Abstract
Cutaneous lesions caused by Leishmania braziliensis infection occasionally heal spontaneously, but with antimonials therapy heal rapidly in approximately 3 weeks. However, about 15% of the cases require several courses of therapy. Matrix metalloproteinase-2 (MMP-2) and MMP-9 are gelatinases that have been implicated in other chronic cutaneous diseases and skin re-epithelialization. These enzymes are controlled by their natural inhibitors [tissue inhibitors of metalloproteinase (TIMPs)] and by some cytokines. Uncontrolled gelatinase activity may result in intense tissue degradation and, consequently, poorly healing wounds. The present study correlates gelatinase activity to therapeutic failure of cutaneous leishmaniasis (CL) lesions. Our results demonstrate an association between gelatinase activity and increased numbers of cells making interferon (IFN)-γ, interleukin (IL)-10 and transforming growth factor (TGF)-β in lesions from poor responders. Conversely, high levels of MMP-2 mRNA and enhanced MMP-2 : TIMP-2 ratios were associated with a satisfactory response to antimonials treatment. Additionally, high gelatinolytic activity was found in the wound beds, necrotic areas in the dermis and within some granulomatous infiltrates. These results indicate the importance of gelatinase activity in the skin lesions caused by CL. Thus, we hypothesize that the immune response profile may be responsible for the gelatinase activity pattern and may ultimately influence the persistence or cure of CL lesions.
Keywords: American tegumentary leishmaniasis, cytokines, leishmaniasis immunology, matrix metalloproteinases, therapeutic failure
Introduction
American cutaneous leishmaniasis (ACL) is an important parasitic disease caused by dermatotrophic species of Leishmania spp. Leishmania (Viannia) braziliensis is the most common and widespread species in Brazil [1]. Typical CL skin lesions are inflamed ulcers at the site of the sandfly bite. These wounds tend to be chronic, but usually evolve slowly to healing, even without treatment [2]. Resolution of CL lesions is dependent on a specific cell-mediated immune response [3,4]. ACL lesions are characterized by a robust inflammatory infiltrate of cells including macrophages, Langerhans cells and plasma cells. There is a clear predominance of T lymphocytes [5,6] that includes γδ T lymphocytes. This lymphocyte predominant inflammation is associated with an intense necrotic process [7]. The phenotypic analysis of these T cells demonstrates a mixture of helper-inducer (CD4+CD29+), memory (CD4+CD45RO+), T naive (CD4+CD45RA+), cytotoxic (CD8+) and regulatory (CD4+CD25+) profiles [5,8–10]. Consequently, in situ cytokine expression patterns may vary significantly depending on the time-point of analysis and according to clinical disease evolution. Both types 1 and 2 cytokines are expressed within CL lesions [11]. Despite this, the healing of CL is associated preferentially with a type 1 response, whereas the non-healing lesions or diffuse cutaneous leishmaniasis show a clear predominance of type 2 cytokines [11–13].
Matrix metalloproteinases (MMPs) are a family of endopeptidases involved in the skin regenerative process [14]. These zinc-dependent enzymes are essential to both the synthesis and degradation of matrix compounds involved in proliferative and migratory cellular events. MMP-2 and MMP-9 are members of the gelatinase subfamily and have been implicated in these events [15,16]. Several studies indicate the importance of these two enzymes in cutaneous wound re-epithelization and closure, as they make keratinocyte migration possible through the extracellular matrix (ECM) of injured dermis [17,18]. Positive tissue remodelling resulting in complete skin regeneration occurs only if MMP activity is regulated strongly by tissue inhibitors of metalloproteinase (TIMPs) [19]. In addition, various cytokines present in sites of inflammation have been described previously to influence MMP activity [20]. Both transforming growth factor (TGF)-β and tumour necrosis factor (TNF)-α can stimulate the expression and activation of MMPs [21,22]. Conversely, interleukin (IL)-10 decreases MMPs expression and activation [23], and interferon (IFN)-γ has variable effects on MMP synthesis and activity [24,25]. Loss of MMP activity control might result in pathological tissue degradation. Similarly, excessive MMP activity has been associated with chronic cutaneous wounds and poor wound healing [26,27].
Besides the essential need for an efficient immunological response, little is known about other mechanisms involved in the successful healing of ACL lesions. MMP-9 secreted by macrophages infected with L. chagasi may contribute to the liver injury observed in visceral leishmaniasis [28]. However, to our knowledge, the involvement of MMPs in cutaneous lesions caused by L. (V.) braziliensis has not been investigated previously. In this study, we aim to investigate the participation of gelatinases in the resolution of human CL lesions. In addition, we aim to determine some of the factors that influence gelatinase activity in these lesions and therefore interfere in the resolution process.
