Abstract
Transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig) is a human fusion protein that binds and neutralizes both B lymphocyte stimulator (BLyS), a cytokine shown to be a key regulator of B cell maturation, proliferation and survival, and a proliferation-inducing ligand (APRIL). Rat adjuvant arthritis (AA) is an experimental animal model of rheumatoid arthritis (RA), which is mainly dependent on T cells and neutrophil-mediated cytokine production. The purpose of the present study was to investigate the effects of TACI-Ig on rat AA. Rat AA was induced by intradermal injection of 0·1 ml complete Freund's adjuvant (CFA). TACI-Ig (0·7, 2·1 and 6·3 mg/kg), recombinant human tumour necrosis factor-α receptor (rhTNFR) : Fc (2·8 mg/kg) and IgG-Fc (6·3 mg/kg) were administered subcutaneously every other day from days 16 to 34 after immunization. Arthritis was evaluated by arthritis global assessment and swollen joint count (SJC). The ankle joint and spleen were harvested for histopathological examination. Spleen index and thymus index were calculated. The levels of BLyS, interleukin (IL)-17, interferon (IFN)-γ, IgG1, IgG2a and IgM in AA rat spleen were measured by enzyme-linked immunosorbent assay. Administration of TACI-Ig significantly reduced the arthritis global assessment and SJC, decreased spleen index and ameliorated histopathological manifestations of rat AA. Suppressing the levels of BLyS, IL-17, IFN-γ and Ig in AA rat spleen were observed after administration of TACI-Ig. These results showed that TACI-Ig significantly inhibited the degree of rat AA, and the inhibitory effects might be associated with its ability to reduce BLyS, proinflammatory cytokines and Ig levels in spleen.
Keywords: adjuvant arthritis, immunoglobulin, pro-inflammatory cytokine, spleen, TACI-Ig
Introduction
Rheumatoid arthritis (RA) is a chronic disorder that mainly targets the synovial membrane of joints but can also have systemic manifestations [1]. The disease is characterized by synovial membrane hyperplasia and infiltration of inflammatory cells, including activated B cells. Although it has long been considered a T cell/macrophage-driven pathology, the success of B cell-targeted therapies (for example, rituximab) in the treatment of RA patients has led investigators to reassess the critical role of B cells in RA pathogenesis [2–4]. It is now established that B cells have many more functions in RA than simply producing autoantibodies (rheumatoid factor, anti-cyclic citrullinated proteins, and so on); B cells are also potent antigen-presenting cells (APC) and play a critical role in the activation of T cells in the synovium of RA. Furthermore, B cells not only respond to but also produce proinflammatory cytokines such as tumour necrosis factor (TNF)-α[5–8]. Thus, B cells have emerged as rational targets for new drug development in RA.
B lymphocyte stimulator (BLyS; trademark of Human Genome Sciences, Rockville, MD, USA) is also called BAFF, THANK, TALl-1, TNFSF13b and zTNF4. This cytokine is shown to be a key regulator of B cell maturation, proliferation and survival, and is an identified 285-amino acid member of the TNF ligand superfamily. BLyS is expressed by a few stromal cells, T cells and most myeloid cell lineages, including monocytes, macrophages, dendritic cells and stimulated neutrophils [9,10]. A proliferation-inducing ligand (APRIL), also called TNFSF13a, is another TNF family member that is closely related to BLyS [11,12]. Three TNF receptor-related receptors have been identified that have unique binding affinities for BLyS and APRIL: transmembrane activator or calcium-modulating cyclophylin ligand-interactor (TACI), B cell maturation antigen (BCMA) and B cell activating factor receptor (BAFF-R). TACI and BCMA bind both BLyS and APRIL, while BAFF-R appears to bind only BLyS with high affinity [13,14]. TACI is expressed not only on mature B cells but also on activated T cells, which suggests that BLyS and APRIL may also regulate T cell-mediated immune functions [15].
Mounting evidence from human and animal models supports an important role of BLyS and APRIL in the development of autoimmune disease. Over-expression of BLyS in BLyS-transgenic mice results in B cell hyperplasia, hypergammaglobulinaemia and development of autoimmune-like disease [13,16]. Similarly, over-expression of BLyS accelerates B6.Sle1.BAFF and B6.Nba2.BAFF mice develop a lupus-like phenotype consisting of anti-double-stranded DNA (anti-dsDNA) antibodies and immunoglobulin (Ig) deposition in the kidneys [17]. In addition, circulating heterotrimer complexes of BLyS and APRIL have been identified in serum from patients with rheumatic diseases [18]. Elevated BLyS and APRIL levels are found in serum and in synovial fluid of patients with RA and systemic lupus erythematosus (SLE), and the levels of BLyS and APRIL are higher in RA synovial fluid than in blood, particularly in the presence of significant joint inflammation, suggesting that these ligands may play an important role in the inflamed synovial compartment [19–21].
The above-mentioned compelling observations in human and animal models have led to the development of several BLyS and APRIL antagonists, including Ig fusion proteins for three receptors (TACI, BCMA, BAFF-R) and monoclonal antibody against BLyS. Among these antagonists, clinical researches of TACI-Ig fusion protein (atacicept) and monoclonal antibody (belimumab) for the treatment of SLE and RA are under way.
