8+ T cells may also play a role against Brucella sp. persistent infection [28–30]. In BALB/c mice, in vivo depletion of CD8+ T cells leads to increased bacterial load in the spleen [30]. In humans, peripheral blood CD8+ T cells that are stimulated with heat-killed B. abortus or lipopolysaccharide produce IFN-γ, which elicit a Th1 immune response [29].
C57BL/6 and C57BL/10 mice strains have also been used to evaluate Brucella sp. infection, since they are considered more resistant to Brucella sp. infection than BALB/c mice [16, 17, 22]. Comparative studies among these mice strains helped detecting specific mechanisms of C57BL mice immune response that are defective in BALB/c mice. These mechanisms are likely important for controlling Brucella sp. infection [8, 17, 22]. Additionally, various knockout mice were developed by using C57BL/6 or 129/Sv as background mice. The results obtained in this model seem to have high similarity to host-pathogen interaction mechanisms that were previously described in humans and domestic animals [8, 31, 32]. Moreover, knockout mice with defective production of cytokines related to innate immune response illustrate the crucial role of specific cytokines against Brucella sp. infection in hosts [22, 33, 34]. Interestingly, interferon regulatory factor-1-deficient (IRF-1−/−) mice infected with B. abortus developed an acute hepatitis similar to humans but, unlike the natural hosts, IRF-1−/− mice are unable to control the infection and die within a short period of time. While uncontrolled infection and death are not typical endpoints of Brucella infection, the IRF-1−/− knockout mouse has been useful for identifying and comparing residual virulence of highly attenuated Brucella vaccine candidates [33].
2.2. Routes of Infection
Brucella infection may occur by digestive route, inhalation or through nasal mucosa or conjunctiva [6, 9]. After crossing the mucosal barrier, the organisms reach regional lymph nodes, replicate in macrophages, and establish a systemic and persistent infection. A bacteremic phase of infection results in colonization of the spleen, liver, and osteoarticular tissues, and depending on the Brucella species and host, it may also colonize the mammary gland and the reproductive system [6, 9, 35]. In murine models of Brucella sp. infection, experimental inoculation is performed mostly through three routes: intraperitoneal, digestive, or nasal (aerosol).
The intraperitoneal route of infection is frequently used to establish a persistent infection in the mouse, as it results in a rapid systemic distribution of Brucella sp. and high bacterial loads in the spleen and liver [7, 8, 18]. Initially, Brucella multiplies during the first week, progressing to a slow decrease in bacterial numbers at systemic sites of infection. During the first 5 to 6 weeks after inoculation, C57BL/6 or BALB/c mice infected with 106 CFU of B. abortus strain 19 remain with stable numbers of organisms at systemic sites of infection, and bacteria can be isolated up to two months after infection. However, B. abortus strain 2308 infection in BALB/c mice may persist over 6 months [7, 36]. Murine models of intraperitoneal infection with Brucella sp. allow the identification of pathogenic factors that are required for establishment of chronic infection [37, 38]. For instance, comparison between input and output loads of wild-type and mutant strains of Brucella in mouse models resulted in the identification of pathogenic factors, including the role of the type IV secretion system encoded by the virB operon during Brucella sp. persistent infection in vivo [31, 32, 38, 39]. virB mutant strains of Brucella sp. are not capable of surviving and replicating intracellularly in macrophages and, therefore, are attenuated in mouse models in vivo [37–39].
The digestive tract is the main route of Brucella infection in humans, which is associated with the ingestion of unpasteurized milk and dairy products from infected animals [2, 40]. Murine models of intestinal infection allow the identification of bacterial pathogenic factors that are required to establish infection through the digestive tract [41–44]. Recently, Paixão and colleagues described a murine model for intestinal infection of B. melitensis, in which a high intragastric dose (~1010 CFU per animal) leads to a systemic infection in BALB/cByJ mice, probably due to bacterial translocation through the intestinal mucosa via M cells [44]. Interestingly, this high infectious dose did not result in intestinal inflammation in the mouse. Previous studies have shown that mice can control intestinal Brucella infection when they are previously vaccinated through the same route [13, 45, 46]. Pasquali and colleagues demonstrated that BALB/c mice previously treated with sodium bicarbonate to neutralize gastric acid are more susceptible to B. abortus systemic infection by digestive route than untreated mice [46]. This result suggests that gastric acidity may interfere with Brucella sp. However, a previous work has shown that Brucella sp. challenge through the digestive tract is an inadequate method to produce a uniform and consistent infection in mice [47]. Additionally, it is important to consider experimental issues in murine models, including artificial inoculation using intragastric gavages and gastric acid neutralization, which may significantly differ from Brucella sp. natural infection in humans and animals. Bacterial factors mediating intestinal infection by Brucella sp. and their target molecules at mucosal surfaces of the digestive tract are still poorly understood; so additional studies evaluating carefully this route are required.
Human brucellosis may also be acquired by inhalation. The number of organisms required to establish the infection in humans by this route is low, with an estimated infectious dose of 10 to 100 organisms for humans by aerosol [48]. Therefore, Brucella sp. is considered a potential biological warfare agent [49]. Characterization of murine models for Brucella sp. infection by the nasal route (aerosol) may be used to evaluate vaccines candidates and therapeutics for human brucellosis [19, 50, 51]. A recent study demonstrated that BALB/c female mice immunized with B. melitensis attenuated strain Rev1 followed by aerosol infection with 104 CFU of B. melitensis 16M had a decreased bacterial load in the spleen, suggesting that this animal is a suitable model to evaluate protection during Brucella sp. aerosol infection [51]. Mice infection by aerosol with 106 CFU of B. melitensis or 107 CFU of B. abortus resulted in high bacterial load in the spleen, liver, and lungs. However, infection doses as low as 102 and 103 CFU per animal are also sufficient to establish a systemic infection in the mouse [19, 52, 53]. Apparently the lung is only affected in the mouse during aerosol infection with pathogenic species of Brucella. No histopathological lesions have been described in the lung, but high bacterial loads are recovered from the lungs at early time points during infection, which indicates that Brucella sp. is able to replicate in this organ without eliciting innate immune responses [19, 52]. Although aerosol chambers have been effectively used to study bacterial infections in mouse models [19], it is important to consider that the infection dose that reaches the lung of a mouse may be significantly lower than expected [53]. Additionally, Brucella sp. infection in these models may be established due to coinfection through the conjunctiva or oral mucosa, since it was previously shown that bacteria can be detected also in the fur of infected mice [19]. Therefore, it is essential to critically evaluate the results of Brucella sp. aerosol infection during vaccine studies in murine models. An open question in the field is the identity of the Brucella factors that are important for its efficient infection of the respiratory tract.