Materials and methods
Patient selection
Skin tissue fragments were obtained from cutaneous lesions of 39 subjects before starting the therapy. All the patients were diagnosed positively with ACL. After treatment and cure, the samples were grouped according to therapeutic response in (i) good (24 individuals) and (ii) poor responders (15 individuals). Response to treatment was considered good when lesions showed complete re-epithelialization and absence of erythema, induration or papules 3 months after the end of treatment with Glucantime® (Rhodia Laboratories, Antony, France). Poor responses were defined when healing was incomplete or when scars still showed the presence of erythema 3 months after the end of therapy. Response was also considered poor if reactivation or secondary metastatic lesions appeared. Normal human skin samples were obtained from five healthy individuals submitted to plastic surgery and used as controls. Both groups were similar regarding other clinical parameters and had similar medians of gender, age (37 years), number (one lesion) and size (good: 7·81 cm2, poor: 8·14 cm2) of lesions and duration of disease (3 months). Informed consent was obtained before all biopsies. This study was performed with the approval of the Ethical Committees of the Fundação Oswaldo Cruz and Instituto de Pesquisa Clinica Evandro Chagas.
Analysis of mRNA encoding MMPs and TIMPs
RNA isolation and cDNA synthesis
Total RNA was isolated from frozen tissue specimens using Trizol (Gibco BRL, New York, NY, USA), following the manufacturer's instructions, and cDNA synthesis was performed as described previously [11]. After isolation, first-strand cDNA was synthesized and stored at −20°C until use.
Real-time PCR
Each reaction was performed in duplicate. PCR reactions were performed in a final volume of 25 µl consisting of SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 10 pmoles of combined sense and anti-sense primers and water. Real-time PCR amplifications were carried out in ABI Prism 7000 Sequence Detector (Applied Biosystems) with temperature profiles as follows: initial denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 30 s, annealing at 59°C for 1 min and extension at 72°C for 1 min. A melt curve (95°C for 15 s, 60°C for 20 s, 95°C for 15 s) was generated at the end of each run to verify specificity of amplified products. Standard curves for all targets were performed. For the individual samples, the final value of each target gene is given as a coefficient normalized to constitutive gene values (β-actin). The sequences of 5′ and 3′ primer pairs are as follows: β-actin: TAATGTCACGCACGATTTCCC and TCACCGAGCGCGGCT; MMP-2: TGCTGGAGACAAATTCTGGAGATA and ACTTCACGCTCTTCAGACTTTGG; MMP-9: ACGATGCCTGCAACGTGA and ATACAGCTGGTTCCCAATCTCC; TIMP-1: CCCAGAGAGACACCAGAGAAC and CACGAACTTGGCCCTGATGAC; and TIMP-2: GCACATCACCCTCTGTGACTT and AGCGCGTGATCTTGCACT.
In situ zymography
In situ zymography was performed as described previously [29]. Briefly, microscopic slides were covered with a film of 50 µm thickness of 10% polyacrylamide gel containing gelatin at a final concentration of 15 mg/ml. Frozen tissue sections of 8 µm thickness were cut on a cryostat, placed onto the gels and incubated in a moist chamber for 24 h at 37°C. After incubation, sections were stained with methylene blue and photographed using a Coolpix camera (Media Cybernetics, Bethesda, MD, USA) coupled to a Nikon E600 microscope (Nikon Corporation, Tokyo, Japan) using ImagePro software (Media Cybernetics). Slides were then immersed in 5% sodium dodecyl sulphate (SDS) in phosphate-buffered saline (PBS, pH 7·4) for 30 min at 37°C and the tissue section was removed carefully. Finally, the remaining polyacrylamide gel was stained with Coomassie blue solution (45% methanol and 10% acetic acid). Stained gels were re-photographed using the same magnification and fields and evaluated using ImagePro software. As negative control, an additional polyacrylamide gel without gelatin was used with each section and submitted to the same conditions. The results represented percentage of positive area to gelatinase activity.
