Abstract
Autoantibodies to intracellular targets in mitochondria and nuclei are serological hallmarks of primary biliary cirrhosis (PBC). One of the most recently identified cellular targets of PBC autoantibodies is a novel cytoplasmic structure referred to as GW bodies [GWB, G (glycine) W (tryptophan)-containing bodies (GWB)]. GWB are indentified as discrete cytoplasmic domains that are involved in mRNA processing via the RNA interference (RNAi) pathway. Key components of GWB include the proteins GW182, Ago2, RNA-associated protein 55 (RAP55) and Ge-1/Hedls. The primary objective was to study the frequency and clinical association of antibodies directed to GWB components, in 109 PBC patients. Autoantibodies to mitochondrial antigen–pyruvate dehydrogenase complex (M2), branched-chain 2-oxo-acid dehydrogenase complex and 2-oxo glutarate dehydrogenase complex (3E-BPO), gp210, sp100, promyelocytic leukaemia cell antigen (PML) and liver kidney microsomal-1 antigen (LKM-1) were detected by a line immunoassay and antibodies to GWB (GW182, RAP55, Ge-1, GW2, GW3) and glutamate receptor interacting protein (GRIP)-associated protein-1 (GRASP-1), by an addressable laser bead immunoassay (ALBIA). The most common GWB autoantigen targets were: RAP55-28%, GW182-12%, GW2-2% and antibodies to GRASP-1-17%. By comparison, the frequency of reactivity to established PBC autoantigens was: gp210, 27%; sp100, 27% and PML, 17%. None of the autoantibodies were associated with differences in Mayo risk score or liver decompensation. This study is the first study to show that antibodies to RAP55, GW182 and GRASP-1 are the most common GWB targets in PBC.
Keywords: autoantibodies, GW bodies, primary biliary cirrhosis, RNA interference
Introduction
Primary biliary cirrhosis (PBC) is a chronic, progressive autoimmune disorder of the liver characterized by non-suppurative inflammation of small bile ducts, which may lead ultimately to hepatic failure [1]. In addition to clinical characteristics and liver biopsy, the detection of autoantibodies is an important adjunct for the diagnosis of PBC. Although anti-mitochondrial antibodies (AMA) are specific and sensitive biomarkers for the diagnosis of PBC [2], their prognostic value is not widely accepted [3]. In the last two decades a number of other autoantibodies, including certain anti-nuclear antibodies (ANAs) [4], have been recognized as specific targets of PBC. More recently, some attention has focused upon cytoplasmic target antigens that are identified as a cytoplasmic dot staining (CDS) pattern produced by autoantibodies in PBC sera [5,6]. One of the cellular targets are mRNA processing bodies (P bodies), also known as GW bodies [GWB, G (glycine) W (tryptophan)-containing bodies (GWBs)][7], which have been reported in 5–10% of PBC sera [5,6]. In addition, autoantibodies to another recently described cytoplasmic target antigen, glutamate receptor interacting protein (GRIP)-associated protein-1 (GRASP-1), also gives a CDS pattern [8], and one study that focused upon identifying novel target antigens by immunoscreening protein arrays found that seven of seven of PBC sera reacted with this antigen [9].
A number of studies have indicated that certain autoantibodies seen in PBC are associated with certain clinical features. For example, antibodies to the nuclear envelope protein gp210 have been shown to be associated with severe disease and anti-centromere antibodies with progressive portal hypertension in a Japanese PBC cohort [10]. However, to date there have been no published reports of the association of anti-GWB with clinical features of the disease. Therefore, the goal of this project was to examine the frequency of autoantibodies to components of GWBs and other autoantibodies that give a CDS pattern (i.e. GRASP-1) and to study their relationship to other autoantibodies and clinical features in a cohort of 109 PBC patients.
Material and methods
Patients and sera
All patients were assessed and followed by one of six hepatologists at a single tertiary care medical centre in Southern Alberta, Canada (referral base of ∼1·5 million) between 2006 and 2009. Patients had a clinical diagnosis of PBC and other co-existing liver diseases were excluded by standard testing. The diagnosis of PBC was based on the presence of at least two of the following three criteria: cholestatic liver biochemistry, AMA-positivity and/or compatible liver histopathology (31 patients) [11]. One hundred and nine PBC patients were recruited to the study, and their serum samples were collected and sent to the Mitogen Advanced Diagnostics Laboratory at the University of Calgary (http://www.mitogen.ca) for autoantibody analysis. Written consent was obtained from all patients in accordance with the project approved by the Conjoint Health Ethics Review Board at the University of Calgary. Clinical and demographic data were obtained by retrospective chart review and were used to calculate the Mayo risk score (http://www.mayoclinic.org) [12]. Sera from age-matched normal (n = 500) and other disease controls [20 primary sclerosing cholangitis; 40 liver-kidney-microsome (LKM) antibody-positive autoimmune hepatitis; 50 coeliac disease] were obtained from Mitogen Advanced Diagnostic Laboratory.
Indirect immunofluorescence (IIF)
Anti-mitochondrial antibodies (AMA) were detected routinely by IIF using rodent kidney substrate and conventional techniques, as published previously [13]. In addition, each serum was screened by IIF at a dilution of 1:160 for other autoantibodies utilizing a commercially prepared human epidermoid cancer cell (HEp-2) substrate kit (HEp-2000™; ImmunoConcepts, Inc., Sacramento, CA, USA) and a heavy chain-specific, fluorescein-conjugated goat anti-human immunoglobulin (Ig)G as the secondary antibody as described previously [14]. Antibodies to dsDNA were determined by IIF using a Crithidia luciliae substrate (ImmunoConcepts, Inc.) [15]. Co-localization of cytoplasmic discrete dot staining was performed with a monoclonal antibody to GW182, a component of GW bodies [16].
Addressable laser bead immunoassay (ALBIA)
The reactivity of the sera with Sm, U1-RNP, Ro52, SS-A/Ro60, SS-B/La, ribosomal P (C22 epitope [17]), Jo-1 (histidyl-tRNA synthetase), chromatin and topoisomerase I (Scl-70) autoantigens was determined by ALBIA (QuantaPlex9; INOVA Diagnostics, Inc., San Diego, CA, USA) on a Luminex 100 flow fluorometer (Luminex Corp., Austin, TX, USA), as described elsewhere [18]. Antibodies to GWB components and other cytoplasmic targets including valosin-containing protein (VCP) [19], early endosome antigen 1 (EEA1) [20], Ge-1/Hedls [21], signal recognition particle (SRP) [22], GW182, GW2, GW3 [6], Ribo P2 [23], RNA-associated protein 55/like Sm antigen (Rap55/LSm14) [24] and cytoplasmic linker protein (CLIP-170) [25] were also assayed by ALBIA. All proteins were full-length recombinant human proteins, except GW2 and GW3, which were partial length recombinant proteins.
Line immunoassay (LIA)
Autoantibodies to antigens associated with autoimmune liver disease (M2, 3E-BPO, Sp100, PML, gp210, LKM-1, LC-1, SLA/LP, Ro52) were identified by LIA (Euroimmun, Lübeck, Germany) using the protocol supplied by the manufacturer. Similarly, autoantibodies to a spectrum of common autoantigens seen in systemic autoimmune diseases (RNP68, RNPA, RNPC, SmB, SmD, Ro/SSA60, Ro/SSA52, SSB/La, Rib-P, PCNA, CENP-B, scleroderma, Scl-70, Jo-1, histone, dsDNA) were tested in a commercially available LIA (Mikrogen GmbH, Neuried, Germany).
