Abstract
Our previous study demonstrated that annexin A2 (ANX2) on cell surface could function as a mediator and stimulate tissue factor (TF) expression of monocytes by anti-β2-glycoprotein I/β2-glycoprotein I complex (anti-β2GPI/β2GPI). However, ANX2 is not a transmembrane protein and lacks the intracellular signal transduction pathway. Growing evidence suggests that Toll-like receptor 4 (TLR-4) might act as an ‘adaptor’ for intracellular signal transduction in anti-β2GPI/β2GPI-induced TF expressing cells. In the current study, we investigated the roles of TLR-4 and its related molecules, myeloid differentiation protein 2 (MD-2) and myeloid differentiation factor 88 (MyD88), in anti-β2GPI/β2GPI-induced TF expressing human monocytic-derived THP-1 (human acute monocytic leukaemia) cells. The relationship of TLR-4 and ANX2 in this process was also explored. Along with TF, expression of TLR-4, MD-2 and MyD88 in THP-1 cells increased significantly when treated by anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex. The addition of paclitaxel, which competes with the MD-2 ligand, could inhibit the effects of anti-β2GPI/β2GPI on TLR-4, MD-2, MyD88 and TF expression. Both ANX2 and TLR-4 in THP-1 cell lysates could bind to β2GPI that had been conjugated to a column (β2GPI-Affi-Gel). Furthermore, TLR-4, MD-2, MyD88 and TF expression was remarkably diminished in THP-1 cells infected with ANX2-specific RNA interference (RNAi) lentivirus (LV-RNAi-ANX2), in spite of treatment with a similar concentration of anti-β2GPI/β2GPI complex. These results indicate that TLR-4 and its signal transduction pathway contribute to anti-β2GPI/β2GPI-induced TF expression in THP-1 cells, and the effects of TLR-4 with ANX2 are tightly co-operative.
Keywords: annexin A2, anti-β2-glycoprotein I antibodies, β2-glycoprotein I, monocyte, tissue factor, Toll-like receptor 4
Introduction
Anti-phospholipid syndrome (APS) is a disorder caused by the production of anti-phospholipid antibodies (aPL) which contributes to thrombosis [1]. In addition to anionic phospholipids, aPL also recognizes phospholipid binding proteins, including β2-glycoprotein I (β2GPI) and prothrombin [2]. β2GPI has emerged as a common antigen for aPL. Anti-β2GPI antibodies are found abundantly in the plasma of APS patients, suggesting its important role in the pathological mechanisms of APS [3]. Growing evidence suggests that anti-β2GPI antibodies may have procoagulant effects, and stimulate blood cells and vascular endothelium to express tissue factor (TF) activity [4]. TF is a specific and high-affinity receptor for factor VII/VIIa and functions as a co-factor for factor VIIa enzymatic activity. Exposure of TF to blood triggers physiological blood coagulation and thrombosis in a wide variety of thrombotic diseases [5]. Under normal physiological conditions, blood monocytes do not express functional TF constitutively; however, they are capable of TF synthesis and expression when stimulated with lipopolysaccharide (LPS) or certain inflammatory cytokines [6]. Our previous study showed that certain aPL (mainly anti-β2GPI) with its antigen could induce monocytes TF activity in APS [7].
Currently, the cell surface molecules involved in the interaction between anti-β2GPI/β2GPI and blood monocytes, and the signal transduction pathways leading to TF expression are not understood thoroughly [8]. β2GPI does not bind to cells through a simple positive charge domain, but via specific binding molecules. Previous studies have shown that annexin A2 (ANX2) on certain cell surfaces is capable of binding to β2GPI, which mediates the pathogenic effects of aPL in vivo and in vitro[9–11]. However, ANX2 is not a transmembrane protein, thus it is unlikely to be involved in intracellular signal transduction. It has been proposed that other transmembrane ‘adaptor’ proteins may exist to associate with ANX2 on cell surfaces. Toll-like receptor 4 (TLR-4) was suggested to act as an ‘adaptor’ for ANX2 leading to intracellular signal transduction [12]. TLR-4 and its signal transduction pathway may also play vital roles in the mechanism of aPL-mediated thrombosis in APS.
TLR-4 is a type I transmembrane glycoprotein expressed mainly on the cells of the innate immune system, which is the first line of host defence against pathogens [13]. Lipopolysaccharide (LPS), the main ligand of TLR-4, can activate the innate immune system via TLR-4, and lead ultimately to gene transcription and induce the release of some proinflammatory cytokines [14]. It is widely known that myeloid differentiation protein 2 (MD-2) is the key co-factor of TLR-4 in LPS-induced signalling transduction [15]. TLR-4, together with MD-2, forms a receptor complex for LPS and induces an intracellular signalling cascade through the common adaptor protein–myeloid differentiation factor 88 (MyD88) [16,17]. However, the effects of anti-β2GPI/β2GPI-induced TF expression on TLR-4, MD-2 and MyD88 expression in human monocytic-derived THP-1 human acute monocytic leukaemia (cells) are unknown. In this study, we investigated whether TLR-4, as well as its related proteins, modulate the expression of TF on THP-1 cells and explored the relationship between TLR-4 and ANX2.
Materials and methods
Cell lines and cell culture
The human monocytic-derived THP-1 cell line was from Shanghai Institutes Biological Sciences (Shanghai, China). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, NY, USA) with low-glucose medium supplemented with 1% glutamine, 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) (Gibco BRL). The cells were cultured at 37°C and 5% CO2 in a humidified incubator to near confluence and were deprived of serum for 16 h before they were used in the experiments. All experimental data were obtained for cells passaged between 3 and 10.