Analysis of cells producing cytokines and MMPs in tissue
Evaluation of cells producing cytokines and MMPs was performed as described. Cryosections were fixed in formol–acetone–methanol (FMA; 2:19:19) for 2 min and hydrated in Tris-buffered solution (TBS). Slides were incubated with primary anti-human antibodies for 12 h at 4°C (17 µg/ml for IFN-γ, 10 µg/ml for IL-10 and 25 µg/ml for TGF-β; BD Biosciences Pharmingen, San Diego, CA, USA; 10 µg/ml for MMP-2, and 5 µg/ml for MMP-9; R&D Systems, Minneapolis, MN, USA), followed by incubation for 30 min with Envision anti-mouse antibody kit (Dako, Carpinteria, CA, USA). Every reaction included negative controls, which excluded the specific primary antibodies. The measurement was based on counting the percentage of positive cells into 500 cells per section by two independent observers.
Statistical analysis
To determine group differences we used the Mann–Whitney t-test. Linear regression and/or non-parametric (Spearman's) correlation was used to evaluate the relationship between two groups. Differences were considered significant when P-value was <0·05. All data were expressed as mean + standard error of the mean (s.e.m.). Prism version 5 for Windows was used throughout (GraphPad Software, San Diego, CA, USA; http://www.graphpad.com).
Results
Compared to healthy skin, all ACL lesions showed statistically significant differences in cytokine production, MMP expression and gelatinase activity (not shown).
Lesions from patients with poor response to therapy showed decreased ratios of MMP-2 : TIMP-2 mRNA expression and higher gelatinase activity
To determine if gelatinase activity corresponded to treatment response in human CL, we analysed gelatinases and TIMP gene expression in CL lesions using real-time PCR. According to the group classification, there were significantly higher levels of MMP-2 mRNA in lesions from good responders (n = 24) compared to poor responders (n = 15) (P= 0·04, Fig. 1a). In contrast, MMP-9, TIMP-1 and TIMP-2 mRNA levels were similar in lesions from both groups. An evaluation of the relative amounts of MMPs and TIMPs mRNA was performed. We observed that ratios between the levels of mRNA encoding MMP-2 and TIMP-2 correlated to response to therapy (Fig. 1b). In lesions from good responders, the ratio of MMP-2 : TIMP-2 mRNA levels was higher (median = 9·7) than in the poor responder group (median = 0·6). The ratios of mRNAs for MMP-9 and TIMP-1 were comparable in both groups, with ratio values above 1 in all samples (Fig. 1b). These data suggest that the high ratios of MMP-2 : TIMP-2 are associated with successful healing.
Fig. 1.
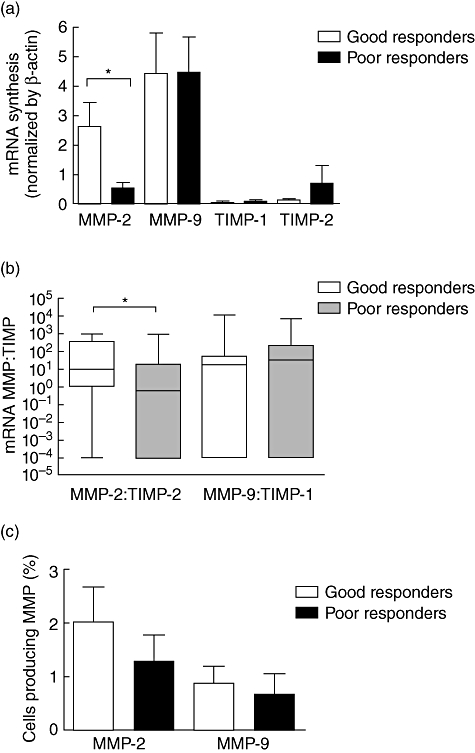
Detection of gelatinases and tissue inhibitors of metalloproteinase (TIMP) in cutaneous leishmaniasis lesions (CL). Matrix metalloproteinase (MMP) and TIMP mRNA levels in 39 CL lesions fragments were evaluated by real-time polymerase chain reaction (PCR). (a) MMP-2 mRNA levels were highly expressed in lesions from patients with good response to therapy (n = 24) compared to poor responders (n = 15). Conversely, MMP-9, TIMP-1 and TIMP-2 mRNA levels showed similar expression between groups. Gelatinase and TIMP mRNA levels were normalized using β-actin expression data. (b) The assay of MMP : TIMP ratios showed that high MMP-2 : TIMP-2 ratios correlate to a satisfactory response to antimonials, suggesting that high ratios values of MMP-2 : TIMP-2 may contribute to an efficient healing process. In contrast, MMP-9 : TIMP-1 ratios were similar between groups. (c) Cells producing MMP-2 and MMP-9 were detected by immunohistochemistry. In lesions from good responders (n = 10) a tendency was observed for more cells to produce MMP-2 than those producing MMP-9 (P = 0·06). The same could not be observed in poor responders (n = 5; P = 0·2). *P< 0·05. Bars represent standard error of the mean.