Western blot
Full-length recombinant GRASP-1 was produced as a polyhistidine tagged protein in pDEST-17 vector (Invitrogen Corporation, Carlsbad, CA, USA) and then purified on a nickel column (Ni-NTA spin kit; Qiagen, Inc., Valencia, CA, USA). Three µg of the purified protein was then loaded in each lane of a 10% sodium dodecyl sulphate polyacrylamide gel and after separation at 120 volts for 1·5 h was transferred to nitrocellulose sheets. Strips of nitrocellulose were then probed for reactivity with sera diluted 1:100 using a conventional immunoblot procedure, as published previously [20].
Clinical outcomes
In addition to the autoantibody profile (including AMA) and Mayo risk score, other clinical outcomes examined included laboratory values of total bilirubin, albumin, alkaline phosphatase (ALP), creatinine (Cr) and prothrombin time (INR). Hepatic decompensation was defined as the presence of ascites, varices or encephalopathy at any time point after diagnosis. Retrospective chart review was conducted to determine the presence of any other autoimmune conditions. Finally, a response to UDCA was defined as a decrease in ALP > 40% from pretreatment level or normalization of ALP after 1 year of treatment [26].
Data analysis
Demographic characteristics of the study cohort and antibody titres were described according to the median [interquartile range (IQR)] and proportions. Comparisons between groups (e.g. antibody-negative versus -positive) were made using Fisher's exact and χ2 tests for categorical variables and Mann–Whitney U-tests for continuous variables, as appropriate. All statistical analyses were performed using stata version 10 software (Stata Corp., College Station, TX, USA). A P-value less than 0·05 was considered statistically significant.
Results
Patient characteristics
The demographics and laboratory values of the 109 PBC patients included in this study are shown in Table 1. The majority (92%) was female and the median ages at diagnosis of PBC and sera collection were 53 (IQR 24–77) and 58 years (IQR 33–90), respectively. Therefore, the median duration of disease follow-up after the diagnosis of PBC was 5 years (IQR 1–28). Fatigue and/or pruritus were present in 45% of patients at the time of diagnosis (n = 49). Retrospective chart review indicated that 94% (n = 103) had a positive conventional AMA test (rodent kidney substrate) at the time of diagnosis. Demographic and clinical features did not differ between AMA-positive and -negative patients (Table 2). The median Mayo risk score at the time of disease diagnosis was 4·06 (available for 95 of 109 patients) (IQR 2·0–6·8). The median Mayo risk score at the time of sera collection was 4·05 (IQR 2·2–7·8), giving an estimated 5-year survival of 91% [12] (Table 1). The median laboratory values at the time of sera collection of total bilirubin, albumin, ALP, Cr and INR are shown in Table 1. Ninety-two per cent (n = 100) of patients were treated with ursodeoxycholic acid (UDCA) at an average daily dose of 14 mg/kg (IQR 7–27 mg/kg). Eight patients (7%) were on additional medications; seven on methotrexate (average dose 11 mg/week), one on Rituximab (1 g, two doses separated by 2 weeks) and three on colchicine (dose 1·2 mg/day). Nineteen per cent (n = 21) had co-morbid autoimmune conditions (Raynaud's, seven; systemic sclerosis, six; autoimmune thyroid disease, four; Sjögren's syndrome, four; rheumatoid arthritis, four; systemic lupus erythematosus, one; antineutrophil cytoplasmic antibodies-related glomerulonephritis, one; autoimmune hepatitis, one; coeliac disease, one; sarcoidosis, one; ulcerative colitis, one; subacute cutaneous lupus, one). Seven patients (6%) had evidence of hepatic decompensation at some point in their disease course.
Table 1.
Clinical features and laboratory findings in 109 primary biliary cirrhosis (PBC) patients at the time of sera collection.
| Female/male (%) | 92/8 |
| Age at diagnosis, years | 53 (24–77)* |
| Length of disease, years | 5 (1–28)* |
| AMA+ (%) | 94 |
| IIF+ (%) | 100 |
| Mayo risk score | 4·05 (2·2–7·8)* |
| Bilirubin, µmol/l (normal = 0–20) | 8 (2–60)* |
| Albumin, g/l (normal = 33–48) | 38 (24–44)* |
| ALP, U/L (normal = 30–145) | 126 (39–753)* |
| Cr, µmol/l (normal = 50–120) | 71 (37–157)* |
| INR (normal = 0·9–1·1) | 1 (0·9–1·2)* |
| Treatment with UDCA (%) | 92 |
| Other medications for PBC (%) | 7 |
| Other autoimmune conditions (%) | 21 |
| Liver biopsy (%) | 31 |
| Hepatic decompensation (%) | 6 |
Median (IQR) laboratory values. ALPL, alkaline phosphatase; AMA, anti-mitochondrial antibodies; Cr, creatinine; IIF, indirect immunofluorescence; INR, prothrombin time; IQR, interquartile range n, normal value; UDCA, ursodeoxycholic acid.
Table 2.
Clinical and biochemical features of anti-mitochondrial antibodies (AMA)-positive and AMA-negative primary biliary cirrhosis (PBC) patients.
| AMA-positive (n = 103) (94%) | AMA-negative (n = 6) (6%) | P-value | |
|---|---|---|---|
| Age (years) (median) | 58 (51–65) | 61 (56–75) | 0·34 |
| Female, n (%) | 94 (92) | 6 (100) | 1 |
| Mayo score (median) | 4·0 (3·6–4·6) | 4·5 (3·8–5·1) | 0·33 |
| Associated autoimmune conditions, n (%) | 20 (19) | 1 (17) | 1 |
| Hepatic decompensation, n (%) | 7 (7) | 0 | 1 |
Autoantibody results
At the time of sera collection, all PBC patients displayed a positive IIF as tested on HEp-2 cells, with a variety of staining patterns (Table 3). The most common patterns were cytoplasmic speckled/mitochondrial in 91% followed by nuclear speckled (65%) and nuclear envelope (57%). The CDS pattern was seen in 23% of the sera, and in selected sera this pattern co-localized with markers of GW bodies (Fig. 1). As expected, the most prevalent antigen targets were the mitochondrial M2 and 3E-BPO autoantigens (Table 4): 89 (82%) patients were M2-positive and 80 (73%) were 3E-BPO-positive. By comparison, 71 (65%) had both anti-M2 and anti-3E-BPO; 18 (17%) had antibodies to M2 but not 3E-BPO; nine (8%) had antibodies to 3E-BPO but not M2 and 11 (10%) did not have antibodies to either M2 or 3E-BPO. Also as measured by LIA, the frequency of reactivity to other common autoantigens associated with PBC were: gp210 27%, sp100 27% and PML 17%. None of the sera had antibodies to the liver–kidney–microsome (LKM) antigen and Table 4 shows the frequency of various autoantibodies detected in our study in comparison to the frequencies cited in other publications. Antibodies to GWB were detected in one of 500 age-matched controls, but were not detected in sera from the 20 primary sclerosing cholangitis, 40 LKM antibody-positive autoimmune hepatitis or 50 coeliac disease patient sera.
Table 3.