Real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) analysis
The THP-1 cells were seeded at 2 × 106 cells per ml into six-well plates and were serum-starved for 16 h prior to stimulation with monoclonal anti-β2GPI (10 µg/ml; Chemicon, Temecula, CA, USA)/β2GPI (100 µg/ml; US Biological, Swampscott, MC, USA) complex, isotype control rabbit immunoglobulin G (R-IgG), (10 µg/ml; Santa Cruz Biotechnology, Santa Cruz, CA, USA)/β2GPI (100 µg/ml), anti-β2GPI (10 µg/ml)/bovine serum albumin (BSA) (100 µg/ml; Sigma, St Louis, MO, USA) or 500 ng/ml of LPS (Sigma) for 2 h. Some experiments involved pretreating cells with 1 µM paclitaxel (Tauto Biotech, Shanghai, China) for 1 h. Total RNA was extracted from cells using Trizol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocol. Oligo dT-primers were used for reverse transcription with 2 µg of total RNA in a 25 µl reaction volume (Applied Biosystems, 2720 Thermal Cycler, USA). The levels of target mRNA on cells were analysed by quantitative RT–PCR using SYBR Green I dye (Takara Biotec, Kyoto, Japan) detection. The primers used for PCR were as follows: TLR-4, forward: CCTGTGCAATTTGACCATTG, reverse: AAGCATTCCCACCTTTGTTG, 263 base pairs (bp); MD-2, forward: ATTGGGTCTGCAACTCATCC, reverse: AATCGTCATCAGATCCTCGG, 240 bp; MyD88, forward: TGCAGAGCAAGGAATGTGAC, reverse: AGGATGCTGGGGAACTCTTT, 121 bp; TF, forward: TCAGGTGATCCACCCACCTT, reverse: GCACCCAATTTCCTTCCATTT, 132 bp; ANX2, forward: ACCTGGAGACGGTGATTT, reverse: TGCTCTTCTACCCTTTGC, 260 bp; β-actin, forward: CACGAAACTACCTTCAACTCC, reverse: CATACTCCTGCTTGCTGATC, 262 bp. The PCR assays were performed in triplicate on a Rotor-Gene 2000 qRT–PCR system (Corbett Research, Sydney, Australia). The amplification run was performed for 35 cycles under the following conditions: denaturation (94°C for 30 s), annealing (TLR-4, MD-2, MyD88, TF at 60°C for 30 s and β-actin, ANX2 at 56°C for 30 s), extension (72°C for 30 s). The relative levels of target mRNA were detected using standard curve calculations by control values of β-actin (%).
Western blotting analysis
THP-1 cells (2 × 106/ml) were incubated with anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex for 6 h. When necessary, the cells were pretreated with 1 µM paclitaxel for 1 h. For total cellular protein, cells were collected and lysed in buffer containing 20 mM Tris-HCl, pH 7·4, 1% Triton X-100, 2·5 mM ethylenediamine tetraacetic acid (EDTA), 1 mM phenylmethylsulphonyl fluoride (PMSF). The lysates were centrifuged at 93 g for 30 min (Kubota 6930, Tokyo, Japan) to remove unbroken cells, nuclei and other organelles. The supernatant containing plasma membrane was recovered and stored at −80°C for analysis. Equal amounts of protein sample (5 µg) were electrophoresed in 12% of sodium dodecyl sulphate-polyacrylamide gel electrophoresis gels (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked in fresh 5% dry defatted milk in Tris-buffered saline/0·05% Tween-20 (TTBS) for 1 h at room temperature (RT), washed three times with TTBS, and then incubated with the primary antibodies recognizing TLR-4 (eBioscience, San Diego, CA, USA), MD2 (eBioscience), MyD88 (Santa Cruz), ANX2 (Abnova Cor, Taipei, Taiwan) and β-actin (Proteintech Group, Chicago, IL, USA) overnight at 4°C. Following three washes with TTBS, the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies (Santa Cruz) for 1 h at RT. Finally, the immunoblots were developed, imaged using enhanced chemiluminescence (ECL) Western blotting detection reagents (GE Healthcare, Buckinghamshire, UK), and quantitated using a Bio-Rad Fluor-S MultiImager (Typhoon 9400, Amersham, Sweden).
Analysis of β2GPI binding with molecules on THP-1 cell membrane
To investigate whether β2GPI can bind to the specific molecules on THP-1 cell surface membrane, the β2GPI-affinity column (β2GPI-Affi-Gel) was first prepared using cyanogen bromide (CNBr)-activated Sepharose® 4B (Amersham Pharmacia Biotech, Uppsala, Sweden). Briefly, human β2GPI (3 mg) was added to 1ml of CNBr-activated Sepharose 4B and agitated overnight at 4°C, following the manufacturer's instructions. The coupled gel was then washed with a blocking buffer and equalization buffer. Finally, about 2 mg of β2GPI was coupled to column.
The THP-1 cells (1 × 107) were incubated with anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex for 6 h, lysed with 1 ml of lysate buffer (mentioned above) and centrifuged at 93 g for 30 min. The supernatant (cell surface membrane) was dialyzed overnight by 20 mM Tris-HCl buffer, pH 7·4 and then added to the β2GPI-Affi-Gel column. The column was washed with 20 mM Tris-HCl, pH 7·4/30 mM NaCl and proteins were eluted using 20 mM Tris-HCl, pH 7·4/350 mM NaCl. Each step was monitored by the optical density (OD) value of the fractions up to zero. Three portions of the fractions (flow solution, the washed solution and eluted solution) were collected and analysed by Western blotting using anti-ANX2 or anti-TLR-4 antibodies.