2.3. Histopathological Changes during Brucella Infection
During Brucella sp. infection in the mouse, the spleen is the most heavily colonized organ, and it develops histiocytic infiltrates and multifocal microgranulomas (Figure 1) [7, 18, 54]. BALB/c mice intraperitoneally (i.p.) infected with B. abortus or B. melitensis develop significant splenomegaly, which is more prominent than in mice infected by aerosol (Figure 2) [19, 54]. The liver is also an important site for colonization and replication of Brucella sp. in the mouse [7, 13, 19]. Usually, mice infected with virulent strains of Brucella sp. have mild to moderate hepatitis, which is characterized by neutrophilic infiltrate at early stages of infection, followed by histiocytic infiltrate with epithelioid cells and microgranulomas at chronic stages of infection (Figure 3) with bacteria localizing intracellularly in macrophages within microgranulomatous lesions (Figure 4) [7, 54]. It is noteworthy that Brucella infection in mice results in lesions that mimic those described in chronic infections in humans. Patients with chronic brucellosis may develop splenomegaly and hepatomegaly. Additionally, multifocal granulomas with epithelioid macrophages are observed in the parenchyma of the liver and spleen in biopsy samples from infected patients [55, 56]. However, hepatic and splenic abscess were described as uncommon complication in some patients during the acute phase of Brucella sp. infection [57]. Brucella sp. chronic infection in humans may also lead to osteoarticular disease, including osteoarthritis, spondliytis, and osteomyelitis [1, 2]. A previous study [58] reported that mice may develop bacterial colonization in osteoarticular tissues during chronic stages of B. melitensis infection. In IRF-1−/− mice that survived more than 45 days after i.p. infection with 107 CFU of B. melitensis, a high number (~105 CFU) of bioluminescent B. melitensis were detected in vertebral joints in the tail, suggesting that these mice might be a useful model for the study of human osteoarticular disease. However, a comparison of actual osteoarticular lesions in mice and humans would help to assess the potential utility of this model to study a common clinical presentation of brucellosis in man.
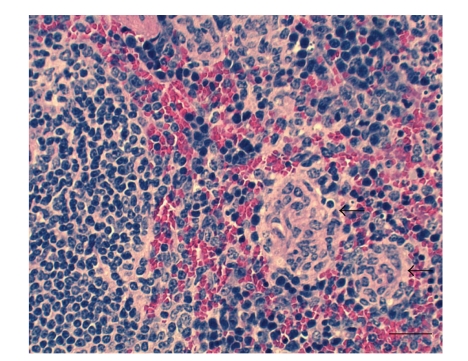
Figure 1.
Spleen of BALB/c mouse at 21 days of infection by Brucella melitensis. The mouse was intragastrically infected with 105 CFU of B. melitensis 16 M. Microgranulomas in red pulp (arrows). HE. Bar: 100 μm.
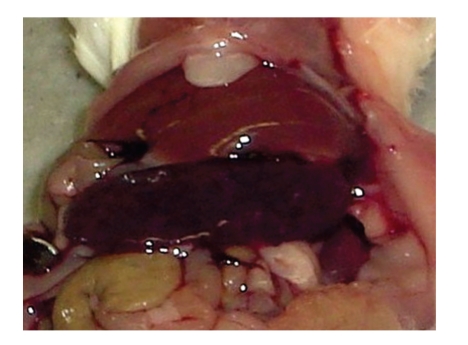
Figure 2.
BALB/c mouse i.p. infected with 106 CFU of Brucella ovis ATCC25840 with severe splenomegaly at 30 days of infection.
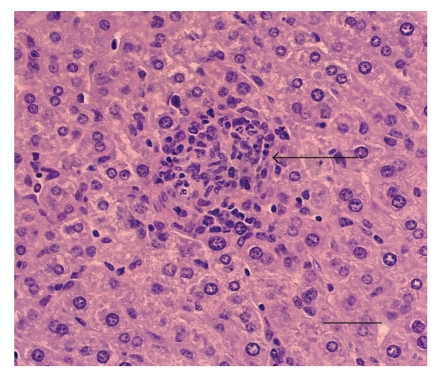
Figure 3.
Liver of BALB/c mouse at 30 days of infection by Brucella ovis. The mouse was i.p. infected with 106 CFU of Brucella ovis ATCC25840. Microgranuloma containing predominantly macrophages and neutrophils (arrow). HE. Bar: 100 μm.
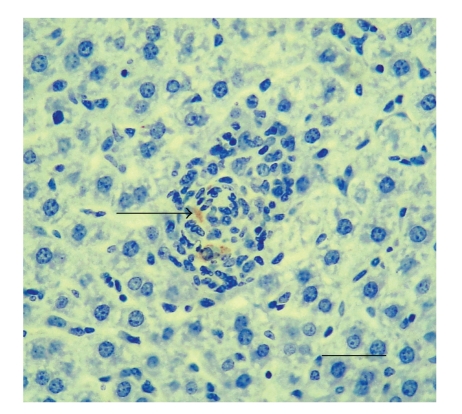
Figure 4.