Prevalence of indirect immunofluorescence staining patterns of 109 primary biliary cirrhosis (PBC) sera on human epidermoid cancer cell (HEp-2) substrate.
| IIF pattern* | % | Reference |
|---|---|---|
| Cytoplasmic speckled/mitochondrial | 91 | [51] |
| Nuclear speckled | 65 | [56] |
| Nuclear envelope | 57 | [4,57,58] |
| Cytoplasmic discrete speckled | 23 | [5,51] |
| Multiple nuclear dots | 22 | [58,59] |
| Centromere | 10 | [56,60] |
| Homogeneous | 4 | [56] |
| Nucleolar | 2 | [61] |
| Midbody | 1 | [62] |
Reviewed in [63]. IIF, indirect immunofluorescence.
Fig. 1.
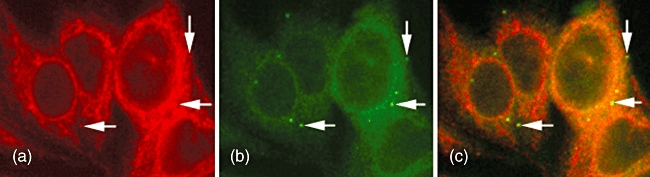
Indirect immunofluorescence (IIF) of a human primary biliary cirrhosis (PBC) serum on a conventional clinical laboratory screening substrate, human epidermoid cancer cells (HEp-2), displayed a cytoplasmic staining pattern typical of anti-mitochondrial antibodies (a). Co-localization with a monoclonal antibody 4B6 (anti-GW182) (b) revealed that the some sera also had a cytoplasmic dot staining (CDS) pattern that was masked by the anti-mitochondrial antibodies (AMA) staining but that co-localized (arrows) with GWB, G (glycine) W (tryptophan)-containing bodies (GWBs) (c). Reactivity with GWB was substantiated by addressable laser bead immunoassay (ALBIA) when it was found that these sera had high titres of antibodies to components of GWB (i.e. RAP55, GW182).
Table 4.
Antibody prevalence in the primary biliary cirrhosis (PBC) cohort compared to the literature.
| Antigen | M2 | 3E-BPO | Ro52 | RAP55 | gp210 | sp100 | PML | GRASP-1 | GW182 |
|---|---|---|---|---|---|---|---|---|---|
| Frequency % | 82 | 73 | 32 | 28 | 27 | 27 | 17 | 17 | 12 |
| Reported Frequency % | 75–96 | 57 | 25–28 | 57 | 17–25 | 20–30 | 19 | 100 | 50 |
| References | [64] | [65] | [51,53] | [9] | [36,64] | [4,35,64] | [42] | [9] | [6] |
| Antigen | CENP-B | VCP | SS-A/Ro60 | SS-B/La | SLA/LP | dsDNA | GW2 | Scl-70 | PCNA |
|---|---|---|---|---|---|---|---|---|---|
| Frequency % | 11 | 11 | 8 | 6 | 3 | 3 | 2 | 2 | 2 |
| Reported Frequency % | 10–30 | 12 | 30 | 7 | 0·6–4 | 10–17 | n.a. | 0 | n.a. |
| References | [48,66,67] | [19,52] | [53] | [66] | [64,68] | [53,69] | [67] |
| Antigen | Ribo-P | RNP | EEA1 | Chromatin | Ge-1 | Sm | Jo-1 | Histone |
|---|---|---|---|---|---|---|---|---|
| Frequency % | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Reported Frequency % | 5 | 5 | n/a | 7–25 | 100 | 24–34 | 24–26 | 81 |
| References | [66] | [67] | [64,66] | [9] | [53,67] | [53,67] | [53] |
EEA1, early endosomal antigen 1; 3E-BPO, branched-chain 2-oxo-acid dehydrogenase complex and 2-oxo glutarate dehydrogenase complex; CENP, centromere; GRASP-1, glutamate receptor interacting protein (GRIP)-associated protein-1; LC-1, liver cytosolic-1 antigen; LKM-1, liver kidney microsomal-1 antigen; M2, mitochondrial antigen–pyruvate dehydrogenase complex; PCNA, proliferating cell nuclear antigen; PML, promyelocytic leukaemia cell antigen; n.a., not available or not published; SLA/LP, soluble liver antigen/liver pancreas antigen; Sm, Smith/U2-U6 ribonucleoprotein antigen; SRP, signal recognition particle; VCP, valosin-containing protein.
As measured by ALBIA, the most common GWB autoantigen targets of the PBC sera were: RAP55 28%, GW182 12% and GW2 2%, but none reacted with Ge-1. The normal serum with anti-GWB antibodies reacted with GW182. Seventeen per cent (n = 18) of the PBC sera had antibodies to GRASP-1, as detected by immunoblotting techniques. Of interest, of the 25 samples that showed the CDS pattern by IIF, only 11 reacted with GWB components included in our ALBIA (nine RAP55; one GW1; one GW2) and three reacted with GRASP-1. Further, not all sera with antibodies to GWB components as detected by ALBIA were identified as having anti-GWB on the basis of IIF on HEp-2 cells primarily because the CDS pattern in some cases was masked or obscured by AMA staining (Fig. 1). Of the 25 samples with the CDS pattern, 20 also had a cytoplasmic speckled/mitochondrial pattern on IIF. In an analysis of the PBC sera with anti-GWB, all were associated with other autoantibodies and hence anti-GWB antibodies were not an independent serological variable.
Associations between autoantibodies and clinical features
For antibodies with a frequency of >10%, a more in-depth analysis of the clinical associations was examined (Table 5). None of the autoantibodies, including gp210 or Sp100, were associated significantly with differences in Mayo risk score or evidence of liver decompensation. As expected, M2 and 3E-BPO autoantibodies were associated with a positive AMA as detected by IIF (P = 0·001, P = 0·005), and CENP-B antibodies were associated with other autoimmune conditions (one Sjögren's syndrome, six systemic sclerosis, three Raynaud's) (P = 0·04). Only antibodies to 3E-BPO were associated significantly with a response to UDCA treatment.
Table 5.
Association of autoantibodies having a prevalence >10% with Mayo risk score, AMA results, associated autoimmune conditions, hepatic decompensation and response of treatment with UDCA in 109 PBC patients.
