Abstract
The colonization, translocation and protective effect of two intestinal bacteria – PR4 (pig commensal strain of Bifidobacterium choerinum) or EcN (probiotic Escherichia coli strain Nissle 1917) – against subsequent infection with a virulent LT2 strain of Salmonella enterica serovar Typhimurium were studied in gnotobiotic pigs after oral association. The clinical state of experimental animals correlated with bacterial translocation and levels of inflammatory cytokines [a chemokine, interleukin (IL)-8, a proinflammatory cytokine, tumour necrosis factor (TNF)-α and an anti-inflammatory cytokine, IL-10] in plasma and intestinal lavages. Gnotobiotic pigs orally mono-associated with either PR4 or EcN thrived, and bacteria were not found in their blood. No significant inflammatory cytokine response was observed. Mono-association with Salmonella caused devastating septicaemia characterized by high levels of IL-10 and TNF-α in plasma and TNF-α in the intestine. Di-associated gnotobiotic pigs were given PR4 or EcN for 24 h. Subsequently, they were infected orally with Salmonella and euthanized 24 h later. Pigs associated with bifidobacteria before Salmonella infection suffered from severe systemic infection and mounted similar cytokine responses as pigs infected with Salmonella alone. In contrast, EcN interfered with translocation of Salmonella into mesenteric lymph nodes and systemic circulation. Pigs pre-associated with EcN thrived and their clinical condition correlated with the absence of IL-10 in their plasma and a decrease of TNF-α in plasma and ileum.
Keywords: Bifidobacterium, cytokine, gnotobiotic pig, Nissle 1917, Salmonella
Introduction
The highly diverse microbiota of the gastrointestinal tract of human and animals forms a unique ecosystem that is highly robust and capable of competing with transient and pathogenic microbes [1,2]. This property was previously named colonization resistance [3]. The intestinal microbiota also contains mutualistic bacterial strains, which confer a health benefit on the host and are known as probiotics [4,5]. The mechanisms of their action are not well understood. It is thought that immunomodulation, competitive exclusion of pathogens and production of different inhibitory compounds (e.g. organic acids, microcins) play an important role. The ban of antibiotics in animal production has encouraged studies of probiotic action and competitive interference in the gut microbiota of domestic animals.
The gastrointestinal tract of mammalian newborns is colonized by the mother's vaginal and intestinal microflora during delivery and progresses from sterility to dense microbial colonization in the first years of life [6]. Bifidobacteria are a regular component of human and animal gut microbiota [7–9]. They belong to the first settlers in the neonatal intestine and reach up to 90% of the microbiota in suckling infants [10]. Newborns delivered by Caesarian section and fed milk replacers have a different composition of gut microbiota characterized by lower numbers of bifidobacteria [6]. Bifidobacteria are present in 10–100-fold lower concentrations in the pig intestine than in humans [7,11–13]. Their number increased after feeding pigs with diet supplement containing prebiotics [14].
Bifidobacterium choerinum is an autochthonous bifidobacterium species of the pig that is well adapted to the gut of pre-weaned piglets and shows potential probiotic properties [15].
Escherichia coli Nissle 1917 (EcN) is a probiotic strain of E. coli[16] isolated originally from stool of a human resistant to infection with Shigella[17]. It is efficient in prevention and cure of dysmicrobia and infant diarrhoea [18] and neonatal calf diarrhoea [19]. It has also been shown that this strain protects pigs against infection with enteropathogenic bacteria [20,21]. EcN produces two microcins which are effective against enterobacteria [22], and reduces invasion of Salmonella into enterocytes [23].
With their simplified, controlled and defined microbiota, gnotobiotic animals are suitable biological models for the study of bacteria–host interactions [24]. These properties have been exploited in studies of Salmonella infection [25,26].
In this work, a possible probiotic effect of autochthonous B. choerinum was compared with that of probiotic E. coli Nissle 1917. Gnotobiotic pigs were used to avoid any effect of interindividual variation in intestinal microflora and rearing environment [27]. The distribution of bacteria, their translocation, the protective effect against subsequent infection with virulent Salmonella Typhimurium, the clinical state of experimental piglets and systemic and local production of two inflammatory cytokines – a chemokine, interleukin (IL)-8, a proinflammatory cytokine, tumour necrosis factor (TNF)-α and an anti-inflammatory cytokine, IL-10, were assessed.
Materials and methods
Animals
Miniature Minnesota-derived sows were treated intramuscularly (i.m.) with 50 mg of medroxyprogesterone acetate (Depo-Promone; Pfizer Manufacturing Belgium, Puurs, Belgium) on the 105th day of gestation. Colostrum-deprived germ-free piglets were obtained by hysterectomy under halothane anaesthesia on the 112th day of gestation. Piglets were reared in positive-pressure microbiologically controlled fibreglass isolators and fed to satiety with autoclave-sterilized milk diet supplemented with minerals and vitamins [28]. Piglets were checked for sterility two times a week and on the day of euthanasia by culturing rectal swabs aerobically and anaerobically and by staining methods [29]. All procedures with animals were approved by the Committee for Animal Protection and Use of the Institute of Microbiology.