TACI-Ig contains the BLyS/APRIL-binding extracellular portion of the TACI molecule fused to the Fc portion of human IgG1, and can be used to neutralize BLyS and APRIL and prevent them from binding to their receptors. Transgenic mice that express TACI-Ig have few mature B cells, reduced concentrations of Ig and a significant decrease in the number of CD4+ and CD8+ T cells in spleens and mesenteric lymph nodes [22]. Administration of TACI-Ig significantly ameliorates the disease symptoms in collagen-induced arthritis (CIA) mice, synovium-severe combined immunodeficient (SCID) mouse chimeras and SLE-prone NZB/NZW F1 mice, but thus far no study has reported on the effects of TACI-Ig on rat adjuvant arthritis (AA), a model which involves primarily T cell autoimmune reaction to joints [22–24]. Recombinant human TNF-α receptor : IgG Fc (rhTNFR : Fc) fusion protein is a soluble TNF receptor fusion protein that binds and inactivates TNF, a proinflammatory cytokine which plays a central role in RA pathogenesis. rhTNFR : Fc reduces symptoms of RA, prevents or slows progressive joint destruction and serves as an ideal positive control in RA research [25,26]. IgG-Fc contains the Fc portion of human IgG1; it has no therapeutic effect on RA. rhTNFR : Fc and IgG-Fc were used as suitable positive and negative controls, respectively, in the present study.
In the present study, we examined the effects of TACI-Ig on secondary inflammation reaction and histopathological injury in AA rats. In addition, we investigated whether TACI-Ig attenuated the progression of rat AA by reducing proinflammatory cytokines and Ig levels in spleen of AA rats, which played an important role in the pathogenesis of RA.
Materials and methods
Animals
Male Sprague–Dawley (SD) rats weighing 150–180 g were purchased from the Experimental Animal Center of Anhui Medical University (Grade II, Certificate no. 2008-0016). The animals were housed in a room with a controlled ambient temperature (22°C ± 2°C) and humidity (50% ± 10%), with food and water ad libitum. Animals were acclimated to the housing conditions for 3 days before experiments. All experimental protocols described in the present study were approved by the Ethics Review Committee for Animal Experimentation of Institute of Clinical Pharmacology of Anhui Medical University.
Reagents
Complete Freund's adjuvant (CFA) was purchased from Sigma (St Louis, MO, USA). Human TACI-Ig fusion protein and IgG-Fc were provided by Shandong Rongchang Biological Medicine Co. Ltd, and both were dissolved in 0·9% saline at different concentrations before use. RhTNFR : Fc fusion protein was purchased from Shanghai CP Guojian Pharmaceutical Co. Ltd. Enzyme-linked immunosorbent assay (ELISA) kits for BLyS, interleukin (IL)-17, interferon (IFN)-γ, IgG1, IgG2a and IgM were purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Induction of rat AA
The rat AA model was induced by a single intradermal injection of 0·1 ml of CFA into the right hind metatarsal rat footpad. The day of CFA injection was designated day 0, and the secondary inflammatory reaction occurred on approximately day 15.
Treatment of rat AA
Animals were divided randomly into seven groups before CFA injection (day 0), in which the AA rats received TACI-Ig (0·7, 2·1 and 6·3 mg/kg, every other day), rhTNFR : Fc (2·8 mg/kg, every other day) and IgG-Fc (6·3 mg/kg, every other day) subcutaneously from days 16 to 34 after immunization. The rats in normal and AA model groups were given an equal volume of 0·9% saline at the same time.
Arthritis assessment
Rats were inspected daily for signs of arthritis under blinded conditions. From day 7, rats were evaluated every 3–4 days for two clinical parameters: arthritis global assessment and swollen joint count (SJC).
Arthritis global assessment was based on symptoms of different AA rat parts (ear: 0 = no nodule and redness, 1 = nodule and redness with one ear, 2 = nodule and redness both ears; nose: 0 = no connective tissue swelling and redness with nose, 1 = evident connective tissue swelling and redness with nose; tail: 0 = no nodule and redness with tail, 1 = evident nodule and redness with tail; paw: 0 = no swelling and redness with paw, 1 = one front or hind paw swelling and redness, 2 = two paws swelling and redness, 3 = three paws swelling and redness, 4 = four paws swelling and redness). The above-mentioned cumulative scores were used as arthritis global assessment, and a maximum value of each rat scored 8.
Each paw had five phalanx joints and one ankle or wrist joint; the maximum SJC for each rat was 24, including three secondary arthritis paws and one primary arthritis paw. Two of the above-mentioned arthritis assessment parameters were evaluated on days 0, 7, 11, 15, 18, 22, 26, 30 and 34.
Histopathological examination
Rats were killed on day 35 for dissection of spleens and left hind paws. Spleens and joints were removed, fixed in formalin, decalcified in 10% ethylenediamine tetraacetic acid (EDTA) and embedded in paraffin for histopathological analysis. Serial paraffin sections were stained with haematoxylin and eosin (H&E) and the changes in spleens and joints were evaluated histopathologically under blinded conditions.