MMP-2 and MMP-9 proteins were detected in situ (Fig. 1c). In good responders, there was a tendency for more cells to produce MMP-2 than those producing MMP-9 (n = 10). However, in the small number of samples examined, this same tendency was not observed in poor responders (n = 5). Nevertheless, because MMPs are released as zymogens and need to be cleaved to have activity, the detection of protein production cannot predict the activity levels of these enzymes. Therefore, to determine the functional activity of these MMPs and localize it within the lesions, we measured gelatinase activity directly in tissues. In situ zymography analysis demonstrated that gelatinase activity was stronger within lesions from poor responders (n = 15) than in lesions from good responders (n = 24) (Fig. 2a). Additionally, within the same group, gelatinolytic activity intensity is similar regardless of the duration of the disease. This indicates that ulcer age does not influence the magnitude of gelatinase activity obtained (not shown).
Fig. 2.

In situ assay of gelatinase activity in cutaneous leishmaniasis lesions. Gelatinolytic activity was measured direct in the tissue by in situ zymography. (a) The measurements demonstrated higher levels of gelatinase activity in lesions from poor responder individuals (n = 15) compared to good responders (n = 24). Data in the graph represent the percentage of the area in the tissue section exhibiting gelatinase activity (corresponding to the clear areas on the gel). The values were obtained by densitometric analysis of the gel. (b–d) Ulcerated areas of sections demonstrated intense gelatinolytic activity. (e–g) Areas of increased gelatinase activity are also associated with necrosis at dermis. (h–j). Granuloma and inflammatory infiltrated cells also show gelatinolytic activity (red arrows). (b,e,h) Haematoxylin and eosin (H&E) staining. (c,f,i) In situ zymography reaction. (d,g,j) Superposition of H&E staining and zymography pictures. Original magnification (b–j): 40×. *P< 0·05. Bars represent standard error of the mean.
The localization of gelatinase activity in the lesion was possible by comparing the results acquired by in situ zymography and haematoxylin and eosin (H&E) staining using sequential sections. There was notable gelatinolytic activity at the epidermis associated with the ulcer (Fig. 2b–d). Moreover, gelatinase activity was present in most necrotic areas at the level of the dermis (Fig. 2e–g). In some cases, gelatinase activity also was detected within granuloma infiltrates (Fig. 2h–j).
Lesions from patients with poor therapeutic response showed higher numbers of cells expressing IFN-γ, TGF-β and IL-10
Cytokines present at inflammatory sites may direct the response to therapy and contribute to resolution of CL lesions. In situ analysis of cytokine expression showed that lesions from poor responders had higher numbers of cells producing IFN-γ (P= 0·003), IL-10 (P= 0·02) and TGF-β (P= 0·01) (Fig. 3a). In addition, we observed that the areas of lesions from poor responders with high rates of cells producing TGF-β also had increased numbers of cells producing IL-10 or IFN-γ. The correlation percentages were 71% for TGF-β and IL-10 and 87% for TGF-β and IFN-γ (Table 1).
Fig. 3.