Bacterial strains
PR4 is a commensal strain of B. choerinum isolated from fecal flora of 8-week-old pigs of (LW × L) × Pn breed using modified trypticase–phytone–yearst (MTPY) agar [30]. The isolate was identified using the random amplified polymorphic DNA–polymerase chain reaction (RAPD-PCR) procedure according to Sakata et al. [31] and compared with porcine bifidobacteria strains from the German Resource Centre for Biological Material.
EcN is E. coli Nissle 1917 (EcN, serovar O6:K5:H1). This serum-sensitive non-virulent E. coli strain is used as a human and veterinary probiotic [16].
LT2 is a serum-resistant LT2 strain of S. enterica serovar Typhimurium causing lethal sepsis in germ-free piglets [26].
Bacterial suspensions
Fresh cultures of bacteria were prepared for each experiment by cultivation at 37°C overnight. PR4 was cultivated in an anaerobic chamber in 10 ml TPY broth (Scharlau, Barcelona, Spain). The cells were harvested by centrifugation at 4000 g for 10 min. The pellet was washed twice with 0·05 M phosphate buffer, pH 6·5 containing 500 mg/l cysteine (PBC). EcN and LT2 were cultivated on meat-peptone agar slopes (blood agar base; Oxoid, Basingstoke, UK). Bacteria were resuspended to the density of 5 × 108 colony-forming units (CFU)/ml and given to gnotobiotic pigs in milk diet. The number of CFU estimated by spectrophotometry at 600 nm was verified by a cultivation method.
Colonization of germ-free pigs with bacteria
One-week-old germ-free pigs were orally associated/infected with 1 × 108 CFU of bacteria in 5 ml of milk diet. Di-associated pigs were infected with S. Typhimurium 24 h after the association with PR4 or EcN, respectively. All experimental pigs were euthanized 24 h after the last bacteria treatment by exsanguination under halothane anaesthesia. The germ-free control group was euthanized at the same age.
Six experimental groups of 1-week-old gnotobiotic pigs (five pigs in each group) from five hysterectomies of miniature sows were investigated: (i) germ-free piglets, (ii) pigs mono-associated with LT2 (LT2 strain of S. enterica serovar Typhimurium), (iii) pigs mono-associated with PR4 (B. choerinum strain PR4), (iv) pigs mono-associated with EcN (E. coli strain Nissle 1917), (v) pigs di-associated with PR4+LT2 and (vi) LT2 pigs di-associated with EcN+ LT2.
Distribution of bacteria
Experimental animals were euthanized and samples of peripheral blood, intestinal lavages and homogenized tissues (from the spleen, mesenteric lymph nodes and liver) were serially diluted in PBC. Appropriate dilutions were transferred to sterile 60 mm Petri dishes, which were immediately filled with the media for bifidobacteria (TPY agar; Scharlau) supplemented with 100 mg/l mupirocin and 1 ml/l of concentrated glacial acetic acid [30]. Bifidobacteria were incubated in an anaerobic jar (Anaerobic Plus System; Oxoid) in CO2/H2 (90/10%) atmosphere at 37°C for 3 days. E. coli and Salmonella sp. were cultivated and enumerated on 90 mm Petri dishes with MacConkey agar (Merck, Darmstadt, Germany) and Brilliant Green agar (Oxoid), respectively. Inoculated plates were incubated aerobically at 37°C for 1 day.
Ileum lavage was obtained by cutting off a 40-cm segment of distal part of the ileum beginning at the ileocaecal orifice and rinsing it with 2 ml of PBC. Colon lavage was obtained by placing the whole colon in a Petri dish, cutting it with scissors into short pieces and adding 4 ml of PBC. Ten-fold serial dilutions of samples were cultivated as above, depending on the target bacteria. A protease inhibitor cocktail (Roche, Mannheim, Germany) was added to the remainder of the intestine lavages for subsequent detection of cytokines according to the manufacturer's recommendations.
Cytokine detection by enzyme-linked immunosorbent assay (ELISA)
IL-8, IL-10 and TNF-α were estimated in citrated blood plasma (1200 g, 10 min., 8°C) or ileum lavage (1500 g, 20 min, 8°C) prepared as above and filtered through 0·2 µm nitrocellulose filters (Sartorius, Göttingen, Germany). All samples with added protease inhibitor cocktail (Roche) were frozen immediately and kept at −70°C until used. The sandwich IL-8 ELISA with a sensitivity of 15 pg/ml is described elsewhere [32]. IL-10 and TNF-α were detected with the same sensitivity of 15 pg/ml using a swine IL-10 CytoSet™ and swine TNF-α CytoSet™ (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The assays were performed in 96-well MaxiSorp™ ELISA plates (Nunc, Roskilde, Denmark) and measured at 450 and 620 nm with Infinite M200 microplate reader (Tecan, Grödig, Austria). The results were evaluated using Magellan version 6.3 software (Tecan).