| Autoantibody (frequency) | Positive (+) | Negative (−) | P-value | |
|---|---|---|---|---|
| M2 (82%) | Mayo risk score, median (IQR) | 4·0 (3·6–4·6) | 4·2 (3·7–4·5) | 0·95 |
| AMA-positive, n (%) | 88 (99) | 15 (75) | 0·001 | |
| Associated autoimmune conditions, n (%) | 18 (20) | 3 (15) | 0·76 | |
| Hepatic decompensation, n (%) | 6 (7) | 1 (5) | 1 | |
| Response to UDCA, n (%) | 41 (55) | 10 (63) | 0·78 | |
| 3E-BPO (73%) | Mayo risk score, median (IQR) | 4·0 (3·6–4·7) | 4·1 (3·7–4·5) | 0·6 |
| AMA-positive, n (%) | 79 (99) | 24 (83) | 0·005 | |
| Associated autoimmune conditions, n (%) | 16 (20) | 5 (17) | 1 | |
| Hepatic decompensation, n (%) | 6 (8) | 1 (3) | 0·67 | |
| Response to UDCA, n (%) | 33 (49) | 18 (82) | 0·007 | |
| Ro52 (32%) | Mayo risk score, median (IQR) | 4·0 (3·7–4·6) | 4·1 (3·6–4·6) | 0·79 |
| AMA-positive, n (%) | 31 (89) | 72 (97) | 0·08 | |
| Associated autoimmune conditions, n (%) | 10 (29) | 11 (15) | 0·12 | |
| Hepatic decompensation, n (%) | 2 (6) | 5 (7) | 1 | |
| Response to UDCA, n (%) | 11 (52) | 40 (58) | 0·8 | |
| RAP55 (28%) | Mayo risk score, median (IQR) | 3·9 (3·3–4·4) | 4·1 (3·7–4·8) | 0·13 |
| AMA-positive, n (%) | 30 (97) | 73 (94) | 0·67 | |
| Associated autoimmune conditions, n (%) | 6 (19) | 15 (19) | 1 | |
| Hepatic decompensation, n (%) | 2 (6) | 5 (6) | 1 | |
| Response to UDCA, n (%) | 17 (59) | 34 (56) | 0·82 | |
| gp210 (27%) | Mayo risk score, median (IQR) | 4·4 (3·8–4·6) | 4·0 (3·6–4·6) | 0·07 |
| AMA-positive, n (%) | 29 (97) | 74 (94) | 1 | |
| Associated autoimmune conditions, n (%) | 4 (13) | 17 (22) | 0·42 | |
| Hepatic decompensation, n (%) | 2 (7) | 5 (6) | 1 | |
| Response to UDCA, n (%) | 12 (52) | 39 (58) | 0·63 | |
| Sp100 (27%) | Mayo risk score, median (IQR) | 4·0 (3·7–4·5) | 4·1 (3·6–4·6) | 0·65 |
| AMA-positive, n (%) | 28 (97) | 75 (94) | 1 | |
| Associated autoimmune conditions, n (%) | 3 (10) | 18 (23) | 0·18 | |
| Hepatic decompensation, n (%) | 1 (3) | 6 (8) | 0·67 | |
| Response to UDCA, n (%) | 12 (48) | 39 (60) | 0·35 | |
| PML (17%) | Mayo risk score, median (IQR) | 4·0 (3·7–4·5) | 4·1 (3·6–4·6) | 0·61 |
| AMA-positive, n (%) | 18 (95) | 85 (94) | 1 | |
| Associated autoimmune conditions, n (%) | 2 (11) | 19 (21) | 0·36 | |
| Hepatic decompensation, n (%) | 1 (5) | 6 (7) | 1 | |
| Response to UDCA, n (%) | 8 (57) | 43 (57) | 1 | |
| GRASP-1 (17%) | Mayo risk score, median (IQR) | 4·0 (3·7–4·8) | 4·1 (3·6–4·6) | 0·85 |
| AMA-positive, n (%) | 17 (94) | 86 (95) | 1 | |
| Associated autoimmune conditions, n (%) | 4 (22) | 17 (19) | 0·75 | |
| Hepatic decompensation, n (%) | 2 (11) | 5 (5) | 0·33 | |
| Response to UDCA, n (%) | 10 (67) | 41 (55) | 0·57 | |
| GW1 (12%) | Mayo risk score, median (IQR) | 4·1 (3·7–4·4) | 4·0 (3·6–4·6) | 0·99 |
| AMA-positive, n (%) | 12 (92) | 91 (95) | 0·54 | |
| Associated autoimmune conditions, n (%) | 1 (8) | 20 (21) | 0·46 | |
| Hepatic decompensation, n (%) | 0 (0) | 7 (7) | 0·6 | |
| Response to UDCA, n (%) | 8 (67) | 43 (55) | 0·54 | |
| CENP-B (11%) | Mayo risk score, median (IQR) | 3·7 (3·3–4·6) | 4·1 (3·7–4·6) | 0·33 |
| AMA-positive, n (%) | 9 (82) | 94 (96) | 0·11 | |
| Associated autoimmune conditions, n (%) | 5 (45) | 16 (16) | 0·04 | |
| Hepatic decompensation, n (%) | 1 (9) | 6 (6) | 0·54 | |
| Response to UDCA, n (%) | 5 (50) | 46 (58) | 0·74 | |
| VCP (11%) | Mayo risk score, median (IQR) | 3·7 (3·0–4·3) | 4·1 (3·7–4·6) | 0·13 |
| AMA-positive, n (%) | 11 (92) | 92 (95) | 0·51 | |
| Associated autoimmune conditions, n (%) | 3 (25) | 18 (19) | 0·7 | |
| Hepatic decompensation, n (%) | 2 (17) | 5 (5) | 0·17 | |
| Response to UDCA, n (%) | 8 (67) | 43 (55) | 0·54 |
AMA, anti-mitochondrial antibodies by IIF; 3E-BPO, branched-chain 2-oxo-acid dehydrogenase complex and 2-oxo glutarate dehydrogenase complex; CENP, centromere protein; GRASP-1, glutamate receptor interacting protein (GRIP)-associated protein-1; PML, promyelocyte leukaemia antigen; UDCA, ursodeoxycholic acid; VCP, valosin-containing protein; IQR, interquartile range.
Discussion
The IIF cytoplasmic discrete speckled (CDS) pattern has been reported in 0·1–9% of sequential sera submitted to a clinical diagnostic laboratory for IIF testing [6,27]. This wide range probably depends upon the substrates used for testing, the awareness of laboratory personnel of this pattern of staining and the referral pattern of the diagnostic laboratory. Autoantibodies producing the CDS pattern react with five to 20 circumscribed cytoplasmic structures, which is distinct from other cytoplasmic staining patterns [5]. In addition to lysosomes and endosomes [27], one of the targets of this particular IIF pattern are mRNA processing bodies known as GW bodies (GWBs) or P bodies [7]. GWBs are known to have a key role in the RNA interference (RNAi) pathway [28]. These structures, having a diameter of 100–300 µM, are somewhat unique in that they do not have a limiting membrane and they contain several autoantigen targets, including GW182 (GW1) [29], Ge-1/Hedls [21], Ago2 [6], diacyl-phosphatidylethanolamine [30] and RAP55/LSm14 [24]. RAP55 (also known as LSm14) is a protein believed to be involved in shuttling mRNAs between GWBs and stress granules [24]. Ge-1 (also known as Hedls) is believed to be involved in the P-body decapping process [21]. In previous studies, the most common clinical associations of autoantibodies to GWBs were Sjögren's syndrome, ataxia and neuropathies [6,18]. Other studies have indicated that approximately 5–10% of PBC patients have autoantibodies against GWBs [5,6].
In our PBC cohort, 23% had a CDS IIF pattern, and we tested all sera for reactivity against an array of GWB antigens components that included RAP55, GW1, GW2, GW3 and Ge-1. A previous study from our laboratory found that the most common target of a serological anti-GWB cohort was Ge-1 (58%), followed by GW1 (40%) [6]. However, in the PBC disease cohort that was the focus of the current study, RAP55 (28%) and GW1 (12%) were the most common targets. This highlights the difference of findings between a serological cohort (i.e. anti-GWB sera) and a disease cohort (i.e. PBC patients) and suggests that perhaps there are disease-specific patterns of the anti-GWB response. Of note, only a minority of samples that had antibodies to GWB components displayed a readily identified CDS staining pattern (RAP55 9, GW1 1, GW2 1). Possible explanations for this may include that the CDS pattern is masked by the more prominent cytoplasmic/mitochondrial pattern commonly seen in PBC sera, indicating that IIF is not a sensitive method to detect these antibodies and, hence, more specific assays such as ALBIA that contain the relevant GWB analytes are needed for their detection. In addition, our data indicate that there are probably other still to be identified CDS antigenic targets, including unknown GWB antigens, which were not assayed in our study.