Liver of BALB/c mouse at 30 days of infection by Brucella ovis. The mouse was i.p. infected with 106 CFU of Brucella ovis ATCC25840. Microgranuloma with immunolabelled B. ovis in macrophages (arrow). IHC. Bar: 100 μm.
2.4. Evaluation of Therapeutic Interventions and Vaccines
The efficiency of different chemotherapies for human brucellosis has also been evaluated in the mouse model [10, 11, 59]. The recommended treatment for human brucellosis is a combination of rifampicin and doxycycline daily for at least six weeks [60]. However, other antibiotic combinations have been tested in animal models and infected patients. Previous studies showed that mice infected with B. melitensis and treated with ciprofloxacin, by subcutaneous (40 mg/kg), digestive (200 mg/kg), or intraperitoneal (20 mg/kg) route, are not able to control the infection [10, 12], whereas mice treated with doxycycline (40 mg/kg) at 24 hours after infection efficiently clear the infection [12]. Additionally, Shasha and coworkers [10] reported that mice treated with rifampin (25 mg/kg) or doxycycline (40 mg/kg) by intraperitoneal route had high levels of antibiotics in the blood (rifampin: 18 μg/ml; doxycycline: 5.4 μg/ml) and were able to clear the infection. Moreover, new antibiotic carriers, like microspheres, have been tested against Brucella sp. infection. Microspheres are phagocytized by monocytes, allowing direct access of the antibiotic to the intracellular site of bacterial replication. However, a previous study showed that mice infected with B. abortus and treated with gentamicin microspheres (100 μg/animal) for three days were not able to reduce bacterial load in the spleen after 1 and 3 weeks after treatment [11].
Additionally, the quality of live vaccines that are commercially used for preventing animal brucellosis is evaluated in murine models [61]. Live B. abortus S19 strain, which is the most widely used vaccine in cattle, has been tested in female CD1 mice 5 to 7 weeks old. Mice are previously treated with 105 CFU of B. abortus reference vaccine (strain S19), a commercial vaccine sample or PBS. After 30 days of vaccination, all mice are i.p. infected with 105 CFU of B. abortus virulent strain. Then, bacterial loads in the spleen are evaluated in each group at 15 days after infection. A commercial vaccine is considered efficient when mice have significantly lower bacterial load than the unvaccinated control group and when the vaccinated group has similar immunogenicity value to mice group vaccinated with S19 reference strain [61].
2.5. Pathogenesis of the Reproductive Tract
Furthermore, murine models were developed to study reproductive changes described in human and animal brucellosis. Previous studies evaluated the occurrence of abortion and placental colonization in female pregnant mice during B. abortus infection [3, 35]. Although B. abortus infection is not characterized by abortion in women [2], it is extremely relevant to study Brucella pathogenesis in pregnant female models, due to its significant economic impact in cattle production as well as in other domestic animal species. Moreover, uterine secretion and products from abortion are the most important source of infection within a herd maintaining the disease and may also represent an occupational source of infection to humans [6, 35].
Previous studies from Bosseray characterized the infection in pregnant CD1 mice with B. abortus strain 544. The infection did not lead to abortion or fetus death at early stages of pregnancy, although high colonization of placenta was described when mice were infected at 7 and 11 days of pregnancy. Additionally, placental and splenic colonization increased with higher challenge accordance to the infection dose and each placenta was considered an independent unit, as some placentas were colonized and others were not in the same uterus [62]. Another study demonstrated the congenital infection of B. abortus in the mouse at 7 days of pregnancy, which resulted in the colonization of 60% of newborns. In this study, newborns remained infected until 30 days and no significant difference of Brucella sp. infection was observed between male and female newborns [63]. Moreover, Bosseray described the kinetics of placental colonization in mice that were intravenously infected with B. abortus at 15 days of pregnancy. Although low bacterial loads were recovered from the placenta at early stages of infection (4–6 hours), apparently local bacterial replication resulted in higher colonization in the placenta at 72 hours after infection [64].
BALB/c female pregnant mice infected with 106 CFU of B. abortus virulent strain 2308 develop a moderate multifocal necrotic placentitis associated with severe neutrophilic infiltrate and intralesional bacteria in trophoblastic cells [54]. The bacterial load and lesions described in the placenta increase throughout the pregnancy, whereas the bacterial load recovered from the spleen was stable during the course of infection in the mouse [54, 65]. The lesions described in female pregnant mice were similar to those observed in cows, which suggests that this model may be useful to study Brucella-induced placental disease, although mice and cattle have different morphological types of placenta [54]. Additionally, Kim and colleagues [65] demonstrated that B. abortus infection may lead to 98% of abortion in female mice at 4.5 days of pregnancy. However, intraperitoneal inoculation of the pathogen at any other time point during the pregnancy does not result in a high abortion rate although placentas from both aborted and live fetuses have intracellular Brucella sp. in trophoblast giant cells. In natural hosts, B. abortus infection leads to abortion in cows at late stages of pregnancy due to placental lesions, which are related to bacterial invasion and intracellular replication in trophoblastic cells [66, 67].
Considering that male genital tract may also be affected during Brucella sp. infection, male mice were characterized to study specific bacterial mechanisms that lead to orchitis and epididymitis in men and animals [3, 6]. Previous studies reported that Brucella sp. may colonize the male genital tract in the mouse [13, 58]. Izadjoo and colleagues demonstrated that B. melitensis infection (1010 CFU) through the digestive tract in sexually mature BALB/c male mice leads to perivascular inflammation of the testes and histiocytosis in inguinal lymph nodes [13]. In addition, use of male mice may be important for testing residual pathogenicity of candidates for vaccine strains, by evaluating histopathologic lesions in the genital tract and the immune response against Brucella sp. [13]. Recently, our laboratory developed a male mouse model for Brucella ovis infection (Silva et al., unpublished data). Although B. ovis is one of the few classical Brucella species that do not have zoonotic potential, this organism is considered a major cause of reproductive failure in sheep, which leads to significant economic losses in the sheep industry [68]. The characterization of a murine model has allowed the study of pathogenic mechanisms used by B. ovis that may determine the bacterial genital tropism in sexually mature rams, causing epididymitis and orchitis exclusively in this animal. Interestingly, B. ovis infection in male mice resulted in early colonization of testes, epididymides, and seminal vesicle. However, colonization of these organs quickly decreased at later time points, and the inflammatory lesions were restricted to peripheral tissues of the genital tract. Therefore, male mice were not considered a good model for B. ovis genital disease in rams, although it may be used as a suitable infection model (Silva et al., unpublished data).
