Abstract
Allergen immunotherapy describes the treatment of allergic disease through administration of gradually increasing doses of allergen. This form of immune tolerance induction is now safer, more reliably efficacious and better understood than when it was first formally described in 1911. In this paper the authors aim to summarize the current state of the art in immunotherapy in the treatment of inhalant, venom and drug allergies, with specific reference to its practice in the United Kingdom. A practical approach has been taken, with reference to current evidence and guidelines, including illustrative protocols and vaccine schedules. A number of novel approaches and techniques are likely to change considerably the way in which we select and treat allergy patients in the coming decade, and these advances are previewed.
Keywords: allergic rhinitis, desensitization, drug desensitization, immunotherapy, hymenoptera venom
ARTICLES PUBLISHED IN THIS CLINICAL IMMUNOLOGY REVIEW SERIES Allergy in childhood, allergy diagnosis by use of the clinical immunology laboratory, anaphylaxis, angioedema, desensitization, management of pulmonary disease in primary antibody deficiency, recurrent infections in childhood, recurrent infections in adulthood, recurrent oro-genital ulceration, recurrent superficial abscesses, urticaria, vasculitis/CTD
Introduction
On 10 June 1911, Leonard Noon published the first short description of allergen-specific immunotherapy by injection [1]. His short paper described increasing tolerance to conjunctival challenge testing with grass pollen extract. His work was completed by Freeman [2], who published a clinical description of improved hay fever symptoms in September of the same year. Between them, these papers described the hypothesis underpinning allergen immunotherapy, the production and standardization of pollen extracts, the use of subcutaneous injections, with short interval up-dosing and longer interval maintenance, and adverse reaction due to overdose. They suggested confirmation of sensitization (by conjunctival challenge) prior to commencing therapy, titration of the starting dose, the choice of the single pollen Phleum pratense from a selection of grass pollen species, and also stated that efficacy is proportional to the duration of prophylactic therapy. At face value it could be argued that these concepts have not changed in the last 100 years. However, the practice of allergen immunotherapy is now supported by a wealth of well-controlled studies, and novel formulations and routes of administration have been investigated. Nonetheless, the gold standard procedure of subcutaneous immunotherapy with P. pratense for hay fever remains alarmingly similar to that described a century ago.
This review of allergen immunotherapy in the treatment of inhalant, venom and drug allergies will focus on patient selection and modalities of administration of this therapy, with specific emphasis on the practicalities of the safe delivery of this service in a specialist centre.
Allergen-specific immunotherapy
Aeroallergens
Allergic rhinoconjuctivitis can be treated effectively with immunotherapy, as demonstrated in recent systematic reviews [3–5]. A wide range of aeroallergens, including pollens, house dust mite, animal danders, mould spores and some occupational allergens have been identified as causing allergic airways disease. Standardized allergen extracts are available and the treatment is currently administered either as subcutaneous injection immunotherapy (SCIT) or sublingual immunotherapy (SLIT), and these are discussed in the following sections.
Indications
Careful patient selection is paramount. Clinical benefit can only be expected if the patient's symptoms are truly attributable to an allergic reaction to the implicated allergen. Allergy testing alone cannot confirm this (as the specificity of allergy tests in isolation is low) [6–8] and a detailed clinical history of allergic symptoms consistent with allergen exposure is also required. Challenge testing can be used to confirm specific allergy, but is not often used in routine practice.
Many patients with allergic rhinoconjunctivitis are sensitized to a number of allergens. Evidence does not support the use of mixed allergen preparations, so that only patients with one significant specific allergy (perhaps two) may be considered for immunotherapy using standardized allergen extract. Patients should also be counselled regarding the expected benefits of treatment for them individually in light of their own symptom severity and triggers.
In the United Kingdom, only patients with clinically significant symptoms not controlled adequately with optimal medical therapy are considered for immunotherapy. This means that in practice many patients are treated under close supervision as per British Society for Allergy and Clinical Immunology guidelines [9], with topical nasal steroids, cromones and antihistamines for a period before enrolment in an immunotherapy programme.
This practice is in contrast to that in other countries, where immunotherapy is often used at an earlier stage, and may even be offered in the hope of modifying disease progression, to prevent the development of new sensitizations and new allergic diseases. A number of recent studies show evidence of such disease modification, but require confirmation in a larger sample size [10–12].
Investigations
Confirmation of sensitization to the specific allergen is a required, but not sufficient, criterion for initiation of immunotherapy. This may be by skin prick testing or detection of serum-specific immunoglobulin (Ig)E. If the patient has mild asthma, verification of adequate control on history and by pulmonary function testing is an important safety consideration.
A guide to evaluation, patient selection and contraindications for allergen-specific immunotherapy in allergic rhinitis is summarized in Table 1.
Table 1.
Guide to evaluation and selection of patients for subcutaneous injection immunotherapy/sublingual immunotherapy (SCIT/SLIT) to aero-allergens for allergic rhinitis (#contraindications for specific allergen immunotherapy to aero-allergens).
 Evaluate symptoms and response to first-line pharmacotherapy (including compliance, nasal spray technique) Evaluate symptoms and response to first-line pharmacotherapy (including compliance, nasal spray technique) |
 Quality of life significantly affected? Quality of life significantly affected? |
 Co-existing local nasal airway pathology such as polyps, deviated septum Co-existing local nasal airway pathology such as polyps, deviated septum |
 Evaluate allergenic trigger from history, e.g. timing of symptoms in the calendar year for hay fever, in case of perennial rhinitis for other factors such as exposure to moulds, animal dander, occupational factors, etc. Evaluate allergenic trigger from history, e.g. timing of symptoms in the calendar year for hay fever, in case of perennial rhinitis for other factors such as exposure to moulds, animal dander, occupational factors, etc. |
 Has the patient followed allergen avoidance measures where appropriate? Has the patient followed allergen avoidance measures where appropriate? |
 Consider environmental factors, both indoor as well as outdoor Consider environmental factors, both indoor as well as outdoor |
 Asthma Asthma |
| ○Mild, moderate# or severe# |
| ○Asthma treatment |
| ○Requirement for short acting β2 agonist per day |
| ○ Recent asthma attacks |
| ○ Nocturnal symptoms |
| ○ Compliance |
| ○ PEFR charts |
| ○ FEV1 |
 Other co-morbid factors and medications Other co-morbid factors and medications |
| ○ Underlying cardiac and respiratory disorders# |
| ○ Beta blockers#, ACE inhibitors# |
| ○ Systemic autoimmune conditions#, oral/parenteral steroids#, immunosuppressive drugs# |
| ○ Pregnancy# |
ACE: angiotensin-converting enzyme; FEV1: forced expiratory volume in 1 s; PEFR: peak expiratory flow rate.
SCIT protocols
SCIT describes the sequential administration of gradually increasing doses of standardized allergen extract up to a maintenance dose, and then continuation of treatment at this dose for a period of time (usually 3 years). Although target maintenance doses are listed for each product by manufacturers, the dose employed is determined by the patient's clinical tolerance to the vaccine. In other words, a lesser dose is recommended if the patient develops an allergic reaction. Evidence from previous studies has shown that a maintenance dose of 5–20 µg can induce clinical benefit [13–15].
Dosage and regimens
Conventional up-dosing protocols use 12–16 weekly injections during up-dosing, and four to six weekly injections during the maintenance phase. Clustered protocols use two or three injections at each weekly visit, thus reducing the total time required to reach maintenance dose (usually in 7–8 weeks). Rush desensitization protocols have been also described, but are used less often for aeroallergens than for hymenoptera venoms (see below) in view of the higher rate of systemic reactions, including anaphylaxis [16]. Dose reductions are made for delayed or missed injections, during a symptomatic period (for example during the pollen season) or following large local reactions (≥ 10 cm) and systemic reactions.
General health, adverse events, changes in medication and peak expiratory flow are monitored prior to administration of SCIT. An observation period of 1 h after the injection is mandatory, with peak expiratory flow testing prior to discharge. However, severe ‘non-immediate’ reactions can occur up to 24 h after allergen injection.
SLIT
SLIT involves placing the vaccine in solution (drop preparation) or tablet form under the tongue for 1–2 min followed by swallowing. Patient selection for sublingual immunotherapy (SLIT) is identical to that for SCIT. The safety profile of SLIT is superior to SCIT, and serious side effects such as anaphylaxis have been extremely rare [17–23]. Many patients develop minor discomfort in the early phase of treatment, including oropharyngeal pruritis and angioedema, which may require treatment with an antihistamine, but these symptoms usually settle with continued administration of the vaccine.
The indications, contraindications and general considerations in administration of SLIT are the same as described under SCIT. However, there are some special considerations listed as follows.
One particular preparation (Grazax; ALK Abello, Denmark) currently licensed in the United Kingdom contains fish gelatin. It may be used cautiously in patients with a history of fish allergy, but is absolutely contraindicated in patients with history of anaphylaxis to fish.
Sublingual vaccines must not be administered if there are raw areas or bleeding in the oral cavity or following dental procedures until the wound is completely healed.
Where there is an up-dosing schedule, this must be re-started if there has been a gap of ≥ 5 days in vaccine administration.
Dosage and regimens
Sublingual immunotherapy has been used for several aeroallergens including pollens, house dust mite and cat. The optimum dosage, duration and frequency of administration have not yet been established. Sublingual immunotherapy involves a much higher dose of allergen than SCIT. The cumulative monthly dosage of SLIT used in clinical studies has been variable, but has been 0·6–500 times greater than customary SCIT [18]. Several dosing regimens have been employed, including daily (fixed or incremental dosing) [24–26], three times per week [27] and weekly [28]. With seasonal allergens such as pollen, treatment has been given preseasonally, co-seasonally, pre- and co-seasonally and perennially. Prolonged preseasonal administration induces greater clinical benefit, and if treatment is continued perennially, clinical and immunological responses improve in subsequent years of treatment [29,30]. A recent large placebo-controlled study of sublingual grass pollen tablets showed evidence of persistence of clinical benefit 1 year after stopping treatment [30], and the results of a further year's follow-up are awaited.