ANX2 RNA interference assay
To evaluate the relationship of ANX2 and TLR-4 in anti-β2GPI/β2GPI complex-induced TF expression on THP-1 cells, the effect of ANX2 RNA interference of THP-1 cells was analysed. The lentiviral expression vector containing ANX2 siRNA gene or the empty vector (Genechem, Shanghai, China) was constructed and packed into HEK 293T cells according to the manufacturer's instructions. The recombinant lentivirus containing ANX2 siRNA (LV-RNAi-ANX2) or empty lentivirus (LV-GFP) harvested from HEK 293T cells were then added into target THP-1 cells at multiplicity of infection (MOI) equal to 100 with enhanced infection solution (ENi.S; Genechem, Shanghai, China) and 5 µg/ml polybrene. After 72 h, the ANX2 mRNA and its protein expression on THP-1 cells were detected by qRT–PCR or Western blot in order to confirm the knock-down of ANX2. The cells were then collected and stimulated by anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) for 2 h or 6 h. The mRNA and protein levels of target molecules were finally assayed.
TF activity measurement
TF activity on cells was determined as factor X activation by TF/VIIa complex. The above cell lysates were collected and assayed using TF activity kits (Assaypro, Greenwich, CT, USA), according to the manufacturer's instructions. Factor VIIa and factor X were, respectively, diluted in the assay diluent, and 60 µl of the mixture (containing VIIa and X) was added to each well of the 96-well plate. Twenty microlitres of lysate samples was then added and incubated at 37°C for 30 min. The activity of TF/FVIIa complex was quantitated by the amount of factor Xa generation, which reacts with a highly specific factor Xa substrate (20 µl/well), releasing a yellow para-nitroaniline (pNA) chromophore. Colour development was monitored by the absorbance at 405 nm using a kinetic microplate reader (Gene Company Limited, Hong Kong, China). The concentration of generated factor Xa was calculated from Vmax (mOD/min) using a standard curve.
Statistical analysis
Normally distributed variables were expressed as means ± standard error of the mean. One-way analyses of variance were used to compare three or more means. Two-factor treatment results were analysed by two-way analyses of variance. All statistical analyses were performed using spss statistical software package version 17·0 (SPSS Inc., Chicago, IL, USA) and the level of significance was set at P < 0·05.
Results
Anti-β2GPI/β2GPI complex induces TF expression in THP-1 cells
Treatment of THP-1 cells with anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex increased TF expression significantly at the mRNA level (Fig. 1a) and enhanced its activity (Fig. 1b) compared to untreated cells (P < 0·05). The effects of anti-β2GPI/β2GPI complex were comparable to that of LPS (500 ng/ml). However, TF expression did not increase in cells when treated with R-IgG at the same concentration as β2GPI or anti-β2GPI (10 µg/ml)/BSA (100 µg/ml). These results were similar to that of our previous study in human peripheral blood monocytes [7].
Fig. 1.
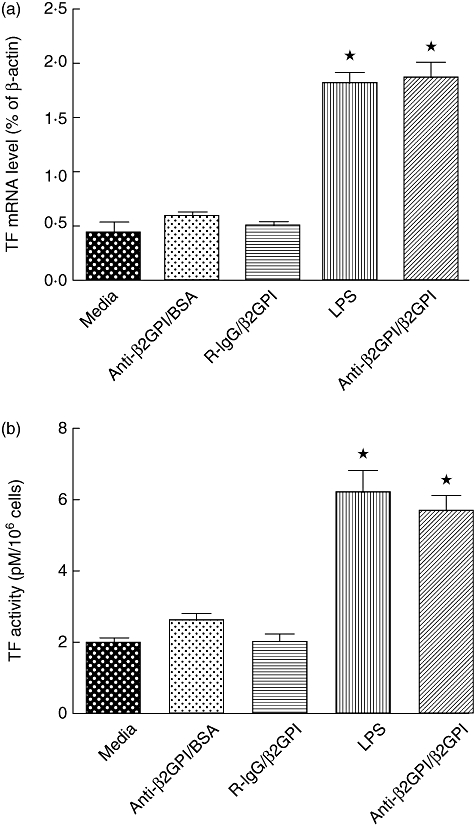
Tissue factor (TF) expression of THP-1 (human acute monocytic leukaemia) cells induced by anti-β2-glycoprotein I/β2-glycoprotein I (anti-β2GPI/β2GPI) complex. The THP-1 cells (2 × 106) were treated with anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex, isotype control rabbit immunoglobulin G (R-IgG) (10 µg/ml)/β2GPI (100 µg/ml), lipopolysaccharide (LPS) (500 ng/ml) and anti-β2GPI (10 µg/ml)/bovine serum albumin (BSA) (100 µg/ml) for 2 h (a) or 6 h (b). TF mRNA (a) and TF activity (b) were detected by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) and TF activity kits, respectively, as described in Materials and methods. Data shown are from three independent experiments. *P < 0·05 versus control of untreated cells.
Anti-β2GPI/β2GPI complex stimulates TLR-4, MyD88 and MD-2 expression in THP-1 cells
We investigated the effects of anti-β2GPI/β2GPI treatment on TLR-4, MyD88 and MD-2 levels in THP-1 cells. Treatment with the anti-β2GPI/β2GPI complex increased TLR-4, MyD88 and MD-2 expression significantly at both the mRNA (Fig. 2a) and protein levels (Fig. 2b) compared to untreated cells (P < 0·05). The observed effects of anti-β2GPI/β2GPI complex were similar to that of LPS (positive control). However, R-IgG treatment of THP-1 cells did not stimulate TLR-4, MyD88 and MD-2 levels.
Fig. 2.