As noted above, in our previous studies of a GWB serology cohort [6] we found that Ge-1 was the most common target autoantigen for anti-GWB sera, with a frequency of 58%. However, of the 38 of 55 patients who were positive for Ge-1 in that study, only one was known to have PBC while three other PBC patients in this cohort had other anti-GWB reactivity. In a study that probed a protein macroarray with seven PBC sera that had a CDS pattern, it was found that anti-RAP55 was present in four (57%) and anti-Ge-1 in four (57%) of sera [9]. In our larger unselected PBC cohort, we found that the frequency of anti-RAP55 (28%) and anti-Ge-1 (0%) was remarkably lower. This discrepancy is probably due to differences in techniques and assays used to detect RAP55 and Ge-1 and also differences in the demographics of the patient cohort, most notably the fact that the former study used preselected PBC sera with the CDS pattern, whereas the PBC patients in our study were not preselected.
GW182 (GW1) was the first autoantigen discovered in GWBs [7], and we found previously that this was a common target autoantigen (40%) for anti-GWB sera, and of the 22 of 55 patients who reacted with GW182 only two had PBC [6]. In the present study of our PBC cohort, we found that the frequency of anti-GW182 was 12% (n = 13). Other target antigens in GWBs are GW2 and GW3, but antibodies to these proteins in our PBC cohort were remarkably uncommon (2% and 0·9%, respectively), suggesting that these are probably not significant autoantibody targets of PBC patients. By comparison, 16% of a cohort of GWB sera reacted with GW2 and 9% with GW3 [6], once again emphasizing the different perspectives that can be gained when studying disease cohorts as opposed to serology cohorts.
Human autoantibodies directed against the novel autoantigen GRASP-1 are also associated with a CDS IIF staining pattern on HEp-2 substrates that does not overlap or co-localize with GWBs [8]. A previous study reported that seven of seven sera from PBC patients preselected on the basis of CDS pattern reacted to GRASP-1 [9]. When examined by immunoblot, the frequency of anti-GRASP-1 in our unselected PBC cohort was 17% (n = 18). GRASP-1 is thought to be expressed primarily in tissues of the nervous system, and has been shown to be involved in the regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor function and receptor synaptic targeting [31]. Of interest with respect to the relevance to PBC, GRASP-1 shares a reactive epitope with component X of the pyruvate dehydrogenase complex (PDC) [8], a common autoantibody target in PBC patients [32].
ANA are found in approximately 30–50% of unselected PBC patients [3,4,33], and are seen even more frequently (up to 85%) in PBC patients who are AMA-negative [3]. Compared to patients who are ANA-negative, patients who are ANA-positive have been reported to be at higher risk of developing liver failure [34]. The most relevant IIF patterns in PBC are nuclear rim [35] and multiple nuclear dots (MND, NSpI) [3,36], which were suggested as surrogate serological markers of the disease in AMA-negative patients [3,37]. The nuclear rim pattern is indicative of antibodies directed against components of the nuclear envelope and nuclear pore complex, with gp210 being the primary target antigen in PBC [38]. Gp210 antibodies were found in approximately 25% of patients with PBC, had a specificity of greater than 95% [36,39] and were associated with an increased risk of hepatic failure [10,40,41] and a higher Mayo risk score [42]. In the present study, 27% of our PBC cohort reacted with gp210, but there was no significant association with a higher Mayo risk score or liver decompensation. A possible explanation for this is that our cohort appeared to have a milder disease course with a lower overall Mayo risk score (median 4·05) than these previous studies. For example, by comparison, other studies of autoantibodies in PBC had Mayo scores of 4·2–4·8 [42], 4·1–4·3 [10], 5·7–6·1 [43] and 4·7 [41]. In addition, due to the relatively short follow-up of our study, decompensation would not necessarily be expected in patients with mild disease.
A primary target of sera that produce the MND pattern of staining is sp100 [3,4] and antibodies directed to Sp100 are reported in 20–30% of PBC patients [4]. Consistent with these studies, 27% of our PBC sera reacted with sp100. PML is a cell growth suppressing protein expressed aberrantly in promyelocytic leukaemia cells [44], and anti-PML antibodies are often seen together (co-autoimmunogenic) with sp100 in patients with PBC [45]. Antibodies to PML were found in 17% of our PBC patients, which is similar to previously reported frequency of 19% by others [42]. Sp100 and PML occurred together in 87% (n = 95) of patients in our cohort (15% dually positive, 72% dually negative). Although it has been reported that antibodies to sp100 and PML may be associated with an unfavourable disease course [46], we found no difference in the Mayo risk score or hepatic decompensation.
Anti-centromere antibodies (ACA) are a hallmark of the limited cutaneous subset of systemic sclerosis (SSc) [47]. ACA have been found in approximately 20–35% of PBC patients [48], and may be associated with increased risk of the development of portal hypertension or liver failure [34]. However, recent studies have found that in patients with SSc and PBC, ACA was associated with less severe hepatic involvement [49]. We did not find a significant difference in Mayo risk score or hepatic decompensation associated with CENP-B antibodies; however, it was associated with other autoimmune conditions. Ten per cent (n = 11) of our cohort showed centromere staining, as detected by IIF on HEp-2 cells, and 11% (n = 12) reacted with CENP-B by LIA. As only one serum with anti-CENP-B antibodies was missed by IIF, it suggests that the IIF test on HEp-2 substrates is a somewhat sensitive assay to detect ACA in PBC sera.
Antibodies to Ro52 have been associated with a number of systemic autoimmune diseases, most notably polymyositis, Sjögren's syndrome and SSc [50]. A recent study found that 28% of PBC patients had antibodies to Ro52 and this was associated with more advanced and active liver disease [51]. We found that the frequency of anti-Ro52 in our cohort was similar (32%), but there was no difference in the Mayo risk score or hepatic decompensation between those positive and negative for anti-Ro52.
VCP plays a role in nuclear envelope assembly and the post-mitotic formation of the endoplasmic reticulum and Golgi apparatus. This antibody was found in 12% of patients with PBC [52], and was suggested to be associated with a milder course [19]. In general agreement with previous reports, our study found that antibodies to VCP were found in 11% of patients and the average Mayo risk score of the 12 patients positive for anti-VCP was 3·7 compared with 4·1 of the entire cohort. While this suggests a trend to milder disease associated with anti-VCP, this difference was not statistically significant (P = 0·13).
Lastly, we noted that in our PBC cohort a positive anti-M2 LIA was more prevalent than anti-3E-BPO LIA. This finding was unexpected, because it has been suggested that the hybrid 3E-BPO analyte comprising major epitopes from oxaloacid dehydrogenase proteins has a higher sensitivity than the more traditional M2 (pyruvate dehydrogenase) antigen [53]. Further, of the six patients who were AMA-negative at diagnosis, one had anti-M2 and one had anti-3E-BPO, confirming that these assays have modestly increased sensitivity compared to the conventional IIF AMA screening test. We have considered that these findings might be related to UDCA therapy, although other studies found that AMA titres were not affected by this therapeutic modality [54,55].