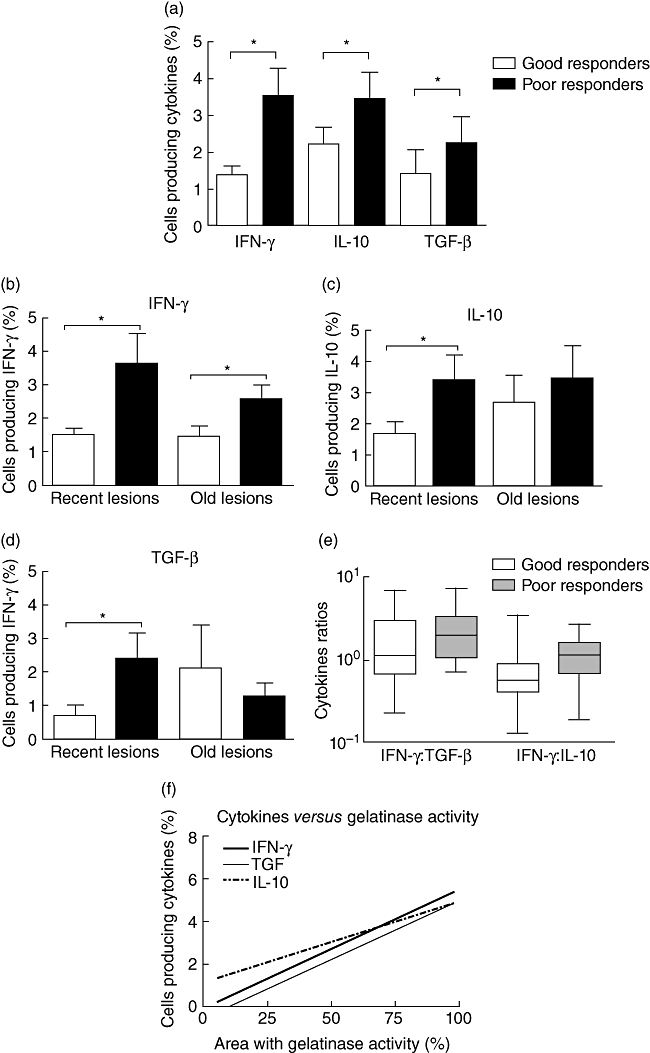
In situ analysis of cytokine production in cutaneous leishmaniasis lesions. Cytokines were assayed by immunohistochemistry in frozen sections of 39 skin lesions. (a) In situ analysis of interferon (IFN)-γ, interleukin (IL)-10 and transforming growth factor (TGF)-β showed that the three cytokines were produced in similar patterns. The Mann–Whitney U-test was used to compare the different cytokines and showed that lesions from poor responders (n = 15) had greater numbers of cells secreting the three cytokines compared to good responders (n = 24). (b–d) Using the ulcer age as an additional parameter, we could observe that recent lesions (<3 months) showed the most important differences of cytokine production. Lesions from poor responders had higher rates of cells producing IFN-γ, IL-10 and TGF-β. (e) Comparing the ratios of cells making the cytokines analysed, there was a predominance of proinflammatory cytokines in lesions from poor responders and a predominance of the anti-inflammatory cytokine IL-10 in lesions from good responders. *P< 0·05. Bars represent standard error of the mean. (f) The non-parametric (Spearman's) correlation test showed a strong and positive association of areas showing gelatinolytic activity and rates of cells making the three cytokines analysed (IFN-γ: P= 0·0002; TGF-β: P< 0·0001; IL-10: P= 0·01).
Table 1.
Evaluation of presence of cells making the different cytokines in the same tissue section.
| Therapeutic response (r2) | ||
|---|---|---|
| Cytokines | Good | Poor |
| IFN-γ× TGF-β | 0·2053 | 0·8774 |
| IFN-γ× IL-10 | 0·2395 | 0·5591 |
| IL-10 × TGF-β | 0·5836 | 0·7111 |
Using linear regression analysis, we found a linear relationship between the numbers of cells expressing two cytokines in the same tissue section. Considering response to therapy, we could observe a strong correlation (high r2) of cells expressing transforming growth factor (TGF)-β and interleukin (IL)-10 or interferon (IFN)-γ in lesions from poor responder patients. The values are expressed in r2.
Besides the response to therapy, the duration of disease also correlated with cytokine response. Recent lesions (<3 months) in poor responders had greater numbers of cells producing the three cytokines compared to recent lesions from good responders (IFN-γ: P= 0·006; IL-10: P= 0·03; TGF-β: P= 0·006). Conversely, the old lesions (>3 months) did not show this difference (Fig. 3b–d).
Finally, we determined that poor responders showed higher ratios of cells making inflammatory cytokines, with IFN-γ : TGF-β ratio equal to 2·0. Interestingly, in lesions from good responders there was a preponderance of anti-inflammatory cytokines, where the ratio of IFN-γ : IL-10 was 0·5 (Fig. 3e).
MMP activity has been correlated with the immune phenotype of inflammatory cells in other systems. Correlating the results obtained by in situ zymography and immunohistochemistry with cytokines, we noticed a strong and positive relationship between gelatinolytic activity and the three cytokines analysed (IFN-γ: P= 0·0002; TGF-β: P< 0·0001; IL-10: P= 0·01) (Fig. 3f).
Discussion
Although cutaneous leishmaniasis (CL) lesions may require more than 6 months for complete cure to occur, treatment with antimonials can reduce the healing time significantly. Most patients are cured clinically approximately 3 weeks after the completion of treatment, but about 15% of the cases require several courses of therapy [30].