Histopathological evaluation in spleen was based on cellularity and size of the B cell area in the white pulp and the appearance of prominent germinal centre (GC) (0 = normal spleen, 1 = slight proliferation of white pulp, 2 = moderate proliferation of white pulp, 3 = severe proliferation of white pulp and prominent GC).
The severity of arthritis in joint was graded from 0 to 4 according to the intensity of the lining layer hyperplasia, mononuclear cell infiltration and pannus formation, as described previously [27] (0 = normal ankle joint, 1 = normal synovium with occasional mononuclear cells, 2 = definite arthritis with a few layers of flat to rounded synovial lining cells and scattered mononuclear cells and dense infiltration with mononuclear cells, 3 = clear hyperplasia of the synovium with three or more layers of loosely arranged lining cells and dense infiltration with mononuclear cells, 4 = severe synovitis with pannus and erosions of articular cartilages and subchondral bones).
Spleen index and thymus index
Rats were killed on day 35 for dissection of their spleens and thymuses and the spleens and thymuses, respectively, were weighed. The ratio of spleen weight to rat body weight represented the spleen index and the ratio of thymus weight to rat body weight represented the thymus index.
Measurement of cytokine and Ig in spleen homogenate
Spleen homogenate was prepared by disrupting the spleen tissue on a sterile fine nylon screen with 0·9% saline. Spleen homogenate was centrifuged and supernatant was collected for ELISA analysis. BLyS, IL-17, IFN-γ, IgG1, IgG2a and IgM concentrations in supernatant of spleen homogenate were assayed by ELISA, respectively, according to the procedures shown in the ELISA kits. Serially diluted samples and a standard were added to the ELISA plate and incubated at 37°C for 30 min. The plate was washed five times following incubation of specific detection antibodies. Horseradish peroxidase (HRP)-conjugated reagent was added and incubated at 37°C for 30 min. After washing, the colour was developed by adding chromogen solution and the reaction was ended with stop solution. The concentration was calculated by absorbance at 450 nm.
Statistical analysis
Data are expressed as mean and standard deviation (s.d.). The analysis of variance (anova) and Student's t-test were used to determine significant differences between groups. Calculations were performed using the spss version 10·0 statistical package. Spearman's correlation coefficients were used to examine the correlations between rat arthritis and spleen BLyS, IL-17 and IFN-γ levels. Values of P < 0·05 were considered significant.
Results
Effects of TACI-Ig on arthritis global assessment
CFA injection was administered on day 0 and the secondary inflammatory reaction occurred on days 14–15. Treatment with TACI-Ig (6·3 mg/kg) and rhTNFR : Fc (2·8 mg/kg) diminished arthritis global assessment significantly in AA rats from days 30 to 34 (P < 0·05) (Fig. 1).
Fig. 1.
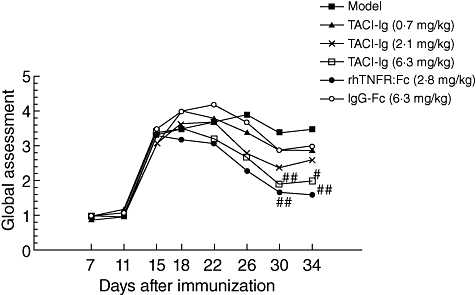
Effects of transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig) on arthritis global assessment. TACI-Ig (6·3 mg/kg) diminished significantly arthritis global assessment from days 30 to 34 (P < 0·05). Data are expressed as mean ± standard deviation from 10 animals for each group. #P < 0·05 versus model group; ##P < 0·01 versus model group.
Effects of TACI-Ig on SJC
After immunization, SJC increased in the AA model group and IgG-Fc group compared with the normal group. Treatment with TACI-Ig (6·3 mg/kg) diminished SJC significantly in AA rats from days 30 to 34 (P < 0·05), and rhTNFR : Fc (2·8 mg/kg) diminished parameters significantly from days 26 to 34 (P < 0·05) (Fig. 2).
Fig. 2.
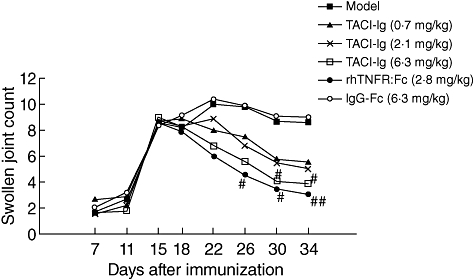
Effects of transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig) on swollen joint count (SJC). TACI-Ig (6·3 mg/kg) diminished SJC significantly from day 30 to day 34 (P < 0·05). Data are expressed as mean ± standard deviation from 10 animals for each group. #P < 0·05 versus model group; ##P < 0·01 versus model group.
Effects of TACI-Ig on histopathology
In the AA model group and IgG-Fc treatment group, immune response resulted in increased cellularity and size in the follicles and marginal zone and the appearance of prominent GC in white pulp. Administration of TACI-Ig (0·7, 2·1 and 6·3 mg/kg) and rhTNFR : Fc (2·8 mg/kg) alleviated these abnormalities in varying degrees (Fig. 3).