3. Other Laboratory Animal Models for Brucellosis
Although the mouse is by far the most often used animal model for brucellosis, it is a good animal model for chronic infection of the reticulo-endothelial system but fails to replicate some features of the clinical disease caused by Brucella in humans, such as fever. Therefore, there are several reports of experimental work employing other laboratory animals, including rats, guinea pigs, and monkeys that are susceptible to experimental infection with Brucella spp.
3.1. Rodent Models Other Than Mice
The rat has been used as a model for human brucellosis due to some peculiarities of this species. Despite the fact that rats do not develop physical signs of infection and are considered more resistant to infection than mice, they develop persistent bacteremia and do not have spontaneous cure after one month of infection [69, 70]. Therefore, rats have been selected as an experimental model for evaluation of increased susceptibility to infection (including Brucella infection) in patients with chronic disorders. Wistar Albino rats with diabetes were used to evaluate the course of infection by B. melitensis. In this case, diabetes is induced by streptozotocin before challenge with B. melitensis. Diabetic rats have higher numbers of bacteria in the liver and spleen when compared to control rats [69]. Other studies investigated the effect of chronic ethanol consumption on the course of B. melitensis infection in a rat model [71–73]. Rats chronically treated with ethanol have an increased susceptibility to B. melitensis due to a decrease in protective cellular immunity [72]. The rat model has also been used to study the efficacy of various antibiotics for treating Brucella infection [74–76]. Sprague Dawley rats were used to evaluate the efficacy of spiramycin, a macrolide antibiotic, for treating brucellosis, since this drug has no teratogenic effect, and therefore it is safe for pregnant women [74]. Similarly to mice, rats are also used to study clinical and pathological effects of B. abortus infection during pregnancy. B. abortus did not affect pregnancy in Sprague Dawley rats, paralleling what happens in women, although necrosis in the periplacentomal chorionic epithelium and metritis were observed in this model [77]. Although rats do not abort even with placentitis, a previous study demonstrated significant protection against systemic B. abortus infection in rats vaccinated with RB51, a vaccine strain against bovine brucellosis [78].
Guinea pigs are probably the most susceptible laboratory animal species to Brucella infection. Early comparative studies of susceptibility in guinea pigs, mice, rats, and sheep demonstrated that guinea pigs developed granulomatous lesions when inoculated with 10 CFU of B. melitensis or B. suis [79]. Lesions were consistently observed in the liver, spleen, lungs, and lymph nodes, resembling those described in humans [80]. Guinea pigs inoculated subcutaneously with infectious doses of B. abortus, B. suis, or B. melitensis develop a persistent bacteremia for 6 weeks after infection, whereas the attenuated B. abortus S19 is cleared from the blood at one week after infection [81]. Therefore, the guinea pig model may be considered valuable for the evaluation of candidate vaccine strains [82, 83]. All classic Brucella species were pathogenic for guinea pigs [70]. Furthermore, the guinea pig has been employed as an animal model for evaluating the efficacy of antibiotics and chemotherapeutic agents for treatment of brucellosis [84, 85].
Although the rabbit is a laboratory animal frequently used as an experimental model, it is not considered a model of choice for Brucella infection. Rabbits are partially susceptible to Brucella infection [70], and only about 20% of infected animals developed a very short and sporadic bacteremia with B. abortus or B. suis [86]. The pregnancy increases systemic susceptibility of rabbits to B. abortus infection but nevertheless the infecting organism was not recovered from the uterus of pregnant female rabbits [70]. Hamsters (Syrian or Golden Hamster) do not appear to be a good animal model for B. abortus infection, due the vast individual differences in susceptibility [70].
3.2. Nonhuman Primate Models
Nonhuman primate models of Brucella infection have been reported in Macaca arctoides and rhesus macaque (Macaca mulatta) infected with B. abortus, B. melitensis, B. suis, and B. canis [87–90]. These animals are susceptible to Brucella organisms administered by digestive, subcutaneous, or respiratory routes and develop persistent bacteremia up to eight weeks after inoculation [89]. The primate infection leads to a multiple-organ disease causing focal granulomatous hepatitis, splenitis, and lymphadenitis, similar to human brucellosis [91]. In a few cases, there is an involvement of the reproductive tract causing granulomatous orchitis, epididymitis, or acute endometritis [89]. Aerosol infection of nonhuman primates has been reported [89, 90], resulting in a number of pathologic changes similar to human brucellosis, suggesting that nonhuman primate model is a suitable model for human brucellosis [90]. It is noteworthy that the aerosol exposure might possibly occur as a result of a bioterrorism event, and studies of dose-dependent infection by this route and animal model are important. Mense and colleagues [89] reported that uninfected macaques, not inoculated with Brucella organisms, became infected when housed in the same room with inoculated macaques, suggesting that the macaque is a good model to study Brucella infection by aerosol route. Moreover, studies on the efficacy of diagnostic methods for Brucella detection after aerosol exposure has been performed, and therefore nonhuman primates could provide an excellent model for testing of diagnostics [90].
4. Conclusions
Human brucellosis results in highly variable clinical manifestations that are not quite paralleled by experimental infections in laboratory animals. However, animal models, particularly the mouse, have been extensively used and allowed for accumulation of valuable information mostly in the past recent years regarding the pathogenesis, immunity, and antibiotic susceptibility of Brucella spp. in vivo. New technologies in mouse genetics will likely bring about even greater insights into the interaction of Brucella spp. with the immune system that lead to disease in humans and in the natural zoonotic reservoir hosts.