Even though the importance of the immunological response (innate and acquired) has been well established in the elimination of Leishmania infection and healing of resulting lesions, the mechanisms involved in skin damage and ulcer resolution are poorly understood. The results of this work demonstrate that low MMP-2 mRNA and high gelatinolytic activity levels were detected, together with an increased number of cells producing IFN-γ, TGF-β and IL-10 in lesions from patients with poor response to antimonial treatment. Additionally, we found that increased MMP2 : TIMP2 mRNA ratios were associated with successful healing during therapy.
Loss of gelatinase synthesis and activity control has been implicated in many destructive diseases, including rheumatoid arthritis, multiple sclerosis, cancer and poor wound healing [31–34]. Furthermore, gelatinase activity also may contribute to pathological events triggered by infectious agents. Infection caused by L. chagasi stimulates murine macrophages to produce MMP-9 [28]. Our group also demonstrated that L. braziliensis infection acutely induces the activation of MMP-9 in primary human macrophages in vitro (submitted for publication elsewhere). In addition, other infectious processes show an intimate relationship between gelatinase activity control and the immunological status of the individual affected. In leprosy, tuberculoid lesions are associated with high levels of TNF-α, IFN-γ, MMP-2 and MMP-9 mRNA and intense gelatinolytic activity. Conversely, lesions from the opposite immunological pole (lepromatous form) do not exhibit this profile [35].
In cutaneous leishmaniasis caused by L. braziliensis, a mix of cytokine profiles can be found in the lesions. At the tissue level, this parasite induces an inflammatory response mediated by T helper type 1 (Th1) cytokines to control the infection [11]. However, TGF-β and IL-10 have been correlated with persistent infection and chronic lesions [36,37]. Although IFN-γ is reported as crucial for CL clinical resolution [38,39], other authors have reported that IFN-γ can have a ‘pro-proteolytic’ impact [40]. These findings corroborate our results, as lesions from poor responders had both higher numbers of cells producing IFN-γ and higher levels of gelatinase activity. Moreover, the observation that there were more cells making these cytokines in recent lesions from poor responders suggests that the first months are the most important to establish an effective immune response that may result in the success or failure of wound healing.
The high ratios of proinflammatory cytokines found in lesions from poor responders (IFN-γ : TGF-β = 2·0) also suggests that the excess of IFN-γ can have the opposite effect and impair wound healing [41]. In contrast, the preponderance of anti-inflammatory cytokines in lesions from good responders (IFN-γ : IL-10 = 0·5) may be responsible for the low gelatinase activity observed in these lesions. IL-10 seems to be unique among the lymphokines in its ability to suppress the production and activation of MMPs, thus having an important matrix-protective role during inflammation [23]. In addition, the high levels of MMP-2 mRNA in lesions from good responders are consistent with other reports, where increased MMP-2 levels were required for cutaneous wound re-epithelialization [42].
Overall, the participation of MMP-2 and MMP-9 in CL skin damage was suggested by detection of gelatinase activity in necrotic areas, wound bed and inflammatory infiltrate. Furthermore, the contribution of gelatinases to therapeutic failure of CL lesions was indicated by (i) the wide gelatinase activity associated with increased numbers of cells producing IFN-γ, TGF-β and IL-10 in lesions from poor responders; (ii) the increased MMP-2 mRNA levels and MMP-2 : TIMP-2 ratios observed in lesions from good responders; (iii) the preponderance of pro-proteolytic cytokine IFN-γ in lesions from poor responders; and (iv) the prevalence of the anti-inflammatory cytokine IL-10 associated with the low intensity of gelatinase activity in lesions from good responders.
In conclusion, we suggest that the immunological profile in response to cutaneous leishmaniasis is established at the beginning of the infection and may directly influence the gelatinase activity patterns in CL lesions. Regulation of MMP and TIMP production resulting from the cytokine repertoire presented at an inflammatory site may ultimately determine the success or failure of wound healing during antimonial therapy for leishmaniasis.
Acknowledgments
We thank Dr C. Mauch's group from University of Cologne, Germany, for providing us with the in situ zymography protocol. Thanks also for the excellent real-time PCR technical support offered by Dr C. Britto and M. A. Cardoso from Fundação Oswaldo Cruz, Brazil. This work was supported by Instituto Oswaldo Cruz (IOC), Conselho Nacional de Pesquisa (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Disclosure
None to disclose.