Fig. 3.
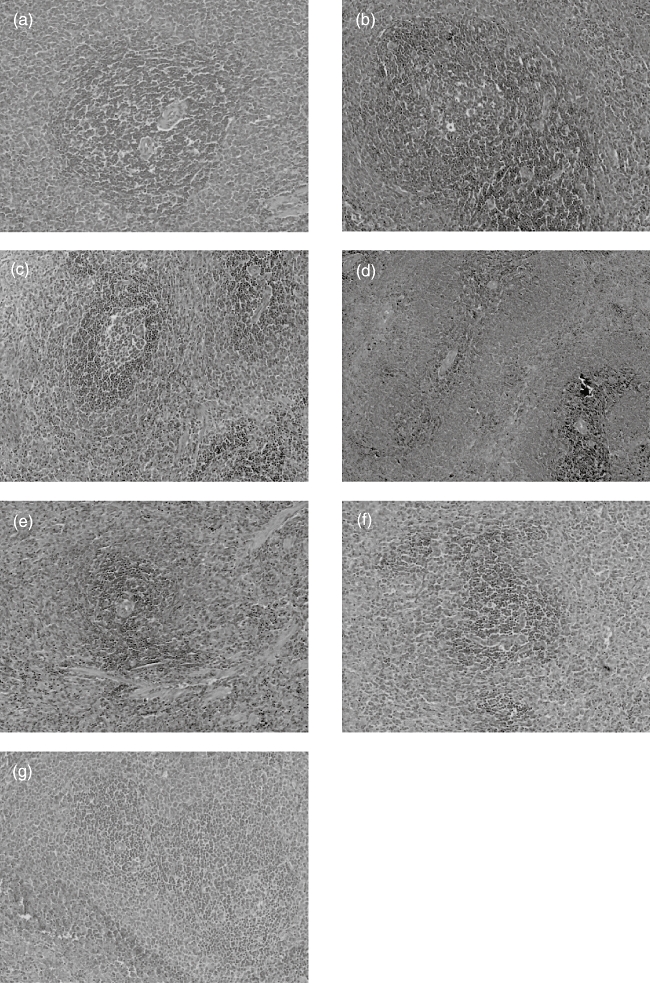
Effects of transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig) on histopathology of spleen (haematoxylin and eosin, ×100). In the adjuvant arthritis (AA) model and IgG-Fc groups, immune response resulted in increased cellularity and size significantly in the white pulp, and with prominent germinal centre (GC) (grade 3, b, g). After administration of TACI-Ig (0·7 mg/kg), the white pulp with prominent GC decreased in cellularity and size when compared to the model group (grade 2, c). Administration of TACI-Ig (2·1 mg/kg) decreased white pulp in cellularity and size (grade 1, d). After administration of TACI-Ig (6·3 mg/kg), the white pulp decreased in cellularity and size and GC disappeared; the spleen architecture of rat AA resembled normal spleen architecture (grade 0, e). For treatment of recombinant human tumour necrosis factor-α receptor (rhTNFR) : Fc (2·8 mg/kg), white pulp decreased in size and GC disappeared (grade 1, f).
In the normal rat ankle joint, synoviocytes were monolayered and there was no infiltration of inflammatory cells. In the AA model group and IgG-Fc treatment group, synoviocytes proliferated over three to eight layers with pannus formation, and articular cartilage was eroded and infiltrated with inflammatory cells. These abnormalities were alleviated significantly in AA rats after administration of TACI-Ig (0·7, 2·1 and 6·3 mg/kg) and rhTNFR : Fc (2·8 mg/kg) (Fig. 4).
Fig. 4.