Statistical analysis
Log10 values of bacteria CFU were compared by unpaired Student's t-test. The pigs infected with S. Typhimurium (LT2) served only as a control group for cytokine levels in plasma and intestine in di-associated groups (PR4+LT2 and EcN+LT2). Differences between groups were compared by analysis of variance (anova) with Dunnett's post-hoc test. The differences were evaluated using InStat version 3.10 (GraphPad Software, San Diego, CA, USA) and considered significant if P < 0·05. Correlations between bacteraemia and plasma cytokine levels were evaluated using Pearson's correlation coefficient (Prism version 5.03, GraphPad Software).
Results
Clinical response to association
All gnotobiotic pigs which were mono-associated with PR4 (bifidobacteria) or EcN (E. coli Nissle 1917) thrived and, together with germ-free pigs, served as the control groups for translocation of beneficial bacteria. Body temperature did not change after mono-association with bifidobacteria, and monoassociation with EcN caused only a subfebrile rise (presumably a lipopolysaccharide effect). The germ-free pigs infected with S. Typhimurium suffered from high fever, anorexia (beginning 8 h after infection), vomiting and/or non-bloody diarrhoea, and showed hallmarks of septicaemia (stupor, tremors, cramps, tachycardia, tachypnoea) 24 h after infection.
In di-associated animals, Salmonella infection caused fever only in gnotobiotic pigs pre-associated with bifidobacteria (mean increase of body temperature 1·6°C), but not in the pigs pre-associated with EcN (subfebrile rise only). The former group showed the same symptoms of septicaemia as pigs infected with Salmonella alone, whereas the latter thrived without any visible symptoms of enteritis or systemic disease.
Distribution of bacteria in mono- and di-associated gnotobiotic pigs
PR4 counts were lower in the colon (P < 0·001) of di-associated pigs (Fig. 1a). The differences between EcN counts in the gut of mono-associated (EcN) and di-associated pigs (EcN+LT2) were not significant (Fig. 1b). Both EcN as well as PR4 reduced Salmonella counts in the ileum (P < 0·01), and also PR4 in the colon (P < 0·05). S. Typhimurium bacteria were present in blood and all organs examined from animals infected with LT2 (Fig. 2). In contrast, neither PR4 nor EcN bacteria were found in blood 24 h after oral administration. EcN also interfered with translocation of S. Typhimurium into mesenteric lymph nodes (P < 0·01) (Fig. 2): S. Typhimurium was absent in blood, liver and lungs of EcN-di-associated pigs. In contrast, all PR4-di-associated pigs suffered from septicaemia.
Fig. 1.
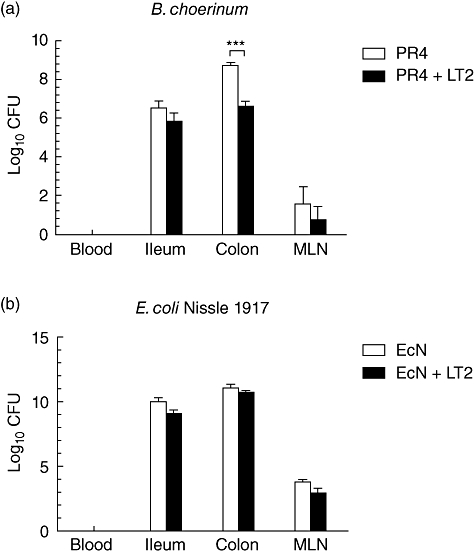
Bacteria counts in blood, intestine and mesenteric lymph nodes (MLN) of gnotobiotic pigs. (a) Bifidobacterium choerinum in mono-associated pigs (PR4) or 1 day after subsequent infection with S. Typhimurium (PR4 + LT2). Bifidobacteria were not found in blood. Salmonella reduced the number of bifidobacteria in the colon (P < 0·001). (b) Escherichia coli Nissle 1917 in mono-associated pigs (EcN) or 1 day after subsequent infection with S. Typhimurium (EcN + LT2). E. coli was not found in blood. Its numbers were not influenced by Salmonella. *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 2.