Acknowledgments
The work in RLS lab is supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasília, Brazil) and FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais, Belo Horizonte, Brazil). T. M. A. Silva, E. A. Costa, and R. L. Santos are recipients of fellowships from CNPq. R. L. Santos is currently a Fellow of the John Simon Guggenheim Memorial Foundation.
References
- 1.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infectious Diseases. 2007;7(12):775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Hartigan PJ. Human brucellosis epidemiology and clinical manifestations. Irish Veterinary Journal. 1997;50(3):179–180. [Google Scholar]
- 3.Xavier MN, Costa ÉA, Paixão TA, Santos RL. The genus Brucella and clinical manifestations of brucellosis. Ciencia Rural. 2009;39(7):2252–2260. [Google Scholar]
- 4.Tiller RV, Gee JE, Lonsway DR, et al. Identification of an unusual Brucella strain (BO2) from a lung biopsy in a 52 year-old patient with chronic destructive pneumonia. BMC Microbiology. 2010;10, article no. 23 doi: 10.1186/1471-2180-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholz HC, Nöckler K, Göllner CG, et al. Brucella inopinata sp. nov., isolated from a breast implant infection. International Journal of Systematic and Evolutionary Microbiology. 2010;60(4):801–808. doi: 10.1099/ijs.0.011148-0. [DOI] [PubMed] [Google Scholar]
- 6.Thoen CO, Enright F, Cheville NF. Brucella . In: Gyles CL, Thoen CO, editors. Pathogenesis of Bacterial Infections in Animals. 2nd edition. Ames, Iowa, USA: Iowa State University Press; 1993. pp. 236–247. [Google Scholar]
- 7.Enright FM, Araya LN, Elzer PH, Rowe GE, Winter AJ. Comparative histopathology in BALB/c mice infected with virulent and attenuated strains of Brucella abortus. Veterinary Immunology and Immunopathology. 1990;26(2):171–182. doi: 10.1016/0165-2427(90)90065-z. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin CL, Parent M. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Veterinary Microbiology. 2002;90(1–4):367–382. doi: 10.1016/s0378-1135(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 9.Ko J, Splitter GA. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clinical Microbiology Reviews. 2003;16(1):65–78. doi: 10.1128/CMR.16.1.65-78.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shasha B, Lang R, Rubinstein E. Therapy of experimental murine brucellosis with streptomycin, co-trimoxazole, ciprofloxacin, ofloxacin, pefloxacin, doxycycline, and rifampin. Antimicrobial Agents and Chemotherapy. 1992;36(5):973–976. doi: 10.1128/aac.36.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prior S, Gander B, Irache JM, Gamazo C. Gentamicin loaded microspheres for treatment of experimental Brucella abortus infection in mice. Journal of Antimicrobial Chemotherapy. 2005;55(6):1032–1036. doi: 10.1093/jac/dki144. [DOI] [PubMed] [Google Scholar]
- 12.Atkins HS, Spencer S, Brew SD, et al. Efficacy of ciprofloxacin versus doxycycline as prophylaxis against experimental murine Brucella melitensis infection. International Journal of Antimicrobial Agents. 2009;34(5):474–476. doi: 10.1016/j.ijantimicag.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Izadjoo MJ, Mense MG, Bhattacharjee AK, Hadfield TL, Crawford RM, Hoover DL. A study on the use of male animal models for developing a live vaccine for brucellosis. Transboundary and Emerging Diseases. 2008;55(3-4):145–151. doi: 10.1111/j.1865-1682.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheers C. Pathogenesis and cellular immunity in experimental murine brucellosis. Developments in Biological Standardization. 1984;56:237–246. [PubMed] [Google Scholar]
- 15.Phillips M, Pugh GW, Jr., Deyoe BL. Duration of strain 2308 infection and immunogenicity of Brucella abortus lipopolysaccharide in five strains of mice. American Journal of Veterinary Research. 1989;50(3):318–322. [PubMed] [Google Scholar]
- 16.Montaraz JA, Winter AJ. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infection and Immunity. 1986;53(2):245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes DM, Jiang X, Jung JH, Baldwin CL. Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus strain 2308. FEMS Immunology and Medical Microbiology. 1996;16(3-4):193–203. doi: 10.1111/j.1574-695X.1996.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 18.Stevens MG, Olsen SC, Pugh GW, Jr., Palmer MV. Immune and pathologic responses in mice infected with Brucella abortus 19, RB51, or 2308. Infection and Immunity. 1994;62(8):3206–3212. doi: 10.1128/iai.62.8.3206-3212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahl-McDonagh MM, Arenas-Gamboa AM, Ficht TA. Aerosol infection of BALB/c mice with Brucella melitensis and Brucella abortus and protective efficacy against aerosol challenge. Infection and Immunity. 2007;75(10):4923–4932. doi: 10.1128/IAI.00451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roux CM, Rolán HG, Santos RL, et al. Brucella requires a functional Type IV secretion system to elicit innate immune responses in mice. Cellular Microbiology. 2007;9(7):1851–1869. doi: 10.1111/j.1462-5822.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infection and Immunity. 1995;63(4):1387–1390. doi: 10.1128/iai.63.4.1387-1390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. Interferon-γ is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology. 2001;103(4):511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed K, Al-Matrouk KA, Martinez G, Oishi K, Rotimi VO, Nagatake T. Increased serum levels of interferon-γ and interleukin-12 during human brucellosis. American Journal of Tropical Medicine and Hygiene. 1999;61(3):425–427. doi: 10.4269/ajtmh.1999.61.425. [DOI] [PubMed] [Google Scholar]