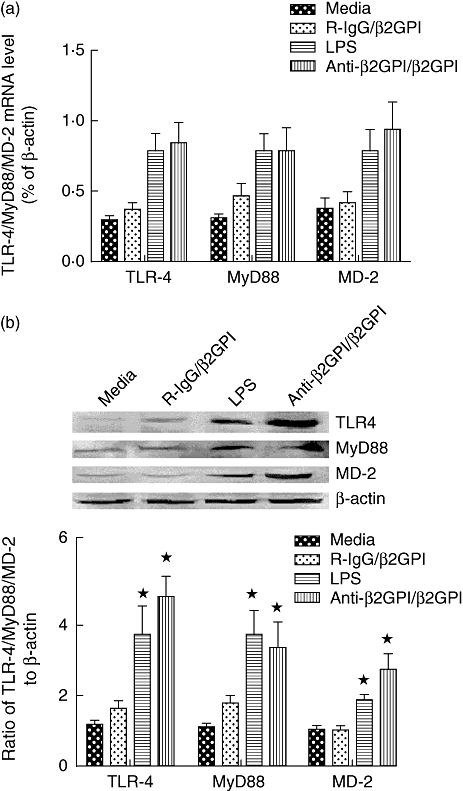
Toll-like receptor (TLR)-4, myeloid differentiation factor 88 (MyD88) and myeloid differentiation protein 2 (MD-2) secretion in THP-1 (human acute monocytic leukaemia) cells stimulated with anti-β2-glycoprotein I/β2-glycoprotein I (anti-β2GPI/β2GPI) complex. The THP-1 cells (2 × 106) were treated with anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex, isotype control rabbit immunoglobulin G (R-IgG) (10 µg/ml)/β2GPI (100 µg/ml) and lipopolysaccharide (LPS) (500 ng/ml) for 2 h (a) or 6 h (b). TLR-4, MyD88 and MD-2 mRNA levels (a) were detected by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR), and their protein levels (b) were analysed by Western blotting. Data shown are from three independent experiments. *P < 0·05 versus control of untreated cells.
ANX2 and TLR-4 interacts with β2GPI in THP-1 cells
Our previous data showed that β2GPI could interact with ANX2 on monocyte surfaces and mediate anti-β2GPI/β2GPI complex-induced TF expression of THP-1 cells. Because ANX2 is not a transmembrane protein and not involved in intracellular signal transduction, we then studied whether β2GPI could bind with ANX2 as well as TLR-4. THP-1 cells were incubated with anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex for 6 h, and cell lysates were prepared. The supernatant from the cell lysates was flowed through a β2GPI-Affi-Gel column, and the fractional sample was analysed by Western blotting using anti-ANX2 or anti-TLR-4 antibodies. As shown in Fig. 3, both ANX2 (approximately 36 kD) and TLR-4 (approximately 100 kD) were detectable in the samples eluted in 20 mM Tris-HCl, pH 7·4/350 mM NaCl solution. However, ANX2 and TLR-4 were undetectable in the flow-away and washed solutions. These data indicate that both ANX2 and TLR-4 could interact with β2GPI on the membranes of THP-1 cells.
Fig. 3.
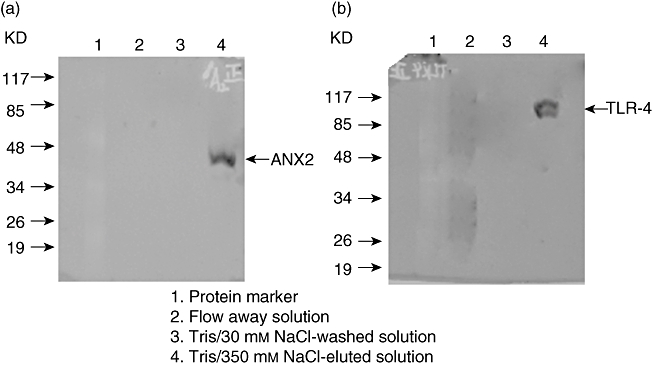
Analysis of β2-glycoprotein I (β2GPI) binding with annexin A2 (ANX2) and Toll-like receptor (TLR)-4 on THP-1 (human acute monocytic leukaemia) cell membrane. The cell lysates were extracted from 1 × 107 of THP-1 cells and subjected to affinity chromatography on β2GPI-Affi-Gel column, then the column was washed and eluted as described in Materials and methods. The proteins in each solution were analysed by Western blotting using anti-ANX2 or anti-TLR-4 antibodies. The protein bands of approximately 36 kD (ANX2) (a) and 100 kD (TLR-4) (b) were found only in Tris/350 mM NaCl-eluted solution.
Paclitaxel inhibits the effects of anti-β2GPI/β2GPI in THP-1 cells
We used paclitaxel, which competes with the MD-2 ligand, to confirm further the effects of the TLR-4 axis in anti-β2GPI/β2GPI-induced TF expression in THP-1 cells. Paclitaxel (1 µM) could decrease TLR-4 (Fig. 4a1,a2), MyD88 (Fig. 4b1,b2) and MD-2 (Fig. 4c1,c2) secretion in THP-1 cells treated with anti-β2GPI/β2GPI complex or LPS. Furthermore, the TF mRNA level and activity were also reduced in the presence of 1 µM paclitaxel, despite cells being pretreated by anti-β2GPI/β2GPI complex or LPS (shown in Fig. 5). Treatment of paclitaxel alone did not affect the expression of TF and TLR-4, MyD88 and MD-2. These data indicate that TLR-4 and its signal transduction pathway are associated with anti-β2GPI/β2GPI-induced TF expression in THP-1 cells.