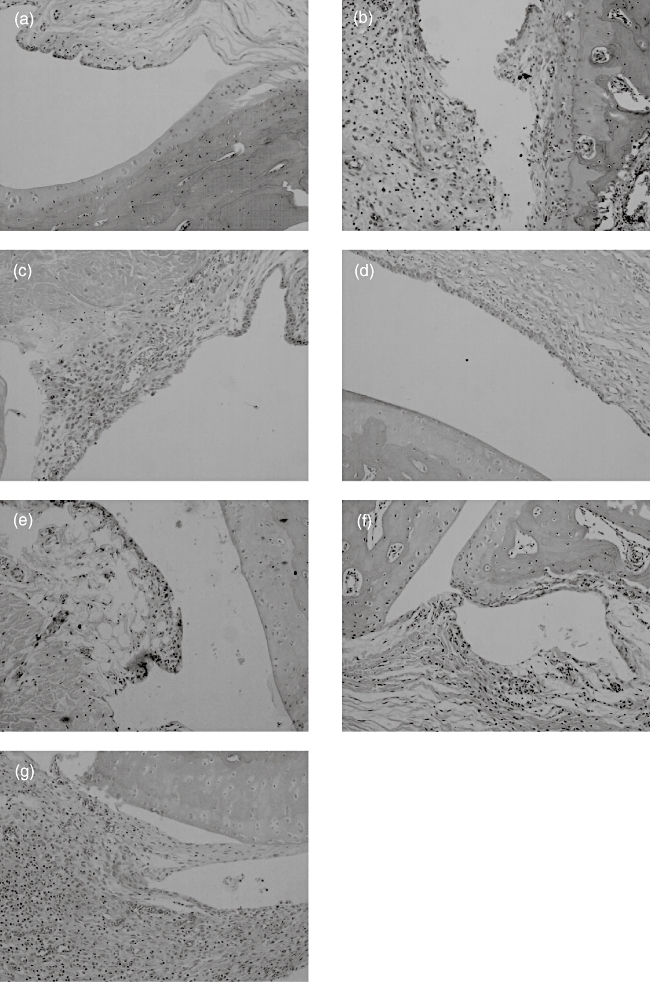
Effects of transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig) on histopathology of joint (haematoxylin and eosin, × 100). Joints of the adjuvant arthritis (AA) model and IgG-Fc groups with synoviocytes proliferated and pannus formation and dense infiltration with mononuclear cells, erosions of articular cartilage (grade 4, b,g). AA rat treated with TACI-Ig (0·7 mg/kg), a clear hyperplasia of the synovium with three or more layers of loosely arranged lining cells and dense infiltration with mononuclear cells (grade 3, c). After treatment with TACI-Ig (2·1 mg/kg), a slight hyperplasia of the synovium, infiltration with scatter mononuclear cells (grade 2, d). AA rat treated with TACI-Ig (6·3 mg/kg), synovium resembled normal synovium with occasional mononuclear cells (grade 1, e). For treatment of recombinant human tumour necrosis factor-α receptor (rhTNFR) : Fc (2·8 mg/kg), synoviocytes proliferated and had eroding articular cartilage and infiltration with mononuclear cells (grade 3, f).
Effects of TACI-Ig on spleen index and thymus index
The spleen index and thymus index of the AA model group and IgG-Fc group increased significantly when compared to the normal group (P < 0·01). After administration of TACI-Ig (2·1 and 6·3 mg/kg), the spleen index decreased significantly compared to the model group (P < 0·01). Administration of TACI-Ig (0·7, 2·1 and 6·3 mg/kg) and rhTNFR : Fc (2·8 mg/kg) had no significant influence on the thymus index in AA rats (Table 1).
Table 1.
Effects of transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig) on spleen index and thymus index.
| Groups | Dose (mg/kg) | Spleen index (mg/g) | Thymus index (mg/g) |
|---|---|---|---|
| Normal | – | 2·09 ± 0·11 | 1·01 ± 0·17 |
| Model | – | 3·30 ± 0·31** | 1·51 ± 0·19** |
| TACI-Ig | 0·7 | 3·00 ± 0·26 | 1·48 ± 0·50 |
| 2·1 | 2·52 ± 0·11*** | 1·48 ± 0·14 | |
| 6·3 | 2·67 ± 0·23*** | 1·22 ± 0·29 | |
| rhTNFR : Fc | 2·8 | 3·00 ± 0·45 | 1·41 ± 0·48 |
| IgG-Fc | 6·3 | 3·15 ± 0·18 | 1·34 ± 0·13 |
Spleen index and thymus index of adjuvant arthritis (AA) model group increased significantly compared to normal group (P < 0·01). After administration of TACI-Ig (2·1 and 6·3 mg/kg), spleen index of AA rats decreased significantly (P < 0·01) and administration of TACI-Ig (0·7, 2·1 and 6·3 mg/kg) had no significant influence on thymus index of AA rats. Data are expressed as mean ± standard deviation from 10 animals for each group.
P < 0·01 versus normal group;
*P < 0·05;
P < 0·01 versus model group. rhTNFR: recombinant human tumour necrosis factor-α receptor.
Effects of TACI-Ig on production of cytokine and Ig in spleen
Figures 5 and 6, respectively, showed the effects of TACI-Ig on BLyS, IL-17, IFN-γ, IgG1, IgG2a and IgM levels in AA rat spleen. BLyS, IL-17, IFN-γ, IgG1, IgG2a and IgM levels in the AA modeland IgG-Fc treatment group were significantly higher when compared to the normal group (P < 0·01). Administration of TACI-Ig (0·7, 2·1 and 6·3 mg/kg) and rhTNFR : Fc (2·8 mg/kg) decreased BLyS, IL-17, IFN-γ, IgG1, IgG2a and IgM levels in varying degrees.
Fig. 5.