- 24.Dornand J, Gross A, Lafont V, Liautard J, Oliaro J, Liautard JP. The innate immune response against Brucella in humans. Veterinary Microbiology. 2002;90(1–4):383–394. doi: 10.1016/s0378-1135(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 25.Rafiei A, Ardestani SK, Kariminia A, Keyhani A, Mohraz M, Amirkhani A. Dominant Th1 cytokine production in early onset of human brucellosis followed by switching towards Th2 along prolongation of disease. Journal of Infection. 2006;53(5):315–324. doi: 10.1016/j.jinf.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Salmeron I, Rodriguez-Zapata M, Salmeron O, Manzano L, Vaquer S, Alvarez- Mon M. Impaired activity of natural killer cells in patients with acute brucellosis. Clinical Infectious Diseases. 1992;15(5):764–770. doi: 10.1093/clind/15.5.764. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes DM, Benson R, Baldwin CL. Lack of a role for natural killer cells in early control of Brucella abortus 2308 infections in mice. Infection and Immunity. 1995;63(10):4029–4033. doi: 10.1128/iai.63.10.4029-4033.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araya LN, Elzer PH, Rowe GE, Enright FM, Winter AJ. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. Journal of Immunology. 1989;143(10):3330–3337. [PubMed] [Google Scholar]
- 29.Zaitseva MB, Golding H, Betts M, et al. Human peripheral blood CD4 and CD8 T cells express Th1-like cytokine mRNA and proteins following in vitro stimulation with heat-inactivated Brucella abortus. Infection and Immunity. 1995;63(7):2720–2728. doi: 10.1128/iai.63.7.2720-2728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy EA, Parent M, Sathiyaseelan J, Jiang X, Baldwin CL. Immune control of Brucella abortus 2308 infections in BALB/c mice. FEMS Immunology and Medical Microbiology. 2001;32(1):85–88. doi: 10.1111/j.1574-695X.2001.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 31.Rolán HG, Tsolis RM. Mice lacking components of adaptive immunity show increased Brucella abortus virB mutant colonization. Infection and Immunity. 2007;75(6):2965–2973. doi: 10.1128/IAI.01896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolán HG, Xavier MN, Santos RL, Tsolis RM. Natural antibody contributes to host defense against an attenuated Brucella abortus virB mutant. Infection and Immunity. 2009;77(7):3004–3013. doi: 10.1128/IAI.01114-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko J, Gendron-Fitzpatrick A, Ficht TA, Splitter GA. Virulence criteria for Brucella abortus strains as determined by interferon regulatory factor 1-deficient mice. Infection and Immunity. 2002;70(12):7004–7012. doi: 10.1128/IAI.70.12.7004-7012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajashekara G, Krepps M, Eskra L, et al. Unraveling Brucella genomics and pathogenesis in immunocompromised IRF-1−/− mice. American Journal of Reproductive Immunology. 2005;54(6):358–368. doi: 10.1111/j.1600-0897.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 35.Neta AVC, Mol JPS, Xavier MN, Paixão TA, Lage AP, Santos RL. Pathogenesis of bovine brucellosis. Veterinary Journal. 2010;184(2):146–155. doi: 10.1016/j.tvjl.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Birmingham JR, Jeska EL. Characterization of macrophage functions in mice infected with Brucella abortus. Infection and Immunity. 1981;32(3):1079–1083. doi: 10.1128/iai.32.3.1079-1083.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Celli J, De Chastellier C, Franchini D-M, Pizarro-Cerda J, Moreno E, Gorvel J-P. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. Journal of Experimental Medicine. 2003;198(4):545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong PC, Tsolis RM, Ficht TA. Identification of genes required for chronic persistence of Brucella abortus in mice. Infection and Immunity. 2000;68(7):4102–4107. doi: 10.1128/iai.68.7.4102-4107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sieira R, Comerci DJ, Sanchez DO, Ugalde RA. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. Journal of Bacteriology. 2000;182(17):4849–4855. doi: 10.1128/jb.182.17.4849-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godfroid J, Cloeckaert A, Liautard JP, et al. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Veterinary Research. 2005;36(3):313–326. doi: 10.1051/vetres:2005003. [DOI] [PubMed] [Google Scholar]
- 41.Bandara AB, Contreras A, Contreras-Rodriguez A, et al. Brucella suis urease encoded by ure1 but not ure2 is necessary for intestinal infection of BALB/c mice. BMC Microbiology. 2007;7, article no. 57 doi: 10.1186/1471-2180-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delpino MV, Marchesini MI, Estein SM, et al. A bile salt hydrolase of Brucella abortus contributes to the establishment of a successful infection through the oral route in mice. Infection and Immunity. 2007;75(1):299–305. doi: 10.1128/IAI.00952-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sangari FJ, Seoane A, Rodríguez MC, Agüero J, Lobo JMG. Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infection and Immunity. 2007;75(2):774–780. doi: 10.1128/IAI.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paixão TA, Roux CM, den Hartigh AB, et al. Establishment of systemic Brucella melitensis infection through the digestive tract requires urease, the type IV secretion system, and lipopolysaccharide O antigen. Infection and Immunity. 2009;77(10):4197–4208. doi: 10.1128/IAI.00417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens MG, Olsen SC, Palmer MV, Pugh GW., Jr. Immune responses and resistance to brucellosis in mice vaccinated orally with Brucella abortus RB51. Infection and Immunity. 1996;64(11):4534–4541. doi: 10.1128/iai.64.11.4534-4541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasquali P, Rosanna A, Pistoia C, Petrucci P, Ciuchini F. Brucella abortus RB51 induces protection in mice orally infected with the virulent strain B. abortus 2308. Infection and Immunity. 2003;71(5):2326–2330. doi: 10.1128/IAI.71.5.2326-2330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen TH, Elberg SS. Immunization against Brucella infections: immune response of mice, guinea pigs, and Cynomolgus philipinensis to live and killed Brucella melitensis strain Rev. I administered by various methods. Journal of Infectious Diseases. 1970;122(6):489–500. doi: 10.1093/infdis/122.6.489. [DOI] [PubMed] [Google Scholar]