Fig. 4.
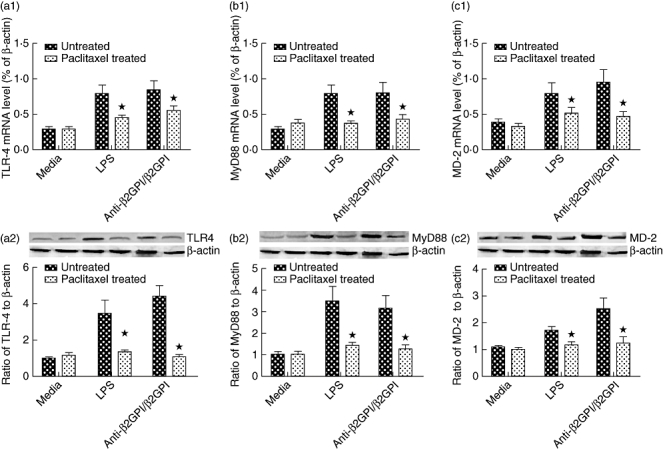
Paclitaxel decreased anti-β2-glycoprotein I/β2-glycoprotein I (anti-β2GPI/β2GPI)-induced Toll-like receptor (TLR)-4, myeloid differentiation factor 88 (MyD88) and myeloid differentiation protein 2 (MD-2) expression in THP-1 (human acute monocytic leukaemia) cells. The THP-1 cells were pretreated with paclitaxel (1 µM) for 1 h, then stimulated by anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex, isotype control rabbit immunoglobulin G (R-IgG) (10 µg/ml)/β2GPI (100 µg/ml) and lipopolysaccharide (LPS) (500 ng/ml) for 2 h (a) or 6 h (b). The mRNA levels and the protein expression of TLR-4 (a1, a2), MyD88 (b1, b2) and MD-2 (c1, c2) were detected by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) and Western blotting. Data shown are from three separate experiments. *P < 0·05 versus no paclitaxel-treated cells.
Fig. 5.
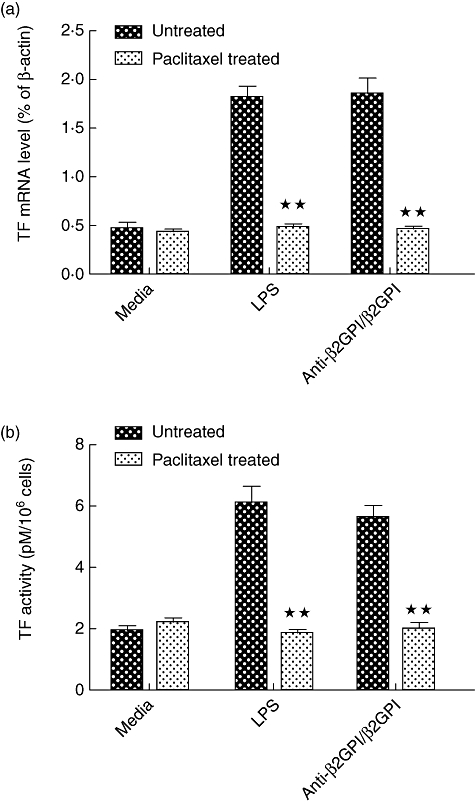
Paclitaxel inhibited anti-β2-glycoprotein I/β2-glycoprotein I (anti-β2GPI/β2GPI)-stimulated tissue factor (TF) expression on THP-1 (human acute monocytic leukaemia) cells. The THP-1 cells were pretreated with paclitaxel (1 µM) for 1 h, then stimulated by anti-β2GPI (10 µg/ml)/β2GPI (100 µg/ml) complex, isotype control rabbit immunoglobulin G (R-IgG) (10 µg/ml)/β2GPI (100 µg/ml) and lipopolysaccharide (LPS) (500 ng/ml) for 2 h (a) or 6 h (b). The TF mRNA (a) and TF activity (b) were measured by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) and TF activity kits, respectively. Data shown are from three separate experiments. **P < 0·01 versus no paclitaxel-treated cells.
Effects of ANX2 knock-down in THP-1 cells
To study further the relationship between ANX2 and TLR-4 in anti-β2GPI/β2GPI-induced TF expression, we used LV-RNAi-ANX2 to knock down ANX2 in THP-1 cells. The RNA interference efficiency was examined by qRT–PCR and Western blot analysis. ANX2 mRNA levels were almost abolished in THP-1 cells compared to the cells transfected with the control siRNA or LV-GFP (Fig. 6a). Meanwhile, ANX2 protein was not detectable in these cells by Western blot analysis (Fig. 6b).
Fig. 6.
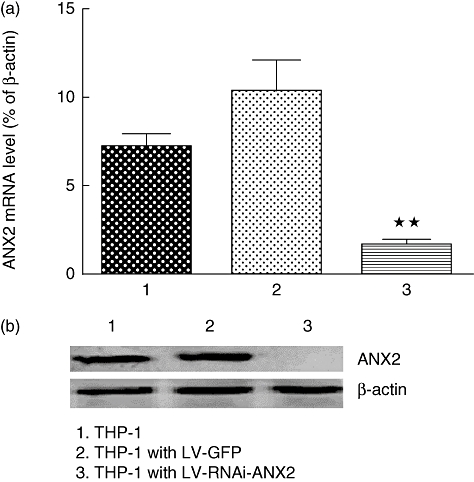
Annexin A2 (ANX2) expression in lentiviral-infected THP-1 (human acute monocytic leukaemia) cells. The empty lentivirus (LV-GFP) and ANX2 siRNA (LV-RNAi-ANX2) were transferred into target THP-1 cells at multiplicity of infection (MOI) equal to 100 with enhanced infection solution (ENi.S) and 5 µg/ml polybrene. After 72 h, the ANX2 mRNA (a) and its protein (b) levels on the cells were detected by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) or Western blot. **P < 0·01 versus no lentivirus cells. Data shown are from three separate experiments.