The first dose is given under observation in the clinic and, if tolerated, the patient can then self-administer the treatment daily at home. Clinical follow-up to encourage compliance, monitor for adverse events and to adjust any medical treatment is still recommended.
Efficacy parameters
There are no efficacy parameters or biomarkers that reliably predict or indicate response to treatment [18]. Responses in clinical trials have been assessed using symptom and medication scores and measuring quality of life using a validated questionnaire.
Long-term efficacy has been shown with SCIT to grass pollen. Patients who received treatment for a period of 3 years showed sustained benefit for 7–9 years following discontinuation of desensitization [13,31–34].
Hymenoptera venom immunotherapy (VIT)
VIT is the only specific treatment currently available to reduce the severity and prevent the recurrence of systemic reactions (SR) in patients with a previous history of life-threatening SR or anaphylaxis to hymenoptera insect sting [35–39]. It is highly effective, providing more than 90% protection from reactions to subsequent stings [35,40–42]. Furthermore, it induces a clinically significant improvement in health-related quality of life both in patients with a history of anaphylaxis as well as those with non-life-threatening SRs to hymenoptera stings [43,44]. For a successful clinical outcome in VIT a systematic approach with a good clinical history, and in some cases scrutiny of hospital records relating to previous reactions, are paramount. Knowledge of the insect involved is valuable in making the correct choice of venom. Honey bees usually leave the barbed stinger behind, whereas wasps and hornets usually do not. Details of the circumstances surrounding the sting episode may also provide useful pointers with respect to the nature of the insect.
Indications (Table 2)
Table 2.
Indications for venom immunotherapy (VIT).
| Symptoms | VIT decision | Comment |
|---|---|---|
| Large local reaction | No | – |
| Anaphylaxis or grade 4 reaction (Muller grading) | Yes | |
| Upper airway or laryngeal oedema and/or severe bronchospasm | Yes | |
| Generalized urticaria and/or non-life threatening angioedema or mild bronchospasm | Consider VIT | Make a decision after considering occupational risk, hobbies, co-morbid conditions, social circumstances, access to emergency care and patient choice |
Anaphylaxis to hymenoptera sting represents a clear indication for VIT [36–38]. However, in patients with non-life-threatening reactions other risk factors such as age, co-morbid conditions, occupation, hobbies, social circumstances and the patient's own choice must be considered carefully prior to making a decision about pursuing VIT. Demonstration of venom-specific IgE is mandatory prior to initiating VIT. Venom immunotherapy is not indicated in patients with local reactions, irrespective of their severity, and further investigations are not warranted [36–38].
VIT must not be attempted in patients with history of non-IgE-mediated systemic reactions such as Guillain–Barré syndrome, peripheral neuritis, haematological and renal complications.
Investigations
Skin prick tests (SPT) are the first-line investigation and are carried out at a concentration of 0–100 µg/ml of standardized venom extract [39]. If SPT are negative, intradermal testing (IDT) should be carried out starting at ≤ 0·001 µg/ml and increasing up to a maximum concentration of 1·0 µg/ml [39]. Skin tests have greater sensitivity and specificity than in vitro tests measuring serum venom-specific IgE (SSIgE) [39]. Levels of SSIgE and skin test responses do not correlate with clinical reactivity. Venom-specific IgE can also be measured by a basophil activation test (BAT), but the latter is currently a research tool and does not have significant advantages over routinely employed enzyme immunoassays.
In patients with a history of moderate–severe SR reaction, plasma baseline tryptase should also be measured to screen for underlying disorders of mast cell overload, such as telangectasia macularis eruptiva perstans (TMEP) and other forms of cutaneous (urticaria pigmentosa) and systemic mastocytosis which may warrant further investigation, including bone marrow studies, tissue biopsy and appropriate management [45–50]. Elevated baseline tryptase is an important risk factor for anaphylaxis [45–50] and will have implications for VIT, as discussed in the following sections.
Choice of venom for VIT
This is dictated by clinical history and demonstration of venom-specific IgE. There is no significant cross-reactivity between clinically significant antigens of Apidae and Vespidae (honey bee and wasp/hornet) venoms [51–53]. Within the Vespidae family, there is significant overlap between wasps and hornet venoms [54–56]. However, there is little cross-reactivity between wasps/hornet and paper wasps (not encountered in the United Kingdom) [56]. These facts, as well as knowledge of local entomology of hymenoptera insects, have to be taken into consideration carefully to make a correct choice of the venom for immunotherapy. For example, in a British patient with a history of hornet sting anaphylaxis during a visit to mainland Europe, the ideal choice for immunotherapy would be wasp venom, as the prevalence of wasps is greater in the United Kingdom and wasp venom immunotherapy will protect the patient from either insect sting.
VIT protocols
Different protocols (Example 1), including conventional, clustered, rush and ultra-rush, have been described in the literature. A conventional protocol involves weekly up-dosing, reaching the maintenance dose in 12 weeks [57–60]. Maintenance dose is reached in 4–7 days in a rush up-dosing [61–63] protocol and 1–2 days in an ultra-rush schedule [61,64,65]. A recent national audit in the United Kingdom has shown that more than 90% of allergy specialists employ the conventional protocol, as services in this country are primarily out-patient-based [66]. Accelerated protocols are popular in North America and Europe, and have been shown to be safe as well as efficacious [61,63,64,67–69].
Example 1.1.Conventional venom immunotherapy (VIT) (as per manufacturer's recommendation – ALK Pharmalgen bee and wasp venom; for a more detailed description refer to the manufacturer's product information).
| Week no. | Dosage in micrograms of bee or wasp venom subcutaneously |
|---|---|
| 1 | 0·01* |
| 2 | 0·1 |
| 3 | 1·0 |
| 4 | 5 |
| 5 | 10 |
| 6 | 20 |
| 7 | 30 |
| 8 | 40 |
| 9 | 50 |
| 10 | 60 |
| 11 | 80 |
| 12 | 100 |
May be lower depending on patient's sensitivity.
Example 1.Protocols for venom immunotherapy (VIT)
Example 1.2.Rush venom immunotherapy (VIT) (injections at 60-min intervals each day).
| Day no. | Dosage in micrograms of bee or wasp venom subcutaneously |
|---|---|
| 1 | 0·001 |
| 0·01 | |
| 0·1 | |
| 0·2 | |
| 0·4 | |
| 2 | 0·8 |
| 1·0 | |
| 2·0 | |
| 4·0 | |
| 6·0 | |
| 3 | 8·0 |
| 10 | |
| 20 | |
| 40 | |
| 60 | |
| 4 | 80 |
| 100 |
Example 1.3.Ultra-rush venom immunotherapy (VIT) (nine injections over 2 days, day 1: seven injections at 30–60-min intervals; day 2: two injections at 2–4-h intervals; days 1 and 2 as in-patient; maintenance dose of 100 µg given on days 7, 14, 28, 42, 63, 84 and then monthly as out-patient) [61].
| Day no. | Dosage in micrograms of bee or wasp venom subcutaneously |
|---|---|
| 1 | 0·01 |
| 0·1 | |
| 1·0 | |
| 10 | |
| 20 | |
| 40 | |
| 80 | |
| 2 | 100 |
| 100 |
The target maintenance dosage is 100 µg and this is administered at 4-, 6- and 8-weekly intervals during the maintenance phases of years 1, 2 and 3 respectively [37]. Appropriate dose reductions have to be undertaken as with SCIT for aero-allergens in the context of missed injections and large local and systemic reactions. In patients who develop field sting-induced systemic reactions, suggesting treatment failure or inadequate tolerance, escalation of the maintenance dose to 150–200 µg has been shown to be beneficial [37,70].
The safety and efficacy of VIT has not yet been established in patients with elevated plasma baseline tryptase. There are two published reports [46,47] involving a relatively small cohort of patients with urticaria pigmentosa and indolent systemic mastocytosis, showing somewhat conflicting observations and utilizing conventional and clustered up-dosing protocols. It is difficult to make definitive conclusions from these studies, but it is recommended that VIT is carried out cautiously in this group of patients [71].
When to stop VIT
The optimal duration of VIT in UK practice is 3 years. This is seldom prolonged to 5 years or more, but this approach is not evidence-based. It has been recommended that a more prolonged programme of VIT should be considered in patients with history of anaphylactic shock resulting in loss of consciousness, those with history of treatment failure/s (i.e. development of systemic reaction/s or anaphylaxis to field stings while undergoing VIT) or with elevated baseline plasma tryptase (bT) and mastocytosis [36,37,72].
There is little benefit in checking venom-specific IgE at the end of the VIT schedule, as up to 75% of patients continue to demonstrate sensitization [73]. Similarly, while venom-specific IgG4 is induced with VIT, this is not correlated with treatment success [74–77]. Long-term follow-up studies in North America and Europe have shown prolonged efficacy of VIT, with a cumulative risk of 10–15% for the development of SR at 15 years following a treatment period of 3–5 years [73,78].
Practical aspects of SCIT
SCIT must be undertaken only by a specialist with adequate knowledge and experience in this field and in a clinical setting where support for cardiopulmonary resuscitation is readily available. Immunotherapy employing 12-week conventional and 7–8-week cluster protocols can be undertaken in an out-patient facility, but accelerated regimens must be administered in an intensive care or high dependency unit. Protocols for safe delivery of the service (Example 2) must be in place, with particular emphasis on confirmation of identity of the patient, allergen extract and dosage during each visit. A 60-min period of observation is mandatory following each injection in order to monitor the patient closely for development of symptoms of type 1 hypersensitivity reaction. Previous surveys have shown that common causes of allergic reactions during SCIT are misidentification of the patient, administration of the incorrect allergen and dosage errors [79]. Therefore, it is recommended that the injection vial and dosage are checked with another health care professional with experience in SCIT.
Example 2.Safety checks prior to injections in an immunotherapy clinic
Check patient identity.
Confirm the allergen involved in treatment.