- 48.Bossi P, Tegnell A, Baka A, et al. Bichat guidelines for the clinical management of brucellosis and bioterrorism-related brucellosis. Euro surveillance. 2004;9(12):E15–E16. [PubMed] [Google Scholar]
- 49.Pappas G, Panagopoulou P, Christou L, Akritidis N. Brucella as a biological weapon. Cellular and Molecular Life Sciences. 2006;63(19-20):2229–2236. doi: 10.1007/s00018-006-6311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izadjoo MJ, Bhattacharjee AK, Paranavitana CM, Hadfield TL, Hoover DL. Oral vaccination with Brucella melitensis WR201 protects mice against intranasal challenge with virulent Brucella melitensis 16M. Infection and Immunity. 2004;72(7):4031–4039. doi: 10.1128/IAI.72.7.4031-4039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smither SJ, Perkins SD, Davies C, Stagg AJ, Nelson M, Atkins HS. Development and characterization of mouse models of infection with aerosolized Brucella melitensis and Brucella suis. Clinical and Vaccine Immunology. 2009;16(5):779–783. doi: 10.1128/CVI.00029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mense MG, Van De Verg LL, Bhattacharjee AK, et al. Bacteriologic and histologic features in mice after intranasal inoculation of Brucella melitensis. American Journal of Veterinary Research. 2001;62(3):398–405. doi: 10.2460/ajvr.2001.62.398. [DOI] [PubMed] [Google Scholar]
- 53.Olsen SC, Waters WR, Stoffregen WS. An aerosolized Brucella spp. challenge model for laboratory animals. Zoonoses and Public Health. 2007;54(8):281–285. doi: 10.1111/j.1863-2378.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 54.Tobias L, Cordes DO, Schurig GG. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Veterinary Pathology. 1993;30(2):119–129. doi: 10.1177/030098589303000204. [DOI] [PubMed] [Google Scholar]
- 55.Colmenero JDD, Queipo-Ortuo MI, Reguera JM, Suarez-Muoz MA, Martín-Carballino S, Morata P. Chronic hepatosplenic abscesses in brucellosis. Clinico-therapeutic features and molecular diagnostic approach. Diagnostic Microbiology and Infectious Disease. 2002;42(3):159–167. doi: 10.1016/s0732-8893(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 56.Akritidis N, Tzivras M, Delladetsima I, Stefanaki S, Moutsopoulos HM, Pappas G. The liver in brucellosis. Clinical Gastroenterology and Hepatology. 2007;5(9):1109–1112. doi: 10.1016/j.cgh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Yayli G, Işler M, Oyar O. Medically treated splenic abscess due to Brucella melitensis. Scandinavian Journal of Infectious Diseases. 2002;34(2):133–135. doi: 10.1080/00365540110077335. [DOI] [PubMed] [Google Scholar]
- 58.Rajashekara G, Glover DA, Krepps M, Splitter GA. Temporal analysis of pathogenic events in virulent and avirulent Brucella melitensis infections. Cellular Microbiology. 2005;7(10):1459–1473. doi: 10.1111/j.1462-5822.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 59.Arda B, Tunçel M, Yaimazhan T, Gökengin D, Gürel Ö. Efficacy of oral levofloxacin and dirithromycin alone and in combination with rifampicin in the treatment of experimental murine Brucella abortus infection. International Journal of Antimicrobial Agents. 2004;23(2):204–207. doi: 10.1016/j.ijantimicag.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization. 6th Report. Geneva, Switzerland: WHO; 1986. Joint FAO/WHO Expert Committee on Brucellosis. [Google Scholar]
- 61.OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Bovine brucellosis. 6th edition. OIE; 2009. [Google Scholar]
- 62.Bosseray N. Colonization of mouse placentas by Brucella abortus inoculated during pregnancy. British Journal of Experimental Pathology. 1980;61(4):361–368. [PMC free article] [PubMed] [Google Scholar]
- 63.Bosseray N. Mother to young transmission of Brucella abortus infection in mouse model. Annales de Recherches Veterinaires. 1982;13(4):341–349. [PubMed] [Google Scholar]
- 64.Bosseray N. Kinetics of placental colonization of mice inoculated intravenously with Brucella abortus at day 15 of pregnancy. British Journal of Experimental Pathology. 1983;64(6):612–616. [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S, Dong SL, Watanabe K, Furuoka H, Suzuki H, Watarai M. Interferon-γ promotes abortion due to Brucella infection in pregnant mice. BMC Microbiology. 2005;5 doi: 10.1186/1471-2180-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carvalho Neta AV, Stynen APR, Paixão TA, et al. Modulation of the bovine trophoblastic innate immune response by Brucella abortus. Infection and Immunity. 2008;76(5):1897–1907. doi: 10.1128/IAI.01554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xavier MN, Paixão TA, Poester FP, Lage AP, Santos RL. Pathological, immunohistochemical and bacteriological study of tissues and milk of cows and fetuses experimentally infected with Brucella abortus. Journal of Comparative Pathology. 2009;140(2-3):149–157. doi: 10.1016/j.jcpa.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 68.Blasco JM. Brucella ovis . In: Nielsen K, Duncan JR, editors. Animal Brucellosis. Boca Raton, Fla, USA: CRC Press; 1990. pp. 351–378. [Google Scholar]