Furthermore, ANX2 knock-down of anti-β2GPI/β2GPI complex-treated THP-1 cells decreased TLR-4, MyD88 and MD-2 expression significantly at both the mRNA and protein levels (Fig. 7). These results indicate that TLR-4 and its related molecules could be linked or regulated via ANX2 in THP-1 cells. Finally, a remarkable decrease was observed of TF mRNA expression (Fig. 8a) and TF activity (Fig. 8b) on ANX2 RNAi-cells treated with anti-β2GPI/β2GPI complex. The effects of LPS on TF expression in THP-1 cells were not affected by LV-RNAi-ANX2 transfection.
Fig. 7.
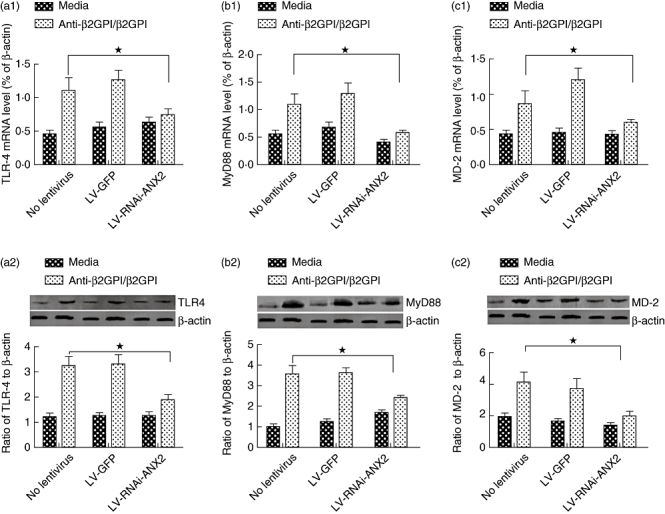
Toll-like receptor (TLR)-4, myeloid differentiation factor 88 (MyD88) and myeloid differentiation protein 2 (MD-2) expression in lentiviral-infected THP-1 (human acute monocytic leukaemia) cells. The THP-1 cells infected with LV-GFP or LV-RNAi-annexin A2 (ANX2) were incubated with anti-β2-glycoprotein I (β2GPI) (10 µg/ml)/β2GPI (100 µg/ml) for 2 h or 6 h. The mRNA levels and the protein expression of TLR-4 (a1, a2), MyD88 (b1, b2) and MD-2 (c1, c2) were detected by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) and Western blotting. Data shown are from three separate experiments. *P < 0·05 versus no lentivirus cells.
Fig. 8.
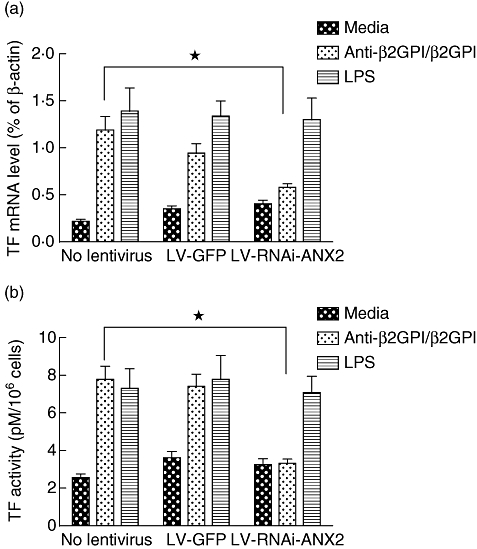
Tissue factor (TF) expression in lentiviral-infected THP-1 (human acute monocytic leukaemia) cells. The THP-1 cells infected with LV-GFP or LV-RNAi-annexin A2 (ANX2) were incubated with anti-β2-glycoprotein I (β2GPI) (10 µg/ml)/β2GPI (100 µg/ml), lipopolysaccharide (LPS) (500 ng/ml) for 2 h or 6 h. TF mRNA (a) and TF activity (b) were examined by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) and and TF activity kits, respectively. Data shown are from three separate experiments. *P < 0·05 versus no lentivirus cells.
Discussion
APS is a devastating disease with significant morbidity and mortality; aPL antibodies, such as anti-β2GPI, are associated closely with thrombotic events (recurrent venous and arterial thrombosis and/or repeated fetal loss) in APS. A large number of evidence demonstrates that β2GPI is the most common target for aPL [18]. de Laat et al. demonstrated that anti-β2GPI antibodies binding to a cryptic epitope on domain I of β2GPI (G40-R43) mediated the pathophysiology of APS [19]. Anti-β2GPI/β2GPI can bind at the surface membranes of monocytes and endothelial cells, promoting TF activity on these cells and thereby increasing the risk of thrombosis. Therefore, TF is considered as an important factor that contributes to hypercoagulability in APS [20]. It has been shown that β2GPI interacts with cell surfaces through negatively charged phospholipids. In addition, β2GPI is able to bind membrane receptors involved in activation of endothelial cells, monocytes and platelets, which includes β2GPI cell receptors, ANX2, megaline and apolipoprotein E receptor 2′ (apoER2′) [9,10,21,22].