References
- 1.Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. doi: 10.4269/ajtmh.1989.41.687. [DOI] [PubMed] [Google Scholar]
- 2.Costa JML, Netto EM, Vale KC, Osaki NK, Tada MS, Marsden PD. Spontaneous healing of cutaneous Leishmania brasiliensis brasiliensis ulcers. Trans R Soc Trop Med Hyg. 1987;81:606. doi: 10.1016/0035-9203(87)90424-x. [DOI] [PubMed] [Google Scholar]
- 3.Castes M, Agnelli A, Rondon AJ. Mechanisms associated with immunoregulation in human American cutaneous leishmaniasis. Clin Exp Immunol. 1984;57:279–86. [PMC free article] [PubMed] [Google Scholar]
- 4.Mendonca SC, Coutinho SG, Amendoeira RR, Marzochi MC, Pirmez C. Human American cutaneous leishmaniasis (Leishmania b. braziliensis) in Brazil: lymphoproliferative responses and influence of therapy. Clin Exp Immunol. 1986;64:269–76. [PMC free article] [PubMed] [Google Scholar]
- 5.Pirmez C, Cooper C, Paes-Oliveira M, Schubach A, Torigian VK, Modlin RL. Immunologic responsiveness in American cutaneous leishmaniasis lesions. J Immunol. 1990;145:3100–4. [PubMed] [Google Scholar]
- 6.Da Cruz AM, Bertho AL, Oliveira-Neto MP, Coutinho SG. Flow cytometric analysis of cellular infiltrate from American tegumentary leishmaniasis lesions. Br J Dermatol. 2005;153:537–43. doi: 10.1111/j.1365-2133.2005.06647.x. [DOI] [PubMed] [Google Scholar]
- 7.Ridley MJ, Ridley DS. Cutaneous leishmaniasis: immune complex formation and necrosis in the acute phase. Br J Exp Pathol. 1984;65:327–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Campanelli AP, Roselino AM, Cavassani KA, et al. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis. 2006;193:1313–22. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]
- 9.Pirmez C. Immunopathology of American cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 1992;87(Suppl 5):105–9. doi: 10.1590/s0074-02761992000900016. [DOI] [PubMed] [Google Scholar]
- 10.Esterre P, Dedet JP, Frenay C, Chevallier M, Grimaud JA. Cell populations in the lesion of human cutaneous leishmaniasis: a light microscopical, immunohistochemical and ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1992;421:239–47. doi: 10.1007/BF01611181. [DOI] [PubMed] [Google Scholar]
- 11.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceicao-Silva F, Modlin RL. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–5. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutinho SG, Oliveira MP, Da Cruz AM, et al. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–55. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 13.Caceres-Dittmar G, Tapia FJ, Sanchez MA, et al. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin Exp Immunol. 1993;91:500–5. doi: 10.1111/j.1365-2249.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res. 2004;299:465–75. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Agren MS. Matrix metalloproteinases (MMPs) are required for re-epithelialization of cutaneous wounds. Arch Dermatol Res. 1999;291:583–90. doi: 10.1007/s004030050459. [DOI] [PubMed] [Google Scholar]
- 16.Woessner JF., Jr The family of matrix metalloproteinases. Ann NY Acad Sci. 1994;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- 17.Makela M, Larjava H, Pirila E, et al. Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp Cell Res. 1999;251:67–78. doi: 10.1006/excr.1999.4564. [DOI] [PubMed] [Google Scholar]
- 18.Salo T, Makela M, Kylmaniemi M, Autio-Harmainen H, Larjava H. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab Invest. 1994;70:176–82. [PubMed] [Google Scholar]
- 19.Murphy G, Willenbrock F, Crabbe T, O'Shea M, Ward R, Atkinson S. Regulation of matrix metalloproteinase activity. Ann NY Acad Sci. 1994;732:31–41. doi: 10.1111/j.1749-6632.1994.tb24722.x. [DOI] [PubMed] [Google Scholar]
- 20.Abraham M, Shapiro S, Karni A, Weiner HL, Miller A. Gelatinases (MMP-2 and MMP-9) are preferentially expressed by Th1 vs. Th2 cells. J Neuroimmunol. 2005;163:157–64. doi: 10.1016/j.jneuroim.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Seomun Y, Kim JT, Joo CK. MMP-14 mediated MMP-9 expression is involved in TGF-beta1-induced keratinocyte migration. J Cell Biochem. 2008;104:934–41. doi: 10.1002/jcb.21675. [DOI] [PubMed] [Google Scholar]
- 22.Makela M, Salo T, Larjava H. MMP-9 from TNF alpha-stimulated keratinocytes binds to cell membranes and type I collagen: a cause for extended matrix degradation in inflammation? Biochem Biophys Res Commun. 1998;253:325–35. doi: 10.1006/bbrc.1998.9641. [DOI] [PubMed] [Google Scholar]