- 69.Yumuk Z, Küçükbasmaci Ö, Boral OB, Anğ MK, Dundar V. The effects of streptozotocin-induced diabetes on brucellosis of rats. FEMS Immunology and Medical Microbiology. 2003;39(3):275–278. doi: 10.1016/S0928-8244(03)00257-8. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Carrillo C. Laboratory animal model for brucellosis studies. In: Nielsen K, Duncan JR, editors. Animal Brucellosis. Boca Raton, Fla, USA: CRC Press; 1990. pp. 422–423. [Google Scholar]
- 71.Uzbay IT, Kayaalp SO. A modified liquid diet of chronic ethanol administration: validation by ethanol withdrawal syndrome in rats. Pharmacological Research. 1995;31(1):37–42. doi: 10.1016/1043-6618(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 72.Yumuk Z, Ozdemirci S, Erden BF, Dundar V. The effect of long-term ethanol feeding on Brucella melitensis infection of rats. Alcohol and Alcoholism. 2001;36(4):314–317. doi: 10.1093/alcalc/36.4.314. [DOI] [PubMed] [Google Scholar]
- 73.Davis CC, Mellencamp MA, Preheim LC. A model of pneumococcal pneumonia in chronically intoxicated rats. Journal of Infectious Diseases. 1991;163(4):799–805. doi: 10.1093/infdis/163.4.799. [DOI] [PubMed] [Google Scholar]
- 74.Geyik MF, Dikici B, Kokoglu OF, et al. Therapeutic effect of spiramycin in brucellosis. Pediatrics International. 2003;45(1):31–34. doi: 10.1046/j.1442-200x.2003.01672.x. [DOI] [PubMed] [Google Scholar]
- 75.Yumuk Z, Dundar V. The effect of long-term ethanol feeding on efficacy of doxycycline plus rifampicin in the treatment of experimental brucellosis caused by Brucella melitensis in rats. Journal of Chemotherapy. 2005;17(5):509–513. doi: 10.1179/joc.2005.17.5.509. [DOI] [PubMed] [Google Scholar]
- 76.Sezak N, Kuruuzum Z, Cakir N, Yuce A. Comparison of rifampicin and moxifloxacin efficacy in an experimental model of animal brucellosis. Journal of Chemotherapy. 2008;20(1):58–62. doi: 10.1179/joc.2008.20.1.58. [DOI] [PubMed] [Google Scholar]
- 77.Siddiqur RM, Kirl BB. Clinical and pathological findings in experimental brucellosis in pregnant rats. Journal of infection in developing countries. 2008;2(3):226–229. doi: 10.3855/jidc.267. [DOI] [PubMed] [Google Scholar]
- 78.Islam MDA, Khatun MM, Baek BK, Lee SI. Efficacy of strain RB51 vaccine in protecting infection and vertical transmission against Brucella abortus in Sprague-Dawley rats. Journal of veterinary science. 2009;10(3):211–218. doi: 10.4142/jvs.2009.10.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taran IF, Rybasov NA. Comparative study of the susceptibility and infectious sensitivity of laboratory animals and sheep to different species of the causative agent of brucellosis. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii. 1971;48(10):97–101. [PubMed] [Google Scholar]
- 80.Braude AI. Studies in the pathology and pathogenesis of experimental brucellosis. I. A comparison of the pathogenicity of Brucella abortus, Brucella melitensis, and Brucella suis for guinea pigs. The Journal of infectious diseases. 1951;89(1):76–86. doi: 10.1093/infdis/89.1.76. [DOI] [PubMed] [Google Scholar]
- 81.Cruickshank JC. The duration of bacteraemia in relation to the virulence of Brucella strains. The Journal of hygiene. 1957;55(1):140–147. doi: 10.1017/s0022172400061313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao WR, Wendoso , Hasi , Qin YX, Weng W, Lu SL. Selection of a Brucella vaccine strain of low residual virulence by chemical mutagenesis. Journal of Medical Microbiology. 1989;30(2):143–148. doi: 10.1099/00222615-30-2-143. [DOI] [PubMed] [Google Scholar]
- 83.Oliveira SC, Harms JS, Banai M, Splitter GA. Recombinant Brucella abortus proteins that induce proliferation and gamma-interferon secretion by CD4+ T cells from Brucella-vaccinated mice and delayed-type hypersensitivity in sensitized guinea pigs. Cellular Immunology. 1996;172(2):262–268. doi: 10.1006/cimm.1996.0241. [DOI] [PubMed] [Google Scholar]
- 84.Chinn BD. The use of sulfanilamide in experimental brucellosis. Journal of Infectious Diseases. 1939;64:78–82. [Google Scholar]
- 85.Weimer HE, Boak RA, Carpenter CM. Serum glycoprotein studies in experimental brucellosis of the guinea pig. Journal of Infectious Diseases. 1955;96(1):19–23. doi: 10.1093/infdis/96.1.19. [DOI] [PubMed] [Google Scholar]
- 86.Thorpe BD, Sidwell RW, Lundgren DL. Experimental studies with four species of Brucella in selected wildlife, laboratory, and domestic animals. American Journal of Tropical Medicine and Hygiene. 1967;16(5):665–674. doi: 10.4269/ajtmh.1967.16.665. [DOI] [PubMed] [Google Scholar]
- 87.Huddleson F, Hallman ET. The pathogenicity of the species of the genus Brucella for monkeys. Journal of Infectious Diseases. 1929;45:293–303. [Google Scholar]
- 88.Percy DH, Egwu IN, Jonas AM. Experimental Brucella canis infection in the monkey (Macaca arctoides) Canadian Journal of Comparative Medicine. 1972;36(3):221–225. [PMC free article] [PubMed] [Google Scholar]
- 89.Mense MG, Borschel RH, Wilhelmsen CL, Pitt ML, Hoover DL. Pathologic changes associated with brucellosis experimentally induced by aerosol exposure in rhesus macaques (Macaca mulatta) American Journal of Veterinary Research. 2004;65(5):644–652. doi: 10.2460/ajvr.2004.65.644. [DOI] [PubMed] [Google Scholar]
- 90.Yingst SL, Huzella LM, Chuvala L, Wolcott M. A rhesus macaque (Macaca mulatta) model of aerosol-exposure brucellosis (Brucella suis): pathology and diagnostic implications. Journal of Medical Microbiology. 2010;59(6):724–730. doi: 10.1099/jmm.0.017285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hunt AC, Bothwell PW. Histological findings in human brucellosis. Journal of Clinical Pathology. 1967;20(3):267–272. doi: 10.1136/jcp.20.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]