Did the patient tolerate the last injection? If not, assess the severity of local and/or systemic reaction/s. Appropriate dose reduction and patient counselling may be necessary.
In patients premedicated with antihistamine, ensure that they have taken their medication beforehand.
Enquire for intercurrent infections and any change in medical status since last injection.
In female patients, enquire about pregnancy.
In asthmatics, ensure asthma is stable and well controlled.
Check peak expiratory flow rate (PEFR) at baseline and 60 min following injection or earlier if warranted.
Asthma and SCIT to aeroallergens
Previous reports have also highlighted that near-fatal and fatal anaphylaxis are more common in patients with underlying asthma, and therefore SCIT for aero-allergens is contraindicated in patients with poorly controlled, ‘brittle’ or moderate to severe asthma [38,79]. While mild, well-controlled asthma is not a contraindication for SCIT with aero-allergens, injections must not be administered during intercurrent respiratory infection when there is exaggerated bronchial reactivity, which may predispose patients to the development of a systemic reaction. Poor asthma control is suggested by excessive use of short-acting β2 agonist (more than twice a day), nocturnal symptoms, recurrent courses of oral steroids and hospitalization for acute asthma. A more objective evaluation of asthma control can be obtained by reviewing peak flow charts recorded twice daily for 3–4 weeks with documentation of short-acting β2 agonist usage, as well as baseline spirometry [forced expiratory volume in 1 s (FEV1) should be ≥ 70% predicted].
Asthma and VIT
VIT injections must also not be administered during intercurrent respiratory infection. It is imperative to optimize the anti-inflammatory therapy for asthma prior to commencing VIT and to perform an objective evaluation of asthma as above. VIT is contraindicated in severe or ‘brittle’ asthma, but the approach is somewhat different in moderate asthmatics where a careful ‘risk–benefit’ analysis must be performed for VIT, taking into consideration co-morbid factors, occupation, hobbies and social circumstances as well as patient choice.
Immunotherapy and pregnancy
Allergen immunotherapy in any form must not be initiated during pregnancy [38]. Although allergen immunotherapy is not known to have teratogenic effects, it should ideally be avoided in pregnancy, even in patients established on treatment who are in maintenance phase, in view of the rare but real possibility of anaphylaxis which may cause fetal hypoxia [38].
Immunotherapy and beta-blocker therapy
Beta-blocker therapy is generally considered an absolute contraindication during allergen-specific immunotherapy due to the risk of refractory anaphylaxis [36–38,80]. This is related to reduced therapeutic efficacy of adrenalin in anaphylaxis due to underlying beta blockade. Therefore, as far as possible, it is better to avoid beta-blockers during immunotherapy, but there are some special circumstances in patients requiring VIT where withdrawal of beta-blockers may put the patient at risk (such as of underlying tachyarrhythmias) [80,81]. In such circumstances, a careful ‘risk–benefit’ analysis must be undertaken, and liaison with the patient's family physician and cardiologist will be beneficial. Where benefit of continuation of treatment of beta-blocker clearly outweigh the risk of their discontinuation, short-acting beta-blockers may be discontinued temporarily prior to injections or during the induction phase of VIT. Some groups have undertaken VIT successfully alongside treatment with beta-blockers. In such circumstances, glucagon must be readily available to treat refractory anaphylaxis [80].
Immunotherapy and angiotensin-converting enzyme (ACE) inhibitors
ACE inhibitors constitute a relative contraindication for immunotherapy [38]. If possible, these must be replaced with an alternative agent such as angiotensin receptor blocker. While there are some anecdotal reports [82] in the literature of severe anaphylaxis to VIT in patients on concurrent treatment with ACE inhibitors, a recent retrospective study in a small cohort of patients did not confirm this observation [83].
Immunotherapy and premedication with antihistamine
There is some evidence in the literature from studies in a small group of subjects that premedication with antihistamine reduces severity of histamine-mediated local reactions, including erythema and induration, and generalized cutaneous response such as urticaria and angioedema, but they do not prevent or abrogate anaphylaxis [65,84,85]. Some allergists express concern about antihistamines potentially masking early symptoms of an allergic reaction to injections, but this is not evidence-based. It is worth noting that recent large multi-centre SCIT hay fever trials included premedication with a short-acting antihistamine [11].
Allergen standardization and adjuvants
The purpose of allergen standardization is to enhance sensitivity and specificity of the extracts used for diagnosis of allergy as well as to minimize the qualitative and quantitative variation in the composition of the vaccines in order to obtain higher safety standards, efficacy and accuracy. The first international initiative on allergen standardization was the establishment of the Nordic Guidelines, based on Danish Allergen Standardization in 1976 [86]. The World Health Organization (WHO) and European Pharmacopoeia have published guidelines on allergen standardization. In Europe, current guidelines dictate the use of ‘in-house’ reference preparation (IHRP) by all manufacturers for monitoring ‘batch-to-batch’ control [87,88].
The source material for allergy vaccines should represent the allergen to which humans are exposed and should meet the specified criteria for limits on foreign substances and be free of microbial contamination [86]. The manufacturing process must not alter the immunogenicity of the vaccine. A major aspect of allergen standardization is to control for total allergenic potency, which is achieved with international collaboration between manufacturers and control authorities using the same standards that are available from the National Institute of Biological Standards and Control, Herts, UK [86]. The ‘in-house’ reference preparation used by individual laboratories is compared with the international standard and ‘batch-to-batch’ control involves monitoring the quantity of major allergens [86].
Another approach has been to use chemically modified allergens (allergoids) treated with formaldehyde or glutaraldehyde, which reduce allergenicity (IgE binding) but retain immunogenicity, and so theoretically would reduce the incidence of systemic reactions [86]. These are available for a number of allergens on a named patient basis, including pollens, house dust mite, animal dander and fungal spores.
Aqueous vaccine preparations are employed commonly in the United States, and a mixture of allergens tailored to the patient's sensitization pattern is often used in patients with multiple allergies. In the United Kingdom, in contrast, single allergen preparations are used and are usually alum (aluminium hydroxide) adsorbed [e.g. Alutard vaccines (ALK Abello)]. Alum acts as an adjuvant [down-regulates T helper type 2 (Th2) cell response/s], and slows the release of the allergen into the tissue and circulation, thereby reducing the incidence of SRs [89,90].
Drug desensitization
Basic principles
Drug desensitization involves a closely supervised graded administration of a drug to a patient with a history of an immediate hypersensitivity response (IgE-mediated and non-IgE-mediated) to that drug. Although there are no controlled clinical trials to validate the dosage regimens employed, there are a number of published case reports/series supporting the efficacy and safety of this process. Drug desensitization has been carried out successfully for a number of IgE-mediated responses, including penicillins, cephalosporins, carbapenems, insulin and platins, as well as for non-IgE-mediated immediate hypersensitivity reactions including aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), radio contrast media and vancomycin [91–102]. In view of the potential risk of anaphylaxis, this procedure must be considered following a careful ‘risk–benefit’ analysis. There are a few clinical scenarios where such a procedure is indicated (Example 3), and it is prudent to establish that desensitization would be life-saving or significantly improve clinical outcome or quality of life in the patient.
Example 3.Indications or common clinical scenarios for drug desensitization
Life-threatening or serious infections where no alternative antibiotic is available:
Neurosyphillis
Syphilis in pregnancy
Infective endocarditis
Tuberculosis
Multiple antibiotic allergies in cystic fibrosis
Cancer chemotherapy
Acetylsalicylic acid (ASA) hypersensitivity in:
Aspirin-induced asthma
Aspirin-induced nasal polyps
Cardiac stents needing dual anti-platelet prophylaxis
Anti-phospholipid syndrome
Non-steroidal anti-inflammatory drugs (NSAIDs): patients requiring pain control as in arthritis and previous history of hypersensitivity reaction to aspirin and/or NSAID
Protamine hypersensitivity in a patient needing cardiac bypass surgery
Practical aspects
In contrast to desensitization with aero-allergens and venoms, where long-term tolerance can be established following a 3–5-year treatment course, tolerance induced by drug desensitization is lost within a few days of stopping the drug [103]. In other words, the process of desensitization has to be repeated each time the patient is exposed to the specific drug after a period of discontinuation. Drug desensitization is principally carried out orally and intravenously, the former being a safer approach. Rapid desensitization protocols have been developed where the therapeutic dosage can be administered within a few hours. Often the starting dose is ≤ 1/1000th the therapeutic dosage, with escalations being carried out in doubling doses at 15–30-min intervals, monitoring the patient closely for symptoms and signs of an allergic reaction. Intravenous desensitization usually involves preparation of three different concentrations of the drug (solutions A, B, C), with a 10-fold increase in concentration between A and C. The rate of infusion of each solution is regulated with a syringe pump in such a way that there are four incremental dosage steps at 15–30-min intervals for each solution.
Rapid drug desensitization must be carried out under the direction and supervision of a specialist in allergy in a high dependency or intensive care unit, where immediate support for resuscitation is available and with a dedicated trained nurse for instituting the desensitization protocol. If the patient develops an allergic reaction, it must be treated promptly with antihistamine, adrenaline and corticosteroids as appropriate to the severity of the response. In such circumstances, dose reduction followed by careful escalation can be re-attempted to establish tolerance. In some patients, this process of dosage reduction followed by escalation may have to be repeated several times in order to achieve the therapeutic dose.
Contraindications
Drug desensitization must not be attempted in non-immediate-type hypersensitivity such as immune complex reactions, acute interstitial nephritis, haemolytic anaemia, toxic epidermal necrolysis and Stevens–Johnson syndrome.
Some relatively common clinical scenarios, including desensitization with penicillin, aspirin and platins, and practical tips are summarized in Examples 3 and 4, respectively.
Example 4.Practical tips for drug desensitization
Carry out allergy tests where possible and appropriate to demonstrate specific immunoglobulin (Ig)E.
Perform ‘risk–benefit’ analysis to demonstrate the need for the procedure.
Counsel patient/relative/guardian and obtain consent.