ANX2 (formerly called annexin II) is a member of the annexin superfamily proteins which share structural and functional features [23]. Surface expression of ANX2 has been found in a variety of cells, and as a receptor, ANX2 mediates the binding of tissue-type plasminogen activator or plasminogen to cells, contributing to plasminogen activation, fibrinolysis and extracellular matrix degradation [24]. It was reported recently by Zhang et al. that ANX2 also mediates anti-β2GPI/β2GPI complex binding to endothelial cell surfaces, stimulating endothelial activation and increasing the levels of TF, vascular cell adhesion molecule 1 (VCAM-1) and other inflammatory molecules in circulation [9]. Our previous study showed that ANX2 could be expressed on the surface membranes of peripheral blood monocytes and the monocytic THP-1 cells. ANX2 could mediate the binding of anti-β2GPI/β2GPI complex to monocytes and induce TF expression. Furthermore, TF expression was reduced dramatically in ANX2 knock-down cells [25].
Annexins are calcium-dependent phospholipid binding proteins that lack a hydrophobic signalling sequence, thus it is unlikely to be involved in intracellular signal transduction mediated by anti-β2GPI/β2GPI. TLRs are part of the innate immune response that bridge innate and specific immunities [26]. Recently, it has been presumed that TLRs and its related signalling molecules are associated with the pathological mechanisms of APS [27,28]. In particular, Raschi et al. reported that MyD88, an adaptor molecule for TLR-4, could transduce the TLR-mediated intracellular signalling triggered by aPL or anti-β2GPI antibodies in vitro[29]. Morever, TLR-4 also interacts with aPL in endothelial cells in vivo[30].
In the present study, we showed that TF expression in THP-1 cells could be induced by anti-β2GPI/β2GPI complex (Fig. 1). The effects of anti-β2GPI/β2GPI were not Fc-mediated, which has been demonstrated previously by our group [7]. The levels of TF expression induced with LPS (as a positive control) in our assays seemed lower than previously reported studies, suggesting that THP-1 cells may not respond to LPS.
ANX2 is involved in anti-β2GPI/β2GPI-induced TF expression of monocytes [10,25]. Whether TLR-4 can act as a co-repressor for ANX2 in anti-β2GPI/β2GPI-induced TF expression of monocytes is not known. In this study, the anti-β2GPI/β2GPI complex could increase the secretion of TLR-4, MD-2 and MyD88 in THP-1 cells (Fig. 2). Increased TLR-4 expression may be due to binding of a specific ligand or stimulation of specific reagents (such as anti-β2GPI/β2GPI complex) that activates a signal transduction pathway.
We used paclitaxel to confirm further the effects of anti-β2GPI/β2GPI on TF expression in THP-1 cells. Paclitaxel could decrease TLR-4, MD-2 and MyD88 expression significantly at both the mRNA and protein levels (Fig. 4). Meanwhile, TF mRNA and TF activity were also reduced in the pretreated cells with paclitaxel (Fig. 5). Paclitaxel has been reported as a novel approach to block LPS-induced TLR-4 expression in monocytes by binding to MD-2 [31,32]. The inhibition of paclitaxel on the effects of anti-β2GPI/β2GPI in THP-1 cells demonstrated further that TLR-4 and its signal transduction pathway were associated with the β2GPI or anti-β2GPI/β2GPI complex. These results suggest that paclitaxel might be used as an inhibitor of TF expression and as a novel therapeutic approach in APS.
The relationship between TLR-4 and ANX2 was investigated further in our study. The results showed that, along with ANX2, TLR-4 in THP-1 cell lysates could bind to β2GPI which had been conjugated to the CNBr-activated-sepharose-4B column (Fig. 3). Furthermore, ANX2 knock-down showed that the expression of TLR-4, MD-2 and MyD88 stimulated by anti-β2GPI/β2GPI complex was remarkably reduced in LV-RNAi-ANX2-transfected THP-1 cells (Fig. 7). Interestingly, anti-β2GPI/β2GPI-induced TLR-4, MD-2 and MyD88, as well as TF expression was modestly higher in LV-RNAi-ANX2 transfected THP-1 cells than in unstimulated cells. These findings may suggest that other membrane receptors besides ANX2, such as TLR-4, may mediate the effects of anti-β2GPI/β2GPI in cells. It was reported recently that TLR-2, but not TLR-4, interacted with β2GPI on the membrane of endothelial cells [33].
Taken together, our current study demonstrated that anti-β2GPI/β2GPI-stimulated TF expression is mediated by TLR-4, ANX2 and their signalling proteins in THP-1 cells, which may contribute to the pathological processes in APS. Further studies are needed to understand the intracellular signalling transduction pathway activated by TLR-4, which may include interleukin-1 receptor-associated kinases (IRAKs), tumour necrosis factor receptor-associated factors (TRAFs), mitogen-activated protein kinases (MAPKs) and nuclear factor kappaB (NF-κB).
Acknowledgments
This work was supported by grants of National Natural Science Foundation of China (no. 30670907, 30971301) to Hong Zhou.
Disclosure
None.