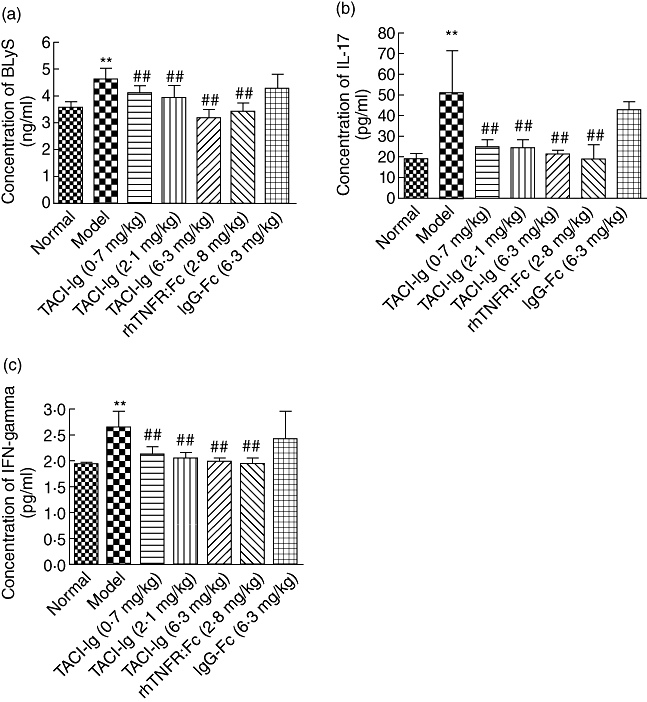
Effects of transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig) on production of B lymphocyte stimulator (BLyS) (a), interleukin (IL)-17 (b) and interferon (IFN)-γ (c), respectively, in spleen of adjuvant arthritis (AA) rats. Administration of TACI-Ig (0·7, 2·1 and 6·3 mg/kg) and recombinant human tumour necrosis factor-α receptor (rhTNFR) : Fc (2·8 mg/kg) significantly reduced BLyS (a), IL-17 (b) and IFN-γ (c) levels in AA rats, respectively (P < 0·01). Data are expressed as mean ± standard deviation (n = 8) for each group. **P < 0·01 versus normal group; #P < 0·05; ##P < 0·01 versus model group.
Fig. 6.

Effects of transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig) on production of IgG1(a), IgG2a(b) and IgM(c) in spleen of adjuvant arthritis (AA) rats. After administration of TACI-Ig (0·7, 2·1 and 6·3 mg/kg) and recombinant human tumour necrosis factor-α receptor (rhTNFR) : Fc (2·8 mg/kg), IgG1 (a), IgG2a (b) and IgM (c) levels reduced, respectively, in varying degrees. Data are expressed as mean ± standard deviation (n = 8) for each group. **P < 0·01 versus normal group; #P < 0·05; ##P < 0·01 versus model group.
To examine the correlations between the severity of arthritis and the levels of cytokines in AA rat spleen, we measured correlation coefficients between the arthritis global assessment and the BLyS, IL-17 and IFN-γ levels. The results showed that the arthritis global assessment correlated significantly with the above cytokines (Table 2).
Table 2.
The correlations between the arthritis global assessment and the spleen B lymphocyte stimulator (BLyS), interleukin (IL)-17 and interferon (IFN)-γ levels.
| BLyS | IL-17 | IFN-γ | |
|---|---|---|---|
| Regression equation | y = 2·59106X–8·14141 | y = 0·14972x–2·1400 | y = 3·79596x–6·64395 |
| Correlation coefficient | 0·85305 | 0·9272 | 0·89727 |
| P-value | <0·0001 | <0·0001 | <0·0001 |
A significant positive relationship between the arthritis global assessment and the BLyS, IL-17 and IFN-γ levels in spleen of adjuvant arthritis (AA) rats, which includes the model group and the transmembrane activator and calcium modulator and cyclophilin ligand interactor-immunoglobulin (TACI-Ig)-treated (6·3 mg/kg) group (n = 6).
Discussion
RA is a chronic cytokine-mediated inflammatory disease, characterized by both cellular and humoral immune responses. Mounting evidence in animal and human studies supports a key role for BLyS and APRIL in the development of autoimmune disease [16–21], and these studies have led to the development of Ig fusion proteins for three receptors (TACI, BCMA, BAFF-R) as BLyS antagonists, including TACI-Ig. Studies in a series of animal models, including TACI-Ig transgenic mice, human synovium-SCID mouse chimeras, SLE-prone NZB/NZW F1 mice and CIA mice show that TACI-Ig inhibits proliferation and development of B cells and ameliorates autoimmune disease symptoms [22–24]. A series of studies indicate that B cell depletion therapy inhibits antigen-specific CD4+ T cell expansion, activation and proliferation in CIA mice [28]. TACI-Ig transgenic mice show a decrease in the number of CD4+ and CD8+ T cells in the spleens and mesenteric lymph nodes [22], and TACI-Ig administration in mice also blocks activation of T cells in vitro and inhibits antigen-specific activation and priming of T cells in CIA mice [29]. These results provide direct evidence that BLyS and/or APRIL contribute to T cell activation and expansion in autoimmune disease. Although TACI can be expressed on activated T cells, the role of BLyS and/or APRIL-TACI pathways in the regulation of T cell function in RA is unknown.
The rat AA model resembles human RA in both clinical and histopathological features. It serves widely as a mature model to demonstrate the effects of new therapeutic agents on human RA. Moreover, the rat AA model, which is mainly dependent upon T cell- and neutrophil-mediated cytokine production, is different from the animal models of autoimmune disease reported previously, such as CIA.