Procedure to be performed in a intensive care or high dependency unit where patient can be closely monitored and support for resuscitation is readily available.
Discontinue beta-blockers as far as possible.
Involve an experienced pharmacist to prepare the relevant concentrations of the drug.
It is preferable to have a dedicated nurse to facilitate administration of the drug and provide a written protocol.
Recognize and treat allergic reactions early and promptly.
Penicillin allergy and desensitization
There are only a few indications for the use of penicillin or related beta lactams in patients with previous history of type 1 hypersensitivity. This applies to infections where no other therapeutically efficacious alternatives are available, and these are summarized in Example 3. Successful oral and intravenous penicillin desensitization protocols have been reported [93,104] (Example 5).
| Dose number | Time (min) | #Amount (units/ml) | ml | Units | Cumulative dose in units |
|---|---|---|---|---|---|
| 1 | 0 | 1000 | 0·1 | 100 | 100 |
| 2 | 15 | 1000 | 0·2 | 200 | 300 |
| 3 | 30 | 1000 | 0·4 | 400 | 700 |
| 4 | 45 | 1000 | 0·8 | 800 | 1500 |
| 5 | 60 | 1000 | 1·6 | 1600 | 3100 |
| 6 | 75 | 1000 | 3·2 | 3200 | 6300 |
| 7 | 90 | 1000 | 6·4 | 6400 | 12 700 |
| 8 | 105 | 10 000 | 1·2 | 12 000 | 24 700 |
| 9 | 120 | 10 000 | 2·4 | 24 000 | 48 700 |
| 10 | 135 | 10 000 | 4·8 | 48 000 | 96 700 |
| 11 | 150 | 80 000 | 1 | 80 000 | 176 700 |
| 12 | 165 | 80 000 | 2 | 160 000 | 336 700 |
| 13 | 180 | 80 000 | 4 | 320 000 | 656 700 |
| 14 | 195 | 80 000 | 8 | 640 000 | 1 296 700 |
| 15 | 225 | Parenteral (intravenous) penicillin is given, after which regular doses should be administered for the duration of treatment. Benzathine penicillin (intramuscular) can be given after step 14. Further doses are usually given every 1–3-weekly but a test dose with penicillin V (400 000 U) is given prior to administration of each dose of benzathine penicillin including the first dose after step 14, patient is observed for an hour before administration of benzathine penicillin and further monitoring in hospital overnight is required | |||
Adapted from Wendel et al. [104]. #This treatment must be delivered in an intensive care or high dependency unit. +Obtain informed consent, check pulse, blood pressure and peak expiratory flow rate and repeat prior to every step. Also, monitor patient for signs and symptoms of allergic reaction.
Example 5. A protocol+ for oral penicillin desensitization [104]
In patients with history of type 1 hypersensitivity to penicillin, aminopenicillins and first- and second-generation cephalosporins must be avoided, but aztreonam, imipenem and third-, fourth- and fifth-generation cephalosporins are usually well tolerated (although these must be administered cautiously) [103,105,106].
Aspirin desensitization
Immediate reactions to aspirin and other NSAIDs are not IgE-mediated and several terms have been used to describe these responses, including pseudo-allergy, intolerance, aspirin/NSAID hypersensitivity and idiosyncracy. This is caused by an abnormal shift of arachidonic acid towards the lipoxygenase pathway due to inhibition of cycloxygenase-1, resulting in excessive production of cysteinyl leukotrienes. It was Zeiss and Lockey [107] who first described a paradoxical observation in 1976 that patients with an intolerance are refractory to aspirin for 3 days following aspirin provocation or challenge. This led to the development of several desensitization protocols.
Indications for aspirin desensitization [92,101,108,109]
Patients with aspirin-exacerbated airways disease (AERD) with moderate–severe asthma with or without associated intractable nasal congestion that is not responding to topical steroids and leukotriene modifiers (antagonists or 5-lipoxygenase inhibitors) should be considered for desensitization.
Similarly, patients with AERD and moderate–severe asthma requiring recurrent courses of oral corticosteroids may be considered for desensitization.
Patients with AERD and nasal polyps requiring recurrent surgical resection.
Patients with AERD or aspirin-induced urticaria, angioedema or anaphylaxis requiring anti-platelet prophylaxis in the context of coronary stent surgery or anti-phospholipid syndrome.
Previous studies have shown that aspirin desensitization improves olfactory function, reduces the need for topical and systemic corticosteroids and reduces infective sinusitis episodes as well as emergency room visits for asthma exacerbations [110,111].
Oral aspirin desensitization protocol is summarized in Example 6. For a more detailed description of preparation of patients for this procedure and treatment of allergic reactions the reader is directed to recently published practice parameter [108]
Example 6.Oral aspirin desensitization protocol [109]
Begin early in the morning and establish intravenous access.
Administer 20·25 mg aspirin orally followed by 40·5 mg, 81 mg, 162·5 mg and 325 mg at 90-min intervals.
Review patient's symptoms and check pulse, blood pressure (BP) and forced expiratory volume in 1 s (FEV1) prior to each step of escalation (dose intervals may have to be extended depending on patient's history).
A decline of FEV1 by ≥ 15% is significant.
After stabilization of the patient, and at least 3 h after the last dose, provoking dose is repeated. Dose escalation can be continued if well tolerated.
If decline in FEV1 or symptoms are persistent, i.e. last > 3 h, abandon the process for the day starting on day 2, with previously tolerated dose.
Usual maintenance dose is 650 mg or 325 mg twice a day. Adapted from Stevenson and Simon [109].
Desensitization with carboplatin
Carboplatin represents the main drug in the management of ovarian cancer, including treatment of relapses. It is usually well tolerated, but up to 27% of patients treated with seven or more cycles with this agent develop type 1 hypersensitivity with cutaneous manifestations in > 90% of patients, and up to 77% show cardiovascular compromise [112,113]. The non-irritant concentration for skin test is 1–10 mg/ml [114,115]. Rapid desensitization with carboplatin has been carried out successfully (Example 7) in these patients, and this is associated with disappearance of skin test reactivity.
Example 7.Carboplatin desensitization [96]
| Step | Solution | Rate (ml/h) | Time | Dose (mg) | Cumulative dose (mg) |
|---|---|---|---|---|---|
| 1 | A | 2 | 15 | 0·01 | 0·01 |
| 2 | A | 5 | 15 | 0·025 | 0·035 |
| 3 | A | 10 | 15 | 0·05 | 0·085 |
| 4 | A | 20 | 15 | 0·1 | 0·185 |
| 5 | B | 5 | 15 | 0·25 | 0·435 |
| 6 | B | 10 | 15 | 0·5 | 0·935 |
| 7 | B | 20 | 15 | 1·0 | 1·935 |
| 8 | B | 40 | 15 | 2·0 | 3·935 |
| 9 | C | 10 | 15 | 5 | 8·935 |
| 10 | C | 20 | 15 | 10 | 18·935 |
| 11 | C | 40 | 15 | 20 | 38·935 |
| 12 | C | 75 | 184·4 | 461·065 | 500 |
| Total time 5·82 h | Total dose 500 mg |
Reproduced with permission from Lee CW et al. [96]. Solution A: 0·02 mg/ml [total volume 250 ml; total dose 5 mg]; Solution B: 0·2 mg/ml [total volume 250 ml; total dose 50 mg]; Solution C: 2 mg/ml [total volume 250 ml; total dose 500 mg].
Mechanisms underlying desensitization (Fig. 1)
Fig. 1.
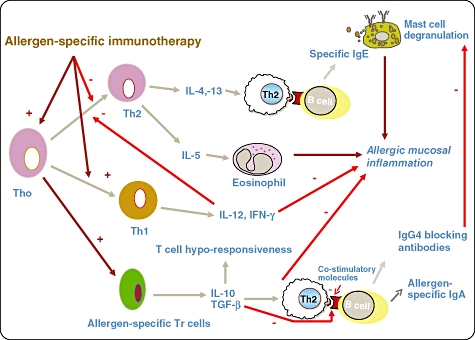
Mechanisms underlying the action of specific immunotherapy (SIT): there is T helper type 2 (Th2) predominance in allergic disease and it is characterized by secretion of interleukin (IL)-4, IL-5 and IL-13. IL-5 is involved in eosinophil survival, activation and maturation and IL-4 and IL-13 play a key role in class-switching of B cells for immunoglobulin (Ig)E production. Th1 response induces IL-12 and interferon (IFN)-γ production and this has inhibitory effects on Th2 cells. SIT up-regulates Th1 response, dampens Th2 response and induces allergen-specific Tr cells to secrete IL-10 and transforming growth factor (TGF)-β. IL-10 induces T cell ‘anergy’ or ‘unresponsiveness’ to the allergen. Allergen-specific immunotherapy induces production of allergen-specific IgG4 and IgA2, the former may play a role in interfering with allergen binding to IgE on the mast cell. The changes in Th1/Th2 balance has been shown to occur within days following initiation of ‘ultra-rush’ bee venom immunotherapy and is associated with loss of T cell responsiveness in vitro to the venom – this response is reversed by anti-IL-10 in the culture medium. A similar pattern of shift from Th2 to Th1 cytokine pattern is seen in the nasal mucosa of patients undergoing grass pollen immunotherapy and is strikingly associated with reduction of Th2 cell and eosinophil influx during the pollen season; this parallels the clinical response.
Although several mechanisms have been delineated, in truth no single mechanism is likely to explain all the observed clinical effects and immunological phenomena; this has been described elegantly in recent reviews [116–120].
Noon's paper cited the work of William Dunbar, who showed that antibodies to the pollen ‘toxin’ were found in hay fever patients and could be induced in animals by injection of pollen. He reasoned that inducing pollen ‘anti-toxins’ in hay fever patients would neutralize the effect of the pollen. Today, IgG4 antibodies directed against the allergen are still measured as evidence of a response to immunotherapy. The precise role of the antibodies is controversial; they are proposed to bind to the allergen and prevent its causing mast cell degranulation via IgE binding. Levels of allergen-specific IgG (total IgG or IgG4) do not predict or correlate with a clinical response to immunotherapy [74–77].