This study of autoantibodies was focused primarily upon the prevalence of anti-GWB in a cohort of unselected PBC patients. Our study may have certain limitations that do not permit wide generalizations for all PBC patients. First, as a group, our PBC cohort seems to represent a milder disease as evidenced by the modest Mayo scores. Our estimated 5-year survival was 91% (based on Mayo risk score), and only 6% had evidence of hepatic decompensation. An epidemiological study by a Canadian PBC cohort, which included some of the current study population, described an overall survival of 80% and transplantation in 4·4% after a median follow-up of 5·8 years [11]. These differences may reflect the retrospective nature of our study and/or the fact that patients with a poor prognosis had already died and were not available to provide sera for the current study. Due to the small number of patients with decompensation in our study, the calculation of clinical associations with specific antibodies is underpowered. In addition, 92% of our patients were on UDCA at the time of sera collection and analysis, although to our knowledge there are limited data available showing that UDCA treatment affects the natural history of autoantibody frequency in PBC. A previous study found that approximately 50% of anti-gp210-positive patients became anti-gp210-negative and that this was associated with a milder disease course [10]. A prospective, longitudinal study examining the effect of medications on autoantibody profiles is needed.
In summary, in patients with PBC, a wide variety of target autoantigens are found in different frequencies. The CDS IIF pattern is relatively common and should alert clinicians to a possible diagnosis of PBC. In some sera the presence of anti-GWB is masked by the presence of cytoplasmic staining, particularly anti-mitochondrial antibodies. Thus, based on IIF analysis alone, the presence and frequency of anti-GWB is probably underestimated. This is the largest study to date showing that the RAP55 and GW182 antigen targets of GWBs and GRASP-1 are found in relatively high frequency in PBC patients. The clinical significance of the GWB antibodies will require further investigation. Identification of anti-GWB could complement the current biomarkers utilized in establishing the diagnosis and estimating the prognosis of PBC.
Acknowledgments
The authors express their appreciation to Haiyan Hou, Maggie Lin, Jane Yang, Mark Fritzler, Riley Sullivan and Pam Crotty for technical assistance. This study was supported through a grant from the Department of Medicine, University of Calgary and the Canadian Institutes of Health Research (CIHR). Dr Myers is supported by a Clinical Investigator Award from the Alberta Heritage Foundation for Medical Research and New Investigator Award from the CIHR.
Glossary
Abbreviations:
- ACA
anti-centromere antibodies
- ALBIA
addressable laser bead immunoassay
- ALP
alkaline phosphatase
- AMA
anti-mitochondrial antibodies by IIF
- ANA
antinuclear antibodies
- 3E-BPO
branched-chain 2-oxo-acid dehydrogenase complex and 2-oxo glutarate dehydrogenase complex
- CDS
cytoplasmic discrete speckled
- CENP
centromere protein
- CLIP
cytoplasmic linker protein
- Cr
creatinine
- dsDNA
double-stranded DNA
- EEA1
early endosomal antigen 1
- GRASP-1
glutamate receptor interacting protein (GRIP)-associated protein -1
- GWB
G (glycine) W (tryptophan)-containing bodies
- IIF
indirect immunofluorescence
- INR
prothrombin time
- IQR
interquartile range
- LC-1
liver cytosolic – 1 antigen
- LIA
line immunoassay
- LKM-1
liver kidney microsomal – 1 antigen
- LSm
like Sm antigen
- M2
mitochondrial antigen pyruvate dehydrogenase complex
- MND
multiple nuclear dots
- mRNA
messenger RNA
- PBC
primary biliary cirrhosis
- PCNA
proliferating cell nuclear antigen
- PDC
pyruvate dehydrogenase complex
- PML
promyelocytic leukemia cell antigen
- n/a
not available or not published
- RNAi
RNA interference
- RNP
ribonucleoprotein
- SLA/LP
soluble liver antigen/liver pancreas antigen
- Sm
Smith/U2-U6 ribonucleoprotein antigen
- SRP
signal recognition particle
- SS
Sjögren's syndrome
- UDCA
ursodeoxycholic acid
- VCP
valosin containing protein
Disclosure
Marvin Fritzler is a consultant to ImmunoConcepts (Sacramento, CA, USA), INOVA Diagnostics (San Diego, CA, USA) and Bio-Rad (Hercules, CA, USA). None of the other authors have any financial conflicts to disclose.
References
- 1.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 2.Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005–13. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- 3.Muratori L, Granito A, Muratori P, Pappas G, Bianchi FB. Antimitochondrial antibodies and other antibodies in primary biliary cirrhosis: diagnostic and prognostic value. Clin Liver Dis. 2008;12:261–76. doi: 10.1016/j.cld.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Worman HJ, Courvalin JC. Antinuclear antibodies specific for primary biliary cirrhosis. Autoimmun Rev. 2003;2:211–17. doi: 10.1016/s1568-9972(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 5.Bloch DB, Yu JH, Yang WH, et al. The cytoplasmic dot staining pattern is detected in a subgroup of patients with primary biliary cirrhosis. J Rheumatol. 2005;32:477–83. [PubMed] [Google Scholar]
- 6.Bhanji RA, Eystathioy T, Chan EK, Bloch DB, Fritzler MJ. Clinical and serological features of patients with autoantibodies to GW/P bodies. Clin Immunol. 2007;125:247–56. doi: 10.1016/j.clim.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eystathioy T, Chan EK, Tenenbaum SA, Keene JD, Griffith K, Fritzler MJ. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol Biol Cell. 2002;13:1338–51. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stinton LM, Selak S, Fritzler MJ. Identification of GRASP-1 as a novel 97 kDa autoantigen localized to endosomes. Clin Immunol. 2005;116:108–17. doi: 10.1016/j.clim.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Yang WH, Bloch DB. Probing the mRNA processing body using protein macroarrays and ‘autoantigenomics’. RNA. 2007;13:704–12. doi: 10.1261/rna.411907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura M, Shimizu-Yoshida Y, Takii Y, et al. Antibody titer to gp210-C terminal peptide as a clinical parameter for monitoring primary biliary cirrhosis. J Hepatol. 2005;42:386–92. doi: 10.1016/j.jhep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Myers RP, Shaheen AA, Fong A, et al. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: a population-based study. Hepatology. 2009;50:1884–92. doi: 10.1002/hep.23210. [DOI] [PubMed] [Google Scholar]