- 23.Stearns ME, Wang M, Hu Y, Garcia FU, Rhim J. Interleukin 10 blocks matrix metalloproteinase-2 and membrane type 1-matrix metalloproteinase synthesis in primary human prostate tumor lines. Clin Cancer Res. 2003;9:1191–9. [PubMed] [Google Scholar]
- 24.Harris JE, Fernandez-Vilaseca M, Elkington PT, Horncastle DE, Graeber MB, Friedland JS. IFNgamma synergizes with IL-1beta to up-regulate MMP-9 secretion in a cellular model of central nervous system tuberculosis. FASEB J. 2007;21:356–65. doi: 10.1096/fj.06-6925com. [DOI] [PubMed] [Google Scholar]
- 25.Fontana VA, Sanchez M, Cebral E, Calvo JC. Interferon-gamma inhibits metalloproteinase activity and cytotrophoblast cell migration. Am J Reprod Immunol. 2010;64:20–6. doi: 10.1111/j.1600-0897.2010.00816.x. [DOI] [PubMed] [Google Scholar]
- 26.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–8. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 27.Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol. 1996;106:1119–24. doi: 10.1111/1523-1747.ep12340167. [DOI] [PubMed] [Google Scholar]
- 28.Costa JD, Nogueira de Melo AC, Vermelho AB, Meirelles MN, Porrozzi R. In vitro evidence for metallopeptidase participation in hepatocyte damage induced by Leishmania chagasi-infected macrophages. Acta Trop. 2008;106:175–83. doi: 10.1016/j.actatropica.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Kurschat P, Wickenhauser C, Groth W, Krieg T, Mauch C. Identification of activated matrix metalloproteinase-2 (MMP-2) as the main gelatinolytic enzyme in malignant melanoma by in situ zymography. J Pathol. 2002;197:179–87. doi: 10.1002/path.1080. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira-Neto MP, Mattos MS, Perez MA, et al. American tegumentary leishmaniasis (ATL) in Rio de Janeiro State, Brazil: main clinical and epidemiologic characteristics. Int J Dermatol. 2000;39:506–14. doi: 10.1046/j.1365-4362.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 31.Chang YH, Lin IL, Tsay GJ, et al. Elevated circulatory MMP-2 and MMP-9 levels and activities in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Biochem. 2008;41:955–9. doi: 10.1016/j.clinbiochem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Dubois B, Opdenakker G, Carton H. Gelatinase B in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neurol Belg. 1999;99:53–6. [PubMed] [Google Scholar]
- 33.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–6. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 34.Mirastschijski U, Impola U, Jahkola T, Karlsmark T, Agren MS, Saarialho-Kere U. Ectopic localization of matrix metalloproteinase-9 in chronic cutaneous wounds. Hum Pathol. 2002;33:355–64. doi: 10.1053/hupa.2002.32221. [DOI] [PubMed] [Google Scholar]
- 35.Teles RM, Teles RB, Amadeu TP, et al. High matrix metalloproteinase production correlates with immune activation and leukocyte migration in leprosy reactional lesions. Infect Immun. 2010;78:1012–21. doi: 10.1128/IAI.00896-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barral A, Teixeira M, Reis P, et al. Transforming growth factor-beta in human cutaneous leishmaniasis. Am J Pathol. 1995;147:947–54. [PMC free article] [PubMed] [Google Scholar]
- 37.Belkaid Y, Hoffmann KF, Mendez S, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harms G, Zwingenberger K, Chehade AK, et al. Effects of intradermal gamma-interferon in cutaneous leishmaniasis. Lancet. 1989;1:1287–92. doi: 10.1016/s0140-6736(89)92686-x. [DOI] [PubMed] [Google Scholar]
- 39.Sadick MD, Locksley RM, Tubbs C, Raff HV. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986;136:655–61. [PubMed] [Google Scholar]
- 40.Galboiz Y, Shapiro S, Lahat N, Miller A. Modulation of monocytes matrix metalloproteinase-2, MT1-MMP and TIMP-2 by interferon-gamma and -beta: implications to multiple sclerosis. J Neuroimmunol. 2002;131:191–200. doi: 10.1016/s0165-5728(02)00266-7. [DOI] [PubMed] [Google Scholar]
- 41.Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172:1848–55. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- 42.Agren MS, Mirastschijski U, Karlsmark T, Saarialho-Kere UK. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp Dermatol. 2001;10:337–48. doi: 10.1034/j.1600-0625.2001.100506.x. [DOI] [PubMed] [Google Scholar]