Alterations of allergen-induced cytokine production profile have been demonstrated in various studies. While the changes seen vary between studies, the overall trend observed is for a switch from a pro-allergenic Th2 profile, including interleukin (IL)-4 and IL-5 production, towards a Th1 profile characterized by increased interferon (IFN)-γ production [119,121,122]. More recently, the induction of allergen-specific regulatory T cells [CD4+ CD25+ forkhead box P3(Foxp3+)] has been demonstrated, with production of anti-inflammatory cytokines such as IL-10 and transforming growth factor (TGF)-β[123–125].
The overall effect of these changes is to reduce the inflammatory response in the target tissue. This was shown as a marked seasonal reduction in mucosal eosinophil recruitment and an increase in IFN-γ and IL-10 production in nasal mucosal biopsy samples after hay fever immunotherapy [126].
Many of the mechanisms described for conventional weekly up-dosing regimens of immunotherapy cannot apply to the initial phase of rush desensitization, where tolerance is induced within days. While the changes described above may eventually supervene, the initial rapid induction of tolerance to the allergen is likely to represent tachyphylaxis, where repeated doses of allergen induce a progressively weaker mediator response. Changes in histamine release, cytokine production by T cells and monocytes and even antibody binding activity have been described within the first days of rush immunotherapy. The tolerant state is maintained by continued administration of allergen, and a long-lasting immune tolerance develops as maintenance therapy continues.
What does the future offer?
Allergen immunotherapy is a unique treatment, one of only a few that can truly be said to fundamentally alter a disease state. Therefore, we approach advances in immunotherapy with caution: what can we improve without losing the core benefits? Clearly, we focus on the disadvantages of standard subcutaneous immunotherapy. It is time-consuming both in frequency of treatments and total duration of therapy, it needs to be administered by trained professionals (and is therefore expensive), it requires injections, which are not acceptable to all patients and it is potentially life-threatening. These factors severely restrict the number of individuals who can take advantage of this treatment. If we are to realize the tantalizing prospect of altering the natural history of allergy in a substantial proportion of allergy patients, and even in the population as a whole, then immunotherapy will need to be dramatically different from what is used routinely today.
Modification of allergen
Allergens extracted from their natural source have been in routine use since the inception of SCIT. Standardization of the potency of these biologically variable products represented a major advance and has led to improved safety and efficacy. Various modifications of the allergen have been attempted to increase potency and specificity and to reduce the risk of acute reactions. Allergoid production by formaldehyde treatment of native antigen has long been used, but is associated with reduced efficacy in allergen immunotherapy.
Short peptides, unable to cross-link IgE and induce mast cell degranulation, but able to activate T cells through presentation on human leucocyte antigen (HLA) class II, were shown to induce Th1 reactivity. Initial clinical studies using these short peptides were hampered by significant delayed adverse reactions, probably related to T cell activation. Lower-dose intradermal treatment has been better tolerated and associated with improvement in airway hyper-responsiveness, late-phase skin test response to whole allergen, reduction in nasal symptoms together with up-regulation of CD4+ T cells producing IFN-γ cells but not regulatory T cells following cat peptide immunotherapy [126–130].
It is also possible to induce in-vivo production of allergen by vaccinating with DNA encoding the allergen. While this often produces a Th1-biased response, it is highly dependent on the DNA construct and mode of delivery. Clinical studies of these agents have not progressed [131]. Recombinant allergens offer the hope of better standardization, but their biological efficacy has been uncertain. Recombinant BetV1 protein has also been proven to be as effective as native BetV1 or conventional birch pollen extract in birch pollen SCIT [132,133], and in a recent clinical trial recombinant grass pollen vaccine has also been shown to be clinically safe and effective [134]. Use of recombinant allergens may not only be safer, but may also allow patient-specific vaccines to be produced based on the individual's in vitro IgE reactivity pattern. While current native allergen vaccines modulate the patient's existing allergen-specific IgE, they can also induce new sensitizations to other epitopes of the allergen, previously not present in the patient's serum. The clinical consequences of this, if any, are not known, so any clinical advantage of vaccines based on component-resolved diagnostics remains to be demonstrated.
Adjuvants
Enhancement of the allergen with adjuvants itself is not new. Enzyme-potentiated immunotherapy represented an early attempt to increase the potency of the allergen by adding a β-glucuronidase, protamine sulphate and cyclohexanediol. It was not widely adopted, and was shown subsequently to be ineffective [135].
Another adjuvant, monophosphoryl lipid A (MPL) has been investigated in allergy vaccines. MPL is a purified lipopolysaccharide extracted from the cell walls of Salmonella minnesota[136–138] and induces a Th1 response via Toll-like receptor-4. A large recent multi-centre study with pollen allergoids adsorbed on L-tyrosine formulated with MPL has shown good efficacy and tolerability. Other adjuvants that have been investigated for their strong Th1-evoking ability include immunostimulatory DNA sequences [139] (ISS) and heat-killed Mycobacterium vaccae[140]. The latter need further investigation in clinical trials.
Many alternative modes of allergen delivery for specific immunotherapy (SIT) aim to induce a T cell response but avoid IgE-binding. Because allergen is presented to T cells in the context of MHC class II, steering allergen towards this pathway is an attractive possibility. Proof of concept and early clinical success has been achieved by fusing recombinant allergen to an intracellular targeting molecule which promotes trafficking into the lysosome, where it is loaded directly onto class II molecules [141].
Intralymphatic allergen immunotherapy
Intralymphatic injection into subcutaneous lymph nodes (ILIT) is a novel and potentially attractive alternative. Randomized controlled trials in more than 200 patients have shown efficacy in reducing symptoms, and immunomodulatory effects have been seen with doses a tiny fraction of those used in conventional SCIT. In a randomized study in hay fever sufferers, a short protocol of three intralymphatic injections of grass pollen extract over 8 weeks resulted in improvements in symptomatic and laboratory parameters comparable to that achieved with conventional SCIT, even after 3 years [142]. No systemic reactions to ILIT occurred during these studies.
Allergen immunotherapy and anti-IgE monoclonal antibody
Another area of interest is the combination of SCIT with anti-IgE humanized monoclonal antibody. There is some evidence that this approach may induce a synergistic effect with respect to clinical efficacy and enhance safety of accelerated protocols [143,144], but cost of treatment would be the important deterrent.
Conclusion
Allergen-specific immunotherapy is a safe and effective method of treatment for allergic rhinitis and hymenoptera venom allergy, provided this is delivered in a safe and controlled environment with robust patient selection criteria and by a specialist with knowledge and experience in this field. There is emerging evidence that allergen-specific immunotherapy may be indicated early in the course of allergic rhinitis in order to prevent progress of ‘allergic march’ and development of newer sensitizations. It is likely that the future will see better vaccines with reduced allergenicity and greater immunogenicity in order to make them even more safe and efficacious. There may be a role for anti-IgE humanized monoclonal antibody alongside allergen immunotherapy, and studies are under way.
Drug desensitization is gaining popularity, as recent reports have highlighted its success across a range of drugs inducing immediate hypersensitivity responses.
Understanding of the precise mechanisms underlying desensitization will pave the way to development of novel immunomodulatory therapies.
Disclosure
Dr M. T. Krishna is a member of Standards of Care Committee of British Society for Allergy and Clinical Immunology and is the lead author of the guideline ‘Diagnosis and Management of Hymenoptera Venom Allergy’ (submitted for publication).