- 12.Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 13.Vergani D, Alvarez F, Bianchi FB, et al. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41:677–83. doi: 10.1016/j.jhep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Fritzler MJ. Autoantibody testing: procedures and significance in systemic rheumatic diseases. Methods Achiev Exp Pathol. 1986;12:224–60. [PubMed] [Google Scholar]
- 15.Smeenk R, Hylkema M. Detection of antibodies to DNA: a technical assessment. Mol Biol Rep. 1992;17:71–9. doi: 10.1007/BF01006401. [DOI] [PubMed] [Google Scholar]
- 16.Eystathioy T, Chan EK, Mahler M, Luft LM, Fritzler ML, Fritzler MJ. A panel of monoclonal antibodies to cytoplasmic GW bodies and the mRNA binding protein GW182. Hybrid Hybridomics. 2003;22:79–86. doi: 10.1089/153685903321947996. [DOI] [PubMed] [Google Scholar]
- 17.Mahler M, Kessenbrock K, Raats J, Williams R, Fritzler MJ, Bluthner M. Characterization of the human autoimmune response to the major C-terminal epitope of the ribosomal P proteins. J Mol Med. 2003;81:194–204. doi: 10.1007/s00109-003-0423-1. [DOI] [PubMed] [Google Scholar]
- 18.Eystathioy T, Chan EK, Takeuchi K, et al. Clinical and serological associations of autoantibodies to GW bodies and a novel cytoplasmic autoantigen GW182. J Mol Med. 2003;81:811–18. doi: 10.1007/s00109-003-0495-y. [DOI] [PubMed] [Google Scholar]
- 19.Miyachi K, Hosaka H, Nakamura N, et al. Anti-p97/VCP antibodies: an autoantibody marker for a subset of primary biliary cirrhosis patients with milder disease? Scand J Immunol. 2006;63:376–82. doi: 10.1111/j.1365-3083.2006.01747.x. [DOI] [PubMed] [Google Scholar]
- 20.Selak S, Chan EK, Schoenroth L, Senecal JL, Fritzler MJ. Early endosome antigen. 1: an autoantigen associated with neurological diseases. J Invest Med. 1999;47:311–18. [PubMed] [Google Scholar]
- 21.Yu JH, Yang WH, Gulick T, Bloch KD, Bloch DB. Ge-1 is a central component of the mammalian cytoplasmic mRNA processing body. RNA. 2005;11:1795–802. doi: 10.1261/rna.2142405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada N, Mimori T, Mukai R, Kashiwagi H, Hardin JA. Characterization of human autoantibodies that selectively precipitate the 7SL RNA component of the signal recognition particle. J Immunol. 1987;138:3219–23. [PubMed] [Google Scholar]
- 23.Magsaam J, Gharavi AE, Parnassa AP, Weissbach H, Brot N, Elkon KB. Quantification of lupus anti-ribosome P antibodies using a recombinant P2 fusion protein and determination of the predicted amino acid sequence of the autoantigen in patients' mononuclear cells. Clin Exp Immunol. 1989;76:165–71. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang WH, Yu JH, Gulick T, Bloch KD, Bloch DB. RNA-associated protein 55 (RAP55) localizes to mRNA processing bodies and stress granules. RNA. 2006;12:547–54. doi: 10.1261/rna.2302706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith KJ, Ryan JP, Senecal JL, Fritzler MJ. The cytoplasmic linker protein CLIP-170 is a human autoantigen. Clin Exp Immunol. 2002;127:533–8. doi: 10.1046/j.1365-2249.2002.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715–20. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Stinton LM, Eystathioy T, Selak S, Chan EK, Fritzler MJ. Autoantibodies to protein transport and messenger RNA processing pathways: endosomes, lysosomes, Golgi complex, proteasomes, assemblyosomes, exosomes, and GW bodies. Clin Immunol. 2004;110:30–44. doi: 10.1016/j.clim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Jakymiw A, Ikeda K, Fritzler MJ, Reeves WH, Satoh M, Chan EK. Autoimmune targeting of key components of RNA interference. Arthritis Res Ther. 2006;8:R87. doi: 10.1186/ar1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eystathioy T, Jakymiw A, Chan EK, Seraphin B, Cougot N, Fritzler MJ. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003;9:1171–3. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurino CC, Fritzler MJ, Mortara RA, Silva NP, Almeida IC, Andrade LE. Human autoantibodies to diacyl-phosphatidylethanolamine recognize a specific set of discrete cytoplasmic domains. Clin Exp Immunol. 2006;143:572–84. doi: 10.1111/j.1365-2249.2006.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong H, Zhang P, Liao D, Huganir RL. Characterization, expression, and distribution of GRIP protein. Ann NY Acad Sci. 1999;868:535–40. doi: 10.1111/j.1749-6632.1999.tb11323.x. [DOI] [PubMed] [Google Scholar]
- 32.Mackay IR. Autoimmunity and primary biliary cirrhosis. Baillières Best Pract Res Clin Gastroenterol. 2000;14:519–33. doi: 10.1053/bega.2000.0101. [DOI] [PubMed] [Google Scholar]
- 33.Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25:298–310. doi: 10.1055/s-2005-916321. [DOI] [PubMed] [Google Scholar]
- 34.Yang WH, Yu JH, Nakajima A, Neuberg D, Lindor K, Bloch DB. Do antinuclear antibodies in primary biliary cirrhosis patients identify increased risk for liver failure? Clin Gastroenterol Hepatol. 2004;2:1116–22. doi: 10.1016/s1542-3565(04)00465-3. [DOI] [PubMed] [Google Scholar]
- 35.Wichmann I, Montes-Cano MA, Respaldiza N, et al. Clinical significance of anti-multiple nuclear dots/Sp100 autoantibodies. Scand J Gastroenterol. 2003;38:996–9. doi: 10.1080/00365520310004876. [DOI] [PubMed] [Google Scholar]
- 36.Worman HJ. Nuclear envelope protein autoantigens in primary biliary cirrhosis. Hepatol Res. 2007;37(Suppl. 3):S406–11. doi: 10.1111/j.1872-034X.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 37.Vergani D, Bogdanos DP. Positive markers in AMA-negative PBC. Am J Gastroenterol. 2003;98:241–3. doi: 10.1111/j.1572-0241.2003.07270.x. [DOI] [PubMed] [Google Scholar]
- 38.Wesierska-Gadek J, Hohenauer H, Hitchman E, Penner E. Autoantibodies from patients with primary biliary cirrhosis preferentially react with the amino-terminal domain of nuclear pore complex glycoprotein gp210. J Exp Med. 1995;182:1159–62. doi: 10.1084/jem.182.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer A, Habior A. Measurement of gp210 autoantibodies in sera of patients with primary biliary cirrhosis. J Clin Lab Anal. 2007;21:227–31. doi: 10.1002/jcla.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh S, Ichida T, Yoshida T, et al. Autoantibodies against a 210 kDa glycoprotein of the nuclear pore complex as a prognostic marker in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 1998;13:257–65. doi: 10.1111/j.1440-1746.1998.01553.x. [DOI] [PubMed] [Google Scholar]
- 41.Muratori P, Muratori L, Ferrari R, et al. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol. 2003;98:431–7. doi: 10.1111/j.1572-0241.2003.07257.x. [DOI] [PubMed] [Google Scholar]
- 42.Gao L, Tian X, Liu B, Zhang F. The value of antinuclear antibodies in primary biliary cirrhosis. Clin Exp Med. 2008;8:9–15. doi: 10.1007/s10238-008-0150-6. [DOI] [PubMed] [Google Scholar]