References
- 1.de Groot PG, Derksen RH. Antiphospholipid antibodies: update on detection, pathophysiology, and treatment. Curr Opin Hematol. 2004;11:165–9. doi: 10.1097/01.moh.0000130313.95291.4a. [DOI] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.Bas de Laat H, Derksen RH, de Groot PG. beta2-glycoprotein I, the playmaker of the antiphospholipid syndrome. Clin Immunol. 2004;112:161–8. doi: 10.1016/j.clim.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Kinev AV, Roubey RA. Tissue factor in the antiphospholipid syndrome. Lupus. 2008;17:952–8. doi: 10.1177/0961203308096662. [DOI] [PubMed] [Google Scholar]
- 5.Morrissey JH. Tissue factor: an enzyme cofactor and a true receptor. Thromb Haemost. 2001;86:66–74. [PubMed] [Google Scholar]
- 6.Steinemann S, Ulevitch RJ, Mackman N. Role of the lipopolysaccharide (LPS)-binding protein/CD14 pathway in LPS induction of tissue factor expression in monocytic cells. Arterioscler Thromb. 1994;14:1202–9. doi: 10.1161/01.atv.14.7.1202. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Wolberg AS, Roubey RA. Characterization of monocyte tissue factor activity induced by IgG antiphospholipid antibodies and inhibition by dilazep. Blood. 2004;104:2353–8. doi: 10.1182/blood-2004-01-0145. [DOI] [PubMed] [Google Scholar]
- 8.Wolberg AS, Roubey RA. Mechanisms of autoantibody-induced monocyte tissue factor expression. Thromb Res. 2004;114:391–6. doi: 10.1016/j.thromres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2005;105:1964–9. doi: 10.1182/blood-2004-05-1708. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Ling S, Yu Y, Wang T, Hu H. Involvement of annexin A2 in anti-beta2GPI/beta2GPI-induced tissue factor expression on monocytes. Cell Res. 2007;17:737–9. doi: 10.1038/cr.2007.33. [DOI] [PubMed] [Google Scholar]
- 11.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, et al. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114:3074–83. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raschi E, Borghi MO, Grossi C, Broggini V, Pierangeli S, Meroni PL. Toll-like receptors: another player in the pathogenesis of the anti-phospholipid syndrome. Lupus. 2008;17:937–42. doi: 10.1177/0961203308095140. [DOI] [PubMed] [Google Scholar]
- 13.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Hoebe K, Beutler B. Forward genetic analysis of TLR-signaling pathways: an evaluation. Adv Drug Deliv Rev. 2008;60:824–9. doi: 10.1016/j.addr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Saitoh S, Tanimura N, et al. Regulatory roles for MD-2 and TLR4 in ligand-induced receptor Clustering. J Immunol. 2006;176:6211–18. doi: 10.4049/jimmunol.176.10.6211. [DOI] [PubMed] [Google Scholar]
- 16.Resman N, Vasl J, Oblak A, et al. Essential roles of hydrophobic residues in both MD-2 and Toll-like receptor 4 in activation by endotoxin. J Biol Chem. 2009;284:15052–60. doi: 10.1074/jbc.M901429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, Fang X, Tong Y, Liu Y, Zhang B. TLR4-mediated MyD88-dependent signaling pathway is activated by cerebral ischemia–reperfusion in cortex in mice. Biomed Pharmacother. 2009;63:442–50. doi: 10.1016/j.biopha.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Kajiwara T, Yasuda T, Matsuura E. Intracellular trafficking of beta2-glycoprotein I complexes with lipid vesicles in macrophages: implications on the development of antiphospholipid syndrome. J Autoimmun. 2007;29:164–73. doi: 10.1016/j.jaut.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 19.de Laat B, Derksen RH, van Lummel M, Pennings MT, Pathogenic GPG. anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycorotein I only after a conformational change. Blood. 2006;107:1916–24. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 20.Boles J, Mackman N. Role of tissue factor in thrombosis in antiphospholipid antibody syndrome. Lupus. 2010;19:370–8. doi: 10.1177/0961203309360810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi K, Matsuura E, Liu Q, et al. A specific ligand for beta(2)-glycoprotein I mediates autoantibody-dependent uptake of oxidized low density lipoprotein by macrophages. J Lipid Res. 2001;42:697–709. [PubMed] [Google Scholar]
- 22.Lutters BC, Derksen RH, Tekelenburg WL, Lenting PJ, Arnout J, de Groot PG. Dimers of beta 2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2. J Biol Chem. 2003;278:33831–8. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- 23.Moss SE, Morgan RO. The annexins. Genome Biol. 2004;5:219. doi: 10.1186/gb-2004-5-4-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falcone DJ, Birth W, Khan KM, Hajjar KA. Plasminogen-mediated matrix invasion and degradation by macrophages is dependent on surface expression of annexin II. Blood. 2001;97:777–84. doi: 10.1182/blood.v97.3.777. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Wang H, Li N, et al. Annexin A2 mediates anti-beta 2 GPI/beta 2 GPI-induced tissue factor expression on monocytes. Int J Mol Med. 2009;24:557–62. doi: 10.3892/ijmm_00000265. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 27.Hurst J, von Landenberg P. Toll-like receptors and autoimmunity. Autoimmun Rev. 2008;7:204–8. doi: 10.1016/j.autrev.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Rauch J, Dieudé M, Subang R, Levine JS. The dual role of innate immunity in the antiphospholipid syndrome. Lupus. 2010;19:347–53. doi: 10.1177/0961203310361492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raschi E, Testoni C, Bosisio D, et al. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101:3495–500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 30.Pierangeli SS, Vega-Ostertag ME, Raschi E, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66:1327–33. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmer SM, Liu J, Clayton JL, Stephens DS, Snyder JP. Paclitaxel binding to human and murine MD-2. J Biol Chem. 2008;283:27916–26. doi: 10.1074/jbc.M802826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resman N, Gradisar H, Vasl J, Keber MM, Pristovsek P, Jerala R. Taxanes inhibit human TLR4 signaling by binding to MD-2. FEBS Lett. 2008;582:3929–34. doi: 10.1016/j.febslet.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 33.Alard JE, Gaillard F, Daridon C, Shoenfeld Y, Jamin C, Youinou P. TLR2 is one of the endothelial receptors for beta2-glycoprotein I. J Immunol. 2010;185:1550–7. doi: 10.4049/jimmunol.1000526. [DOI] [PubMed] [Google Scholar]