References
- 1.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;i:1572–3. [Google Scholar]
- 2.Freeman J. Further observations on the treatment of hay fever by hypodermic inoculation of pollen vaccine. Lancet. 1911;ii:814–7. [PubMed] [Google Scholar]
- 3.Roder E, Berger MY, de Groot H, van Wijk RG. Immunotherapy in children and adolescents with allergic rhinoconjunctivitis: a systematic review. Pediatr Allergy Immunol. 2008;19:197–207. doi: 10.1111/j.1399-3038.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 4.Calamita Z, Saconato H, Pela AB, Atallah AN. Efficacy of sublingual immunotherapy in asthma: systematic review of randomized-clinical trials using the Cochrane Collaboration method. Allergy. 2006;61:1162–72. doi: 10.1111/j.1398-9995.2006.01205.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12. doi: 10.1111/j.1398-9995.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson MD, Sellins S, Grube E, et al. Aeroallergen sensitization in healthy children: racial and socioeconomic correlates. J Pediatr. 2007;151:187–91. doi: 10.1016/j.jpeds.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King MJ, Tamulis T, Lockey RF. Prick puncture skin tests and serum specific IgE as predictors of nasal challenge response to dermatophagoides pteronyssinus in older adults. Ann Allergy Asthma Immunol. 2008;101:12–7. doi: 10.1016/S1081-1206(10)60828-9. [DOI] [PubMed] [Google Scholar]
- 8.Rasanen L, Kuusisto P, Penttila M, Nieminen M, Savolainen J, Lehto M. Comparison of immunologic tests in the diagnosis of occupational asthma and rhinitis. Allergy. 1994;49:342–7. doi: 10.1111/j.1398-9995.1994.tb02279.x. [DOI] [PubMed] [Google Scholar]
- 9.Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19–42. doi: 10.1111/j.1365-2222.2007.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Rienzo V, Marcucci F, Puccinelli P, et al. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy. 2003;33:206–10. doi: 10.1046/j.1365-2222.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 11.Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851–7. doi: 10.1016/j.jaci.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–7. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 13.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 14.Frew AJ, Powell RJ, Corrigan CJ, Durham SR. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319–25. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy. 2003;33:1076–82. doi: 10.1046/j.1365-2222.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 16.Bousquet J, Hejjaoui A, Dhivert H, Clauzel AM, Michel FB. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. Systemic reactions during the rush protocol in patients suffering from asthma. J Allergy Clin Immunol. 1989;83:797–802. doi: 10.1016/0091-6749(89)90017-1. [DOI] [PubMed] [Google Scholar]
- 17.Andre C, Fadel R. Anaphylaxis caused by allergen sublingual immunotherapy? Allergy. 2007;62:1220–1. doi: 10.1111/j.1398-9995.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 18.Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117:1021–35. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 19.Dunsky EH, Goldstein MF, Dvorin DJ, Belecanech GA. Anaphylaxis to sublingual immunotherapy. Allergy. 2006;61:1235. doi: 10.1111/j.1398-9995.2006.01137.x. [DOI] [PubMed] [Google Scholar]
- 20.Eifan AO, Keles S, Bahceciler NN, Barlan IB. Anaphylaxis to multiple pollen allergen sublingual immunotherapy. Allergy. 2007;62:567–8. doi: 10.1111/j.1398-9995.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 21.Gidaro GB, Marcucci F, Sensi L, Incorvaia C, Frati F, Ciprandi G. The safety of sublingual-swallow immunotherapy: an analysis of published studies. Clin Exp Allergy. 2005;35:565–71. doi: 10.1111/j.1365-2222.2005.02240.x. [DOI] [PubMed] [Google Scholar]
- 22.Passalacqua G, Pawankar R, Baena-Cagnani CE, Canonica GW. Sublingual immunotherapy: where do we stand? Present and future. Curr Opin Allergy Clin Immunol. 2009;9:1–3. doi: 10.1097/ACI.0b013e3283196a9b. [DOI] [PubMed] [Google Scholar]
- 23.Lombardi C, Gargioni S, Cottini M, Canonica GW, Passalacqua G. The safety of sublingual immunotherapy with one or more allergens in adults. Allergy. 2008;63:375–6. doi: 10.1111/j.1398-9995.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- 24.Bowen T, Greenbaum J, Charbonneau Y, et al. Canadian trial of sublingual swallow immunotherapy for ragweed rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2004;93:425–30. doi: 10.1016/S1081-1206(10)61408-1. [DOI] [PubMed] [Google Scholar]
- 25.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–9. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 26.Passalacqua G, Albano M, Riccio A, et al. Clinical and immunologic effects of a rush sublingual immunotherapy to Parietaria species: a double-blind, placebo-controlled trial. J Allergy Clin Immunol. 1999;104:964–8. doi: 10.1016/s0091-6749(99)70076-x. [DOI] [PubMed] [Google Scholar]
- 27.Bordignon V, Parmiani S. Variation of the skin end-point in patients treated with sublingual specific immunotherapy. J Invest Allergol Clin Immunol. 2003;13:170–6. [PubMed] [Google Scholar]
- 28.Lombardi C, Gargioni S, Venturi S, Zoccali P, Canonica GW, Passalacqua G. Controlled study of preseasonal immunotherapy with grass pollen extract in tablets: effect on bronchial hyperreactivity. J Invest Allergol Clin Immunol. 2001;11:41–5. [PubMed] [Google Scholar]
- 29.Calderon MA, Birk AO, Andersen JS, Durham SR. Prolonged preseasonal treatment phase with Grazax sublingual immunotherapy increases clinical efficacy. Allergy. 2007;62:958–61. doi: 10.1111/j.1398-9995.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 30.Durham SR, Emminger W, Kapp A, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125:131–8. doi: 10.1016/j.jaci.2009.10.035. e1–7. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen L, Nuchel Petersen B, Wihl JA, Lowenstein H, Ipsen H. Immunotherapy with partially purified and standardized tree pollen extracts. IV. Results from long-term (6-year) follow-up. Allergy. 1997;52:914–20. doi: 10.1111/j.1398-9995.1997.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 32.Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–6. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 33.Mosbech H, Osterballe O. Does the effect of immunotherapy last after termination of treatment? Follow-up study in patients with grass pollen rhinitis. Allergy. 1988;43:523–9. doi: 10.1111/j.1398-9995.1988.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 34.Walker SM, Varney VA, Gaga M, Jacobson MR, Durham SR. Grass pollen immunotherapy: efficacy and safety during a 4-year follow-up study. Allergy. 1995;50:405–13. doi: 10.1111/j.1398-9995.1995.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 35.Hunt KJ, Valentine MD, Sobotka AK, Benton AW, Amodio FJ, Lichtenstein LM. A controlled trial of immunotherapy in insect hypersensitivity. N Engl J Med. 1978;299:157–61. doi: 10.1056/NEJM197807272990401. [DOI] [PubMed] [Google Scholar]
- 36.Moffitt JE, Golden DB, Reisman RE, et al. Stinging insect hypersensitivity: a practice parameter update. J Allergy Clin Immunol. 2004;114:869–86. doi: 10.1016/j.jaci.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Bonifazi F, Jutel M, Bilo BM, Birnbaum J, Muller U. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy. 2005;60:1459–70. doi: 10.1111/j.1398-9995.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 38.Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–85. doi: 10.1016/j.jaci.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339–49. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 40.Muller U, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol. 1992;89:529–35. doi: 10.1016/0091-6749(92)90319-w. [DOI] [PubMed] [Google Scholar]
- 41.Mosbech H, Malling HJ, Biering I, et al. Immunotherapy with yellow jacket venom. A comparative study including three different extracts, one adsorbed to aluminium hydroxide and two unmodified. Allergy. 1986;41:95–103. doi: 10.1111/j.1398-9995.1986.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 42.Gillman SA, Cummins LH, Kozak PP, Jr, Hoffman DR. Venom immunotherapy: comparison of ‘rush’ vs ‘conventional’ schedules. Ann Allergy. 1980;45:351–4. [PubMed] [Google Scholar]
- 43.Oude Elberink JN, De Monchy JG, Van Der Heide S, Guyatt GH, Dubois AE. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. J Allergy Clin Immunol. 2002;110:174–82. doi: 10.1067/mai.2002.125827. [DOI] [PubMed] [Google Scholar]
- 44.Oude Elberink JN, van der Heide S, Guyatt GH, Dubois AE. Immunotherapy improves health-related quality of life of adult patients with dermal reactions following yellow jacket stings. Clin Exp Allergy. 2009;39:883–9. doi: 10.1111/j.1365-2222.2009.03230.x. [DOI] [PubMed] [Google Scholar]
- 45.Bonadonna P, Perbellini O, Passalacqua G, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J Allergy Clin Immunol. 2009;123:680–6. doi: 10.1016/j.jaci.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 46.Bonadonna P, Zanotti R, Caruso B, et al. Allergen specific immunotherapy is safe and effective in patients with systemic mastocytosis and Hymenoptera allergy. J Allergy Clin Immunol. 2008;121:256–7. doi: 10.1016/j.jaci.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez de Olano D, Alvarez-Twose I, Esteban-Lopez MI, et al. Safety and effectiveness of immunotherapy in patients with indolent systemic mastocytosis presenting with Hymenoptera venom anaphylaxis. J Allergy Clin Immunol. 2008;121:519–26. doi: 10.1016/j.jaci.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Haeberli G, Bronnimann M, Hunziker T, Muller U. Elevated basal serum tryptase and hymenoptera venom allergy: relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clin Exp Allergy. 2003;33:1216–20. doi: 10.1046/j.1365-2222.2003.01755.x. [DOI] [PubMed] [Google Scholar]
- 49.Ludolph-Hauser D, Rueff F, Fries C, Schopf P, Przybilla B. Constitutively raised serum concentrations of mast-cell tryptase and severe anaphylactic reactions to Hymenoptera stings. Lancet. 2001;357:361–2. doi: 10.1016/S0140-6736(00)03647-3. [DOI] [PubMed] [Google Scholar]
- 50.Wagner N, Fritze D, Przybilla B, Hagedorn M, Rueff F. Fatal anaphylactic sting reaction in a patient with mastocytosis. Int Arch Allergy Immunol. 2008;146:162–3. doi: 10.1159/000113520. [DOI] [PubMed] [Google Scholar]