- 43.Liu B, Shi XH, Zhang FC, Zhang W, Gao LX. Antimitochondrial antibody-negative primary biliary cirrhosis: a subset of primary biliary cirrhosis. Liver Int. 2008;28:233–9. doi: 10.1111/j.1478-3231.2007.01651.x. [DOI] [PubMed] [Google Scholar]
- 44.Sternsdorf T, Grotzinger T, Jensen K, Will H. Nuclear dots: actors on many stages. Immunobiology. 1997;198:307–31. doi: 10.1016/S0171-2985(97)80051-4. [DOI] [PubMed] [Google Scholar]
- 45.Sternsdorf T, Guldner HH, Szostecki C, Grotzinger T, Will H. Two nuclear dot-associated proteins, PML and Sp100, are often co-autoimmunogenic in patients with primary biliary cirrhosis. Scand J Immunol. 1995;42:257–68. doi: 10.1111/j.1365-3083.1995.tb03652.x. [DOI] [PubMed] [Google Scholar]
- 46.Zuchner D, Sternsdorf T, Szostecki C, Heathcote EJ, Cauch-Dudek K, Will H. Prevalence, kinetics, and therapeutic modulation of autoantibodies against Sp100 and promyelocytic leukemia protein in a large cohort of patients with primary biliary cirrhosis. Hepatology. 1997;26:1123–30. doi: 10.1002/hep.510260506. [DOI] [PubMed] [Google Scholar]
- 47.Assassi S, Fritzler MJ, Arnett FC, et al. Primary biliary cirrhosis (PBC), PBC autoantibodies, and hepatic parameter abnormalities in a large population of systemic sclerosis patients. J Rheumatol. 2009;36:2250–6. doi: 10.3899/jrheum.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyachi K, Miyakawa H, Oda M, Horigome T, Fritzler MJ. Primary biliary cirrhosis and autoantibodies. Nihon Rinsho Meneki Gakkai Kaishi. 2008;31:47–55. doi: 10.2177/jsci.31.47. [DOI] [PubMed] [Google Scholar]
- 49.Rigamonti C, Shand LM, Feudjo M, et al. Clinical features and prognosis of primary biliary cirrhosis associated with systemic sclerosis. Gut. 2006;55:388–94. doi: 10.1136/gut.2005.075002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632–7. doi: 10.1016/j.autrev.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Granito A, Muratori P, Muratori L, et al. Antibodies to SS-A/Ro-52 kD and centromere in autoimmune liver disease: a clue to diagnosis and prognosis of primary biliary cirrhosis. Aliment Pharmacol Ther. 2007;26:831–8. doi: 10.1111/j.1365-2036.2007.03433.x. [DOI] [PubMed] [Google Scholar]
- 52.Miyachi K, Matsushima H, Hankins RW, et al. A novel antibody directed against a three-dimensional configuration of a 95-kDa protein in patients with autoimmune hepatic diseases. Scand J Immunol. 1998;47:63–8. doi: 10.1046/j.1365-3083.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 53.Chou MJ, Lee SL, Chen TY, Tsay GJ. Specificity of antinuclear antibodies in primary biliary cirrhosis. Ann Rheum Dis. 1995;54:148–51. doi: 10.1136/ard.54.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benson GD, Kikuchi K, Miyakawa H, Tanaka A, Watnik MR, Gershwin ME. Serial analysis of antimitochondrial antibody in patients with primary biliary cirrhosis. Clin Dev Immunol. 2004;11:129–33. doi: 10.1080/10446670410001722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kikuchi K, Hsu W, Hosoya N, et al. Ursodeoxycholic acid reduces CpG-induced IgM production in patients with primary biliary cirrhosis. Hepatol Res. 2009;39:448–54. doi: 10.1111/j.1872-034X.2008.00474.x. [DOI] [PubMed] [Google Scholar]
- 56.Czaja AJ, Nishioka M, Morshed SA, Hachiya T. Patterns of nuclear immunofluorescence and reactivities to recombinant nuclear antigens in autoimmune hepatitis. Gastroenterology. 1994;107:200–7. doi: 10.1016/0016-5085(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 57.Enarson P, Rattner JB, Ou Y, Miyachi K, Horigome T, Fritzler MJ. Autoantigens of the nuclear pore complex. J Mol Med. 2004;82:423–33. doi: 10.1007/s00109-004-0554-z. [DOI] [PubMed] [Google Scholar]
- 58.Granito A, Muratori P, Muratori L, et al. Antinuclear antibodies giving the ‘multiple nuclear dots’ or the ‘rim-like/membranous’ patterns: diagnostic accuracy for primary biliary cirrhosis. Aliment Pharmacol Ther. 2006;24:1575–83. doi: 10.1111/j.1365-2036.2006.03172.x. [DOI] [PubMed] [Google Scholar]
- 59.Granito A, Yang WH, Muratori L, et al. PML nuclear body component Sp140 is a novel autoantigen in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:125–31. doi: 10.1038/ajg.2009.596. [DOI] [PubMed] [Google Scholar]
- 60.Makinen D, Fritzler M, Davis P, Sherlock S. Anticentromere antibodies in primary biliary cirrhosis. Arthritis Rheum. 1983;26:914–17. doi: 10.1002/art.1780260714. [DOI] [PubMed] [Google Scholar]
- 61.Fritzler MJ. Autoantibodies in scleroderma. J Dermatol. 1993;20:257–68. doi: 10.1111/j.1346-8138.1993.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 62.Rattner JB. Mapping the mammalian intercellular bridge. [Review] Cell Motil Cytoskeleton. 1992;23:231–5. doi: 10.1002/cm.970230402. [DOI] [PubMed] [Google Scholar]
- 63.Czaja AJ. Autoantibodies as prognostic markers in autoimmune liver disease. Dig Dis Sci. 2010;55:2144–61. doi: 10.1007/s10620-010-1268-4. [DOI] [PubMed] [Google Scholar]
- 64.Milkiewicz P, Buwaneswaran H, Coltescu C, Shums Z, Norman GL, Heathcote EJ. Value of autoantibody analysis in the differential diagnosis of chronic cholestatic liver disease. Clin Gastroenterol Hepatol. 2009;7:1355–60. doi: 10.1016/j.cgh.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 65.Miyakawa H, Abe K, Kitazawa E, et al. Detection of anti-branched chain 2-oxo acid dehydrogenase complex (BCOADC)-E2 antibody in primary biliary cirrhosis by ELISA using recombinant fusion protein. Autoimmunity. 1999;30:11–20. doi: 10.3109/08916939908994755. [DOI] [PubMed] [Google Scholar]
- 66.Agmon-Levin N, Shapira Y, Selmi C, et al. A comprehensive evaluation of serum autoantibodies in primary biliary cirrhosis. J Autoimmun. 2010;34:55–8. doi: 10.1016/j.jaut.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Tishler M, Alosachie I, Barka N, et al. Primary Sjogren's syndrome and primary biliary cirrhosis: differences and similarities in the autoantibody profile. Clin Exp Rheumatol. 1995;13:497–500. [PubMed] [Google Scholar]
- 68.Kanzler S, Bozkurt S, Herkel J, Galle PR, Dienes HP, Lohse AW. [Presence of SLA/LP autoantibodies in patients with primary biliary cirrhosis as a marker for secondary autoimmune hepatitis (overlap syndrome)] Dtsch Med Wochenschr. 2001;126:450–6. doi: 10.1055/s-2001-12906. [DOI] [PubMed] [Google Scholar]
- 69.Tsuchiya K, Kiyosawa K, Imai H, Sodeyama T, Furuta S. Detection of anti-double and anti-single stranded DNA antibodies in chronic liver disease: significance of anti-double stranded DNA antibody in autoimmune hepatitis. J Gastroenterol. 1994;29:152–8. doi: 10.1007/BF02358676. [DOI] [PubMed] [Google Scholar]