It is known that spleen is the largest secondary lymphoid organ in rats, and also the largest peripheral B cell compartment. Immune response results in the proliferation of self-autoreactive B cells and formation of GC in the white pulp, with the spleen index apparently increased. BLyS plays an important role in promoting the inappropriate survival and proliferation of B cell clones, and regulating both the size and repertoire of the peripheral B cell compartment. Over-expression of BLyS in BLyS transgenic mice results in an expansion of the peripheral mature B cell compartment [13,16]. Further study shows fewer transitional T2 B cells, and mature B cells have been found in TACI-Ig transgenic mice [22]. In addition, human synovium-SCID mouse chimeras treated with TACI-Ig result in ectopic GC destruction [23], and in SLE-prone NZB/NZW F1 mice TACI-Ig decreases the frequency of plasma cells in the spleen [24]. In the present study, we found BLyS level increased significantly in AA rat spleen when compared to the normal group, and the spleen index and thymus index of AA rats increased significantly, which implied indirectly that B cells and T cells in peripheral lymphoid organ proliferated severely. Moreover, histopathological examination showed that white pulp of AA rats increased significantly in cellularity and size, and prominent GC emerged. TACI-Ig (2·1 and 6·3 mg/kg) decreased the spleen index significantly (no significant influence on the thymus index) (Table 1) and ameliorated the GC reaction (Fig. 3).
When inappropriate survival and proliferation of mature B cells and plasma cells occur, overproducing autoantibodies and Ig are detected. Hyperglobulinaemia, anti-ssDNA and anti-dsDNA antibodies, and circulating immune complexes present in BLyS transgenic mice [13,16]. Similarly, B6.Sle1.BAFF and B6.Nba2.BAFF mice develop a lupus-like phenotype consisting of anti-dsDNA antibodies and Ig deposition in the kidneys with elevated levels of BLyS [17]. In rat AA, IgG1, IgG2a and IgM levels in spleen increased significantly. TACI-Ig transgenic mice led to a decrease in Ig concentrations and circulating IgG levels. Human synovium-SCID mouse chimeras treated with TACI-Ig result in marked inhibition of Ig transcription [23]. In addition in SLE-prone NZB/NZW F1 mice, TACI-Ig reduces the level of IgM in the serum [24]. In the present study, TACI-Ig reduced IgG1, IgG2a and IgM levels in AA rat spleens (Fig. 6), suggesting that BLyS may play a key role in promoting and sustaining plasma cell dysfunction in rat AA, and absorption of BLyS may result in reduced Ig levels.
IFN-γ is a well-known proinflammatory cytokine. Previous studies show that GC-rich tissues produce significantly higher IFN-γ levels, suggesting that IFN-γ may correlate closely with inappropriate survival and proliferation of self-autoreactive B cells [23,30]. Previous studies have also shown that BLyS gene expression and levels of membrane-associated and soluble BLyS are found to be regulated by IFN-γin vitro[31,32], and further study demonstrates that BLyS also induces significantly higher levels of IFN-γ secretion of CD4+ T lymphocytes [33]. Human synovium-SCID mouse chimeras treated with TACI-Ig results in marked inhibition of IFN-γ transcription [23]. IL-17 is a prominent cytokine produced by T helper type 17 (Th17) cells. IL-17 exerts a strong proinflammatory effect, ensures the differentiation, maturation and activation of neutrophils and stimulates monocyte and fibroblast activation, and it is noticeable that these cells are mainly BLyS producers [34]. Studies show that B cell reduction by BLyS blockade is accompanied by decreased frequencies of pathogenic CD4+CD40+ T cells and reduced IL-17 levels, suggesting that BLyS levels may correlate positively with production of IL-17 [35]. In the present study, we found that levels of IFN-γ, IL-17 and BLyS increased significantly in AA rat spleen and correlated significantly with joint inflammation and clinical manifestations (Fig. 5 and Table 2). TACI-Ig decreased the elevated IFN-γ and IL-17 levels of AA rats. The results indicated that anti-inflammatory action of TACI-Ig might be associated with inhibiting IFN-γ and IL-17 in AA rat spleen.
In addition, BLyS and APRIL exist by circulating heterotrimer complexes and have been identified in serum samples taken from patients with systemic immune-based rheumatic diseases. Among three receptors (TACI, BCMA, BAFF-R)-Ig fusion proteins as BLyS and/or APRIL antagonists, only TACI-Ig is able to block the biological activity of the heterotrimeric complexes [19]. Although we have found that BLyS levels apparently decreased in TACI-Ig-treated AA rat spleen, the role of APRIL in rat AA is still unclear and needs to be elucidated. The level of BLyS is higher in RA synovial fluid than in serum, suggesting that BLyS may play an important role in the inflamed synovial compartment. Further studies found that fibroblast-like synoviocytes (FLS) in synovioum express BLyS and APRIL and BCMA receptors in RA [29,30]. Therefore, it is in our interest to clarify the effect of TACI-Ig on FLS in AA rats and the molecular mechanism of BLyS interacting with IFN-γ and IL-17. Exactly what role is played by BLyS in the complex proinflammatory cytokine network in AA remains to be determined and needs further research.