- 51.Caruso B, Bonadonna P, Severino MG, et al. Evaluation of the IgE cross-reactions among vespid venoms. A possible approach for the choice of immunotherapy. Allergy. 2007;62:561–4. doi: 10.1111/j.1398-9995.2007.01353.x. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman DR. Allergens in Hymenoptera venom. XXV: The amino acid sequences of antigen 5 molecules and the structural basis of antigenic cross-reactivity. J Allergy Clin Immunol. 1993;92:707–16. doi: 10.1016/0091-6749(93)90014-7. [DOI] [PubMed] [Google Scholar]
- 53.King TP, Lu G, Gonzalez M, Qian N, Soldatova L. Yellow jacket venom allergens, hyaluronidase and phospholipase: sequence similarity and antigenic cross-reactivity with their hornet and wasp homologs and possible implications for clinical allergy. J Allergy Clin Immunol. 1996;98:588–600. doi: 10.1016/s0091-6749(96)70093-3. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman DR, Gillman SA, Cummins LH, Kozak PP, Jr, Oswald A. Correlation of IgG and IgE antibody levels to honey bee venom allergens with protection to sting challenge. Ann Allergy. 1981;46:17–23. [PubMed] [Google Scholar]
- 55.Mueller U, Reisman R, Wypych J, et al. Comparison of vespid venoms collected by electrostimulation and by venom sac extraction. J Allergy Clin Immunol. 1981;68:254–61. doi: 10.1016/0091-6749(81)90148-2. [DOI] [PubMed] [Google Scholar]
- 56.Reisman RE, Mueller U, Wypych J, Elliott W, Arbesman CE. Comparison of the allergenicity and antigenicity of yellow jacket and hornet venoms. J Allergy Clin Immunol. 1982;69:268–74. doi: 10.1016/s0091-6749(82)80003-1. [DOI] [PubMed] [Google Scholar]
- 57.Chipps BE, Valentine MD, Kagey-Sobotka A, Schuberth KC, Lichtenstein LM. Diagnosis and treatment of anaphylactic reactions to Hymenoptera stings in children. J Pediatr. 1980;97:177–84. doi: 10.1016/s0022-3476(80)80470-7. [DOI] [PubMed] [Google Scholar]
- 58.Lockey RF, Turkeltaub PC, Olive ES, Hubbard JM, Baird-Warren IA, Bukantz SC. The Hymenoptera venom study. III: Safety of venom immunotherapy. J Allergy Clin Immunol. 1990;86:775–80. doi: 10.1016/s0091-6749(05)80182-4. [DOI] [PubMed] [Google Scholar]
- 59.Ramirez DA, Londono S, Evans R., III Adverse reactions to venom immunotherapy. Ann Allergy. 1981;47:435–9. [PubMed] [Google Scholar]
- 60.Wyss M, Scheitlin T, Stadler BM, Wuthrich B. Immunotherapy with aluminum hydroxide adsorbed insect venom extracts (Alutard SQ): immunologic and clinical results of a prospective study over 3 years. Allergy. 1993;48:81–6. doi: 10.1111/j.1398-9995.1993.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 61.Brehler R, Wolf H, Kutting B, Schnitker J, Luger T. Safety of a two-day ultrarush insect venom immunotherapy protocol in comparison with protocols of longer duration and involving a larger number of injections. J Allergy Clin Immunol. 2000;105:1231–5. doi: 10.1067/mai.2000.105708. [DOI] [PubMed] [Google Scholar]
- 62.Laurent J, Smiejan JM, Bloch-Morot E, Herman D. Safety of Hymenoptera venom rush immunotherapy. Allergy. 1997;52:94–6. doi: 10.1111/j.1398-9995.1997.tb02551.x. [DOI] [PubMed] [Google Scholar]
- 63.Sturm G, Kranke B, Rudolph C, Aberer W. Rush Hymenoptera venom immunotherapy: a safe and practical protocol for high-risk patients. J Allergy Clin Immunol. 2002;110:928–33. doi: 10.1067/mai.2002.129124. [DOI] [PubMed] [Google Scholar]
- 64.Birnbaum J, Ramadour M, Magnan A, Vervloet D. Hymenoptera ultra-rush venom immunotherapy (210 min): a safety study and risk factors. Clin Exp Allergy. 2003;33:58–64. doi: 10.1046/j.1365-2222.2003.01564.x. [DOI] [PubMed] [Google Scholar]
- 65.Reimers A, Hari Y, Muller U. Reduction of side-effects from ultrarush immunotherapy with honeybee venom by pretreatment with fexofenadine: a double-blind, placebo-controlled trial. Allergy. 2000;55:484–8. doi: 10.1034/j.1398-9995.2000.00520.x. [DOI] [PubMed] [Google Scholar]
- 66.Diwakar L, Noorani S, Huissoon AP, Frew AJ, Krishna MT. Practice of venom immunotherapy in the United Kingdom: a national audit and review of literature. Clin Exp Allergy. 2008;38:1651–8. doi: 10.1111/j.1365-2222.2008.03044.x. [DOI] [PubMed] [Google Scholar]
- 67.Bernstein JA, Kagen SL, Bernstein DI, Bernstein IL. Rapid venom immunotherapy is safe for routine use in the treatment of patients with Hymenoptera anaphylaxis. Ann Allergy. 1994;73:423–8. [PubMed] [Google Scholar]
- 68.Birnbaum J, Charpin D, Vervloet D. Rapid Hymenoptera venom immunotherapy: comparative safety of three protocols. Clin Exp Allergy. 1993;23:226–30. doi: 10.1111/j.1365-2222.1993.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 69.Golden DB, Valentine MD, Kagey-Sobotka A, Lichtenstein LM. Regimens of Hymenoptera venom immunotherapy. Ann Intern Med. 1980;92:620–4. doi: 10.7326/0003-4819-92-5-620. [DOI] [PubMed] [Google Scholar]
- 70.Rueff F, Wenderoth A, Przybilla B. Patients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol. 2001;108:1027–32. doi: 10.1067/mai.2001.119154. [DOI] [PubMed] [Google Scholar]
- 71.Niedoszytko M, de Monchy J, van Doormaal JJ, Jassem E, Oude Elberink JN. Mastocytosis and insect venom allergy: diagnosis, safety and efficacy of venom immunotherapy. Allergy. 2009;64:1237–45. doi: 10.1111/j.1398-9995.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 72.Golden DB. Discontinuing venom immunotherapy. Curr Opin Allergy Clin Immunol. 2001;1:353–6. doi: 10.1097/01.all.0000011038.45505.c6. [DOI] [PubMed] [Google Scholar]
- 73.Lerch E, Muller UR. Long-term protection after stopping venom immunotherapy: results of re-stings in 200 patients. J Allergy Clin Immunol. 1998;101:606–12. doi: 10.1016/S0091-6749(98)70167-8. [DOI] [PubMed] [Google Scholar]
- 74.Blaauw PJ, Smithuis LO. The evaluation of the common diagnostic methods of hypersensitivity for bee and yellow jacket venom by means of an in-hospital insect sting. J Allergy Clin Immunol. 1985;75:556–62. doi: 10.1016/0091-6749(85)90029-6. [DOI] [PubMed] [Google Scholar]
- 75.Michils A, Baldassarre S, Ledent C, Mairesse M, Gossart B, Duchateau J. Early effect of ultrarush venom immunotherapy on the IgG antibody response. Allergy. 2000;55:455–62. doi: 10.1034/j.1398-9995.2000.00412.x. [DOI] [PubMed] [Google Scholar]
- 76.Wilson AB, Deighton J, Lachmann PJ, Ewan PW. A comparative study of IgG subclass antibodies in patients allergic to wasp or bee venom. Allergy. 1994;49:272–80. doi: 10.1111/j.1398-9995.1994.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 77.Ewan PW, Deighton J, Wilson AB, Lachmann PJ. Venom-specific IgG antibodies in bee and wasp allergy: lack of correlation with protection from stings. Clin Exp Allergy. 1993;23:647–60. doi: 10.1111/j.1365-2222.1993.tb01791.x. [DOI] [PubMed] [Google Scholar]
- 78.Golden DB, Kagey-Sobotka A, Lichtenstein LM. Survey of patients after discontinuing venom immunotherapy. J Allergy Clin Immunol. 2000;105:385–90. doi: 10.1016/s0091-6749(00)90092-7. [DOI] [PubMed] [Google Scholar]
- 79.Bernstein DI, Wanner M, Borish L, Liss GM. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol. 2004;113:1129–36. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Mueller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337–41. doi: 10.1097/ACI.0b013e328259c328. [DOI] [PubMed] [Google Scholar]
- 81.Muller UR, Haeberli G. Use of beta-blockers during immunotherapy for Hymenoptera venom allergy. J Allergy Clin Immunol. 2005;115:606–10. doi: 10.1016/j.jaci.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 82.Ober AI, MacLean JA, Hannaway PJ. Life-threatening anaphylaxis to venom immunotherapy in a patient taking an angiotensin-converting enzyme inhibitor. J Allergy Clin Immunol. 2003;112:1008–9. doi: 10.1016/s0091-6749(03)01940-7. [DOI] [PubMed] [Google Scholar]
- 83.White KM, England RW. Safety of angiotensin-converting enzyme inhibitors while receiving venom immunotherapy. Ann Allergy Asthma Immunol. 2008;101:426–30. doi: 10.1016/S1081-1206(10)60321-3. [DOI] [PubMed] [Google Scholar]
- 84.Berchtold E, Maibach R, Muller U. Reduction of side effects from rush-immunotherapy with honey bee venom by pretreatment with terfenadine. Clin Exp Allergy. 1992;22:59–65. doi: 10.1111/j.1365-2222.1992.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 85.Brockow K, Kiehn M, Riethmuller C, Vieluf D, Berger J, Ring J. Efficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: a prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol. 1997;100:458–63. doi: 10.1016/s0091-6749(97)70135-0. [DOI] [PubMed] [Google Scholar]
- 86.Spangfort MD, Larsen JN. Standardization of allergen-specific immunotherapy vaccines. Immunol Allergy Clin North Am. 2006;26:191–206. doi: 10.1016/j.iac.2006.02.012. v–vi. [DOI] [PubMed] [Google Scholar]
- 87.Larsen JN, Houghton CG, Lowenstein H, Lombardero M. Manufacturing and standardizing allergen extracts in Europe. Clin Allergy Immunol. 2004;18:433–55. [PubMed] [Google Scholar]
- 88.Lowenstein H. Purification and characterization of allergens. Summing-up. Allergy. 1980;35:210–11. doi: 10.1111/j.1398-9995.1980.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 89.Rueff F, Wolf H, Schnitker J, Ring J, Przybilla B. Specific immunotherapy in honeybee venom allergy: a comparative study using aqueous and aluminium hydroxide adsorbed preparations. Allergy. 2004;59:589–95. doi: 10.1111/j.1398-9995.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 90.Wilcock LK, Francis JN, Durham SR. Aluminium hydroxide down-regulates T helper 2 responses by allergen-stimulated human peripheral blood mononuclear cells. Clin Exp Allergy. 2004;34:1373–8. doi: 10.1111/j.1365-2222.2004.02052.x. [DOI] [PubMed] [Google Scholar]
- 91.Alexander S, Hopewell S, Hunter S, Chouksey A. Rituximab and desensitization for a patient with severe factor IX deficiency, inhibitors, and history of anaphylaxis. J Pediatr Hematol Oncol. 2008;30:93–5. doi: 10.1097/MPH.0b013e31815cf742. [DOI] [PubMed] [Google Scholar]
- 92.Alijotas-Reig J, San Miguel-Moncin M, Cistero-Bahima A. Aspirin desensitization in the treatment of antiphospholipid syndrome during pregnancy in ASA-sensitive patients. Am J Reprod Immunol. 2006;55:45–50. doi: 10.1111/j.1600-0897.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 93.Borish L, Tamir R, Rosenwasser LJ. Intravenous desensitization to beta-lactam antibiotics. J Allergy Clin Immunol. 1987;80:314–9. doi: 10.1016/0091-6749(87)90037-6. [DOI] [PubMed] [Google Scholar]