In summary, the present study showed that TACI-Ig had a therapeutic effect on rat AA by absorption of BLyS/APRIL as a decoy receptor. TACI-Ig decreased the spleen index, ameliorated the proliferation of splenic B cells and the formation of GC. The levels of IL-17, IFN-γ, IgG1, IgG2a and IgM in AA rat spleen also decreased after TACI-Ig treatment. These results suggest that BlyS may play an important role in the pathogenesis of RA, and TACI-Ig represents a potentially new immunotherapeutic agent for the management of autoimmune diseases.
Acknowledgments
The authors would like to thank Doctor Wendi Zhao and Doctor Feng Xiao for technical assistance, and Professor Yumin Xia for English writing. This work was supported by the National Natural Science Foundation of China (no. 30973543), the Young Talents Foundation of the Education Department of Anhui Province (no. 2010SQRL067), the Natural Science Foundation of the Education Department of Anhui Province (no. KJ2010B406, KJ2010B398) and the Financial Assistance Project of Scientific Research Funds of Doctors (no. XJ200808).
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with Rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 3.Dass S, Vital EM, Emery P. Rituximab: novel B-cell depletion therapy for the treatment of rheumatoid arthritis. Exp Opin Pharmacother. 2006;7:2559–70. doi: 10.1517/14656566.7.18.2559. [DOI] [PubMed] [Google Scholar]
- 4.Boumans MJ, Tak PP. Rituximab treatment in rheumatoid arthritis: how does it work? Arthritis Res Ther. 2009;11:134. doi: 10.1186/ar2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugatti S, Codullo V, Caporali R, Montecucco C. B cells in rheumatoid arthritis. Autoimmun Rev. 2007;7:137–42. doi: 10.1016/j.autrev.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Cheromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 7.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–18. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 8.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172:3422–7. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 9.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 11.Hahne M, Kataoka T, Schröter M, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–90. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth W, Wagenknecht B, Klumpp A, et al. APRIL, a new member of the tumor necrosis factor family, modulates death ligand-induced apoptosis. Cell Death Differ. 2001;8:403–10. doi: 10.1038/sj.cdd.4400827. [DOI] [PubMed] [Google Scholar]
- 13.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–9. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–11. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 15.von Bülow GU, Bram RJ. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–41. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- 16.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stohl W, Xu D, Kim KS, et al. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005;52:2080–91. doi: 10.1002/art.21138. [DOI] [PubMed] [Google Scholar]
- 18.Roschke V, Sosnovtseva S, Ward CD, et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol. 2002;169:4314–21. doi: 10.4049/jimmunol.169.8.4314. [DOI] [PubMed] [Google Scholar]
- 19.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–19. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Tan SM, Xu D, Roschke V, et al. Local production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritis. Arthritis Rheum. 2003;48:982–92. doi: 10.1002/art.10860. [DOI] [PubMed] [Google Scholar]
- 21.Assi LK, Wong SH, Ludwig A, et al. Tumor necrosis factor alpha activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum. 2007;56:1776–86. doi: 10.1002/art.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross JA, Dillon SR, Mudri S, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease: impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 23.Seyler TM, Park YW, Takemura S, et al. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115:3083–92. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanujam M, Wang X, Huang W, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116:724–34. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wooley PH, Dutcher J, Widmer MB, Gillis S. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J Immunol. 1993;151:6602–7. [PubMed] [Google Scholar]
- 26.Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Eng J Med. 1997;337:141–7. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 27.Tong T, Zhao W, Wu YQ, et al. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm Res. 2010;59:369–77. doi: 10.1007/s00011-009-0109-4. [DOI] [PubMed] [Google Scholar]
- 28.Bouaziz JD, Yanaba K, Venturi GM, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20878–83. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Marsters SA, Baker T, et al. TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat Immunol. 2001;2:632–7. doi: 10.1038/89782. [DOI] [PubMed] [Google Scholar]
- 30.Klimiuk PA, Yang H, Goronzy JJ, Weyand CM. Production of cytokines and metalloproteinases in rheumatoid synovitis is T cell dependent. Clin Immunol. 1999;90:65–78. doi: 10.1006/clim.1998.4618. [DOI] [PubMed] [Google Scholar]
- 31.Ohata J, Zvaifler NJ, Nishio M, et al. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J Immunol. 2005;174:864–70. doi: 10.4049/jimmunol.174.2.864. [DOI] [PubMed] [Google Scholar]
- 32.Reyes LI, León F, González P, et al. Dexamethasone inhibits BAFF expression in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Cytokine. 2008;42:170–8. doi: 10.1016/j.cyto.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Wen L, Huang X, et al. hsBAFF enhances activity of NK cells by regulation of CD4(+) T lymphocyte function. Immunol Lett. 2008;120:96–102. doi: 10.1016/j.imlet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- 35.Mariño E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4(+)CD25(+) T-cells control autoimmunity in the absence of B-cells. Diabetes. 2009;58:1568–77. doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]