- 94.Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology. 2003;8:359–64. doi: 10.1046/j.1440-1843.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 95.Gea-Banacloche JC, Metcalfe DD. Ciprofloxacin desensitization. J Allergy Clin Immunol. 1996;97:1426–7. doi: 10.1016/s0091-6749(96)70218-x. [DOI] [PubMed] [Google Scholar]
- 96.Lee CW, Matulonis UA, Castells MC. Carboplatin hypersensitivity: a 6-h 12-step protocol effective in 35 desensitizations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol. 2004;95:370–6. doi: 10.1016/j.ygyno.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Lee JY, Simon RA, Stevenson DD. Selection of aspirin dosages for aspirin desensitization treatment in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2007;119:157–64. doi: 10.1016/j.jaci.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 98.Matheu V, Perez E, Hernandez M, et al. Insulin allergy and resistance successfully treated by desensitization with Aspart insulin. Clin Mol Allergy. 2005;3:16. doi: 10.1186/1476-7961-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with Type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23:204–6. doi: 10.1111/j.1464-5491.2006.01811.x. [DOI] [PubMed] [Google Scholar]
- 100.Puchner TC, Kugathasan S, Kelly KJ, Binion DG. Successful desensitization and therapeutic use of infliximab in adult and pediatric Crohn's disease patients with prior anaphylactic reaction. Inflamm Bowel Dis. 2001;7:34–7. doi: 10.1097/00054725-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 101.Silberman S, Neukirch-Stoop C, Steg PG. Rapid desensitization procedure for patients with aspirin hypersensitivity undergoing coronary stenting. Am J Cardiol. 2005;95:509–10. doi: 10.1016/j.amjcard.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 102.Wong JT, Ripple RE, MacLean JA, Marks DR, Bloch KJ. Vancomycin hypersensitivity: synergism with narcotics and ‘desensitization’ by a rapid continuous intravenous protocol. J Allergy Clin Immunol. 1994;94:189–94. doi: 10.1016/0091-6749(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 103.Castells M. Rapid desensitization for hypersensitivity reactions to medications. Immunol Allergy Clin North Am. 2009;29:585–606. doi: 10.1016/j.iac.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 104.Wendel GD, Jr, Stark BJ, Jamison RB, Molina RD, Sullivan TJ. Penicillin allergy and desensitization in serious infections during pregnancy. N Engl J Med. 1985;312:1229–32. doi: 10.1056/NEJM198505093121905. [DOI] [PubMed] [Google Scholar]
- 105.Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Pettinato R, Gueant JL. Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J Med. 2006;354:2835–7. doi: 10.1056/NEJMc053529. [DOI] [PubMed] [Google Scholar]
- 106.Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med. 2001;345:804–9. doi: 10.1056/NEJMra993637. [DOI] [PubMed] [Google Scholar]
- 107.Zeiss CR, Lockey RF. Refractory period to aspirin in a patient with aspirin-induced asthma. J Allergy Clin Immunol. 1976;57:440–8. doi: 10.1016/0091-6749(76)90059-2. [DOI] [PubMed] [Google Scholar]
- 108.Macy E, Bernstein JA, Castells MC, et al. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol. 2007;98:172–4. doi: 10.1016/S1081-1206(10)60692-8. [DOI] [PubMed] [Google Scholar]
- 109.Stevenson DD, Simon RA. Selection of patients for aspirin desensitization treatment. J Allergy Clin Immunol. 2006;118:801–4. doi: 10.1016/j.jaci.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 110.Stevenson DD, Pleskow WW, Simon RA, et al. Aspirin-sensitive rhinosinusitis asthma: a double-blind crossover study of treatment with aspirin. J Allergy Clin Immunol. 1984;73:500–7. doi: 10.1016/0091-6749(84)90361-0. [DOI] [PubMed] [Google Scholar]
- 111.Sweet JM, Stevenson DD, Simon RA, Mathison DA. Long-term effects of aspirin desensitization – treatment for aspirin-sensitive rhinosinusitis-asthma. J Allergy Clin Immunol. 1990;85:59–65. doi: 10.1016/0091-6749(90)90222-p. [DOI] [PubMed] [Google Scholar]
- 112.Kook H, Kim KM, Choi SH, et al. Life-threatening carboplatin hypersensitivity during conditioning for autologous PBSC transplantation: successful rechallenge after desensitization. Bone Marrow Transplant. 1998;21:727–9. doi: 10.1038/sj.bmt.1701161. [DOI] [PubMed] [Google Scholar]
- 113.Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 114.Markman M, Zanotti K, Peterson G, Kulp B, Webster K, Belinson J. Expanded experience with an intradermal skin test to predict for the presence or absence of carboplatin hypersensitivity. J Clin Oncol. 2003;21:4611–4. doi: 10.1200/JCO.2003.05.539. [DOI] [PubMed] [Google Scholar]
- 115.Zanotti KM, Rybicki LA, Kennedy AW, et al. Carboplatin skin testing: a skin-testing protocol for predicting hypersensitivity to carboplatin chemotherapy. J Clin Oncol. 2001;19:3126–9. doi: 10.1200/JCO.2001.19.12.3126. [DOI] [PubMed] [Google Scholar]
- 116.Akdis CA. New insights into mechanisms of immunoregulation in 2007. J Allergy Clin Immunol. 2008;122:700–9. doi: 10.1016/j.jaci.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 117.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. quiz 47–8. [DOI] [PubMed] [Google Scholar]
- 118.Akdis M, Akdis CA. Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov. 2009;8:645–60. doi: 10.1038/nrd2653. [DOI] [PubMed] [Google Scholar]
- 119.Durham SR. Mechanisms of immunotherapy. Drugs Today (Barc) 2008;44(Suppl B):93–4. [PubMed] [Google Scholar]
- 120.James LK, Durham SR. Update on mechanisms of allergen injection immunotherapy. Clin Exp Allergy. 2008;38:1074–88. doi: 10.1111/j.1365-2222.2008.02976.x. [DOI] [PubMed] [Google Scholar]
- 121.Durham SR, Ying S, Varney VA, et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-gamma. J Allergy Clin Immunol. 1996;97:1356–65. doi: 10.1016/s0091-6749(96)70205-1. [DOI] [PubMed] [Google Scholar]
- 122.Hamid QA, Schotman E, Jacobson MR, Walker SM, Durham SR. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J Allergy Clin Immunol. 1997;99:254–60. doi: 10.1016/s0091-6749(97)70106-4. [DOI] [PubMed] [Google Scholar]
- 123.Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121:1120–5. doi: 10.1016/j.jaci.2008.01.072. e2. [DOI] [PubMed] [Google Scholar]
- 124.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111:1255–61. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 125.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–72. doi: 10.1016/j.jaci.2008.03.013. 72 e1. [DOI] [PubMed] [Google Scholar]
- 126.Wachholz PA, Nouri-Aria KT, Wilson DR, et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1:Th2 cytokine ratios. Immunology. 2002;105:56–62. doi: 10.1046/j.1365-2567.2002.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alexander C, Tarzi M, Larche M, Kay AB. The effect of Fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy. 2005;60:1269–74. doi: 10.1111/j.1398-9995.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 128.Alexander C, Ying S, Barry Kay A, Larche M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005;35:52–8. doi: 10.1111/j.1365-2222.2005.02143.x. [DOI] [PubMed] [Google Scholar]
- 129.Smith TR, Alexander C, Kay AB, Larche M, Robinson DS. Cat allergen peptide immunotherapy reduces CD4(+) T cell responses to cat allergen but does not alter suppression by CD4(+) CD25(+) T cells: a double-blind placebo-controlled study. Allergy. 2004;59:1097–101. doi: 10.1111/j.1398-9995.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 130.Larche M. Update on the current status of peptide immunotherapy. J Allergy Clin Immunol. 2007;119:906–9. doi: 10.1016/j.jaci.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 131.Chua KY, Kuo IC, Huang CH. DNA vaccines for the prevention and treatment of allergy. Curr Opin Allergy Clin Immunol. 2009;9:50–4. doi: 10.1097/ACI.0b013e3283207ad8. [DOI] [PubMed] [Google Scholar]
- 132.Purohit A, Niederberger V, Kronqvist M, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–25. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- 133.Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 134.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 135.Radcliffe MJ, Lewith GT, Turner RG, Prescott P, Church MK, Holgate ST. Enzyme potentiated desensitization in treatment of seasonal allergic rhinitis: double blind randomised controlled study. BMJ. 2003;327:251–4. doi: 10.1136/bmj.327.7409.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Drachenberg KJ, Heinzkill M, Urban E, Woroniecki SR. Efficacy and tolerability of short-term specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A (MPL) for children and adolescents. Allergol Immunopathol (Madr) 2003;31:270–7. doi: 10.1016/s0301-0546(03)79195-2. [DOI] [PubMed] [Google Scholar]
- 137.Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- 138.Mothes N, Heinzkill M, Drachenberg KJ, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 139.Tighe H, Takabayashi K, Schwartz D, et al. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J Allergy Clin Immunol. 2000;106:124–34. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- 140.Camporota L, Corkhill A, Long H, et al. The effects of Mycobacterium vaccae on allergen-induced airway responses in atopic asthma. Eur Respir J. 2003;21:287–93. doi: 10.1183/09031936.03.00042103. [DOI] [PubMed] [Google Scholar]
- 141.Crameri R, Kundig TM, Akdis CA. Modular antigen-translocation as a novel vaccine strategy for allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9:568–73. doi: 10.1097/ACI.0b013e3283310fdf. [DOI] [PubMed] [Google Scholar]
- 142.Senti G, Prinz Vavricka BM, Erdmann I, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci USA. 2008;105:17908–12. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kopp MV, Hamelmann E, Zielen S, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–9. doi: 10.1111/j.1365-2222.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 144.Kuehr J, Brauburger J, Zielen S, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109:274–80. doi: 10.1067/mai.2002.121949. [DOI] [PubMed] [Google Scholar]