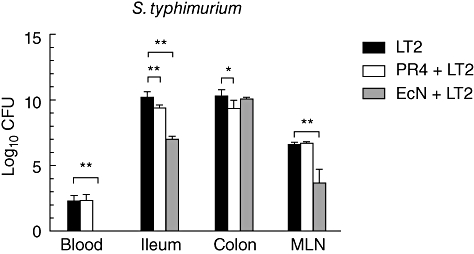
Salmonella Typhimurium counts in blood, intestine and mesenteric lymph nodes (MLN) of gnotobiotic pigs. Salmonella counts in pigs infected with S. Typhimurium alone (LT2), pigs associated with B. choerinum and infected with S. Typhimurium (PR4 + LT2) or pigs associated with Escherichia coli Nissle 1917 and infected with S. Typhimurium (EcN + LT2). EcN protected piglets against bacteraemia. Salmonella counts were reduced in the ileum and mesenteric lymph nodes (P < 0·01) in the presence of EcN, and in the ileum (P < 0·01) and colon (P < 0·05) in the presence of PR4. *P < 0·05; **P < 0·01; ***P < 0·001.
Cytokines
The concentrations of IL-8, TNF-α and IL-10 were measured in plasma, ileum and colon lavages of germ-free pigs, gnotobiotic pigs mono-associated with LT2 strain of S. Typhimurium, gnotobiotic pigs di-associated with EcN and LT2 and gnotobiotic pigs di-associated with PR4 and LT2. No inflammatory cytokines were found in samples from germ-free pigs (Fig. 3a–c).
Fig. 3.
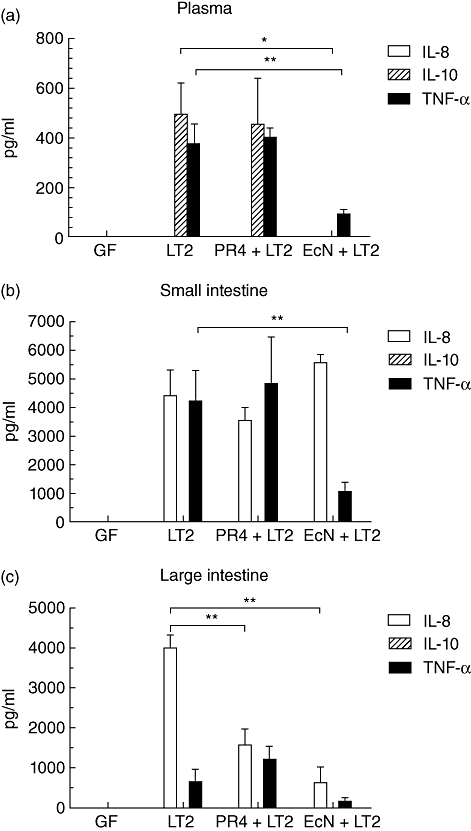
Cytokine levels. Inflammatory cytokines were absent in plasma and intestinal lavages of germfree (GF) pigs. Interleukin (IL)-8 was absent in all plasma samples and IL-10 was absent in all intestinal lavages. (a) Plasma. High levels of IL-10 and tumour necrosis factor (TNF)-α were detected in gnotobiotic pigs infected with S. Typhimurium alone (LT2) and pigs associated with Bifidobacterium choerinum and infected with S. Typhimurium (PR4 + LT2). Lower levels of IL-10 (P < 0·05) and TNF-α (P < 0·01) were detected in pigs associated with Escherichia coli Nissle 1917 and infected with S. Typhimurium (EcN + LT2). (b) Ileum. High levels of IL-8 and TNF-α were found in gnotobiotic pigs infected with S. Typhimurium alone (LT2) and pigs associated with B. choerinum and infected with S. Typhimurium (PR4 + LT2). Lower levels of TNF-α (P < 0·01) were found in pigs associated with E. coli Nissle 1917 and infected with S. Typhimurium (EcN + LT2). (c) Colon. High levels of IL-8 were detected in pigs infected with Salmonella alone (LT2). Lower levels of IL-8 (P < 0·01) were found both in pigs associated with E. coli Nissle 1917 (EcN + LT2) or B. choerinum (PR4 + LT2) and infected with S. Typhimurium. *P < 0·05; **P < 0·01; ***P < 0·001.
Plasma cytokines
IL-8 was not found in any plasma sample (Fig. 3a). LT2 induced significant IL-10 and TNF-α responses in circulation. There was no significant difference between the levels of both cytokines in plasma samples from pigs infected with LT2 alone and those from pigs associated with PR4 and LT2. Bacteraemia in piglets infected with Salmonella (Fig. 2) was correlated highly with plasma IL-10 (r = 0·909, Fig. 4a) and TNF-α (r = 0·769, Fig. 4b) levels. A marked decrease was observed in pigs di-associated with EcN and LT2 compared to LT2 alone: IL-10 was absent in their plasma and TNF-α levels were significantly lower (Fig. 3a).
Fig. 4.

Correlation between bacteraemia and plasma cytokines in piglets infected with Salmonella Typhimurium. Colony-forming units (CFU)/ml of S. Typhimurium were highly correlated with (a) interleukin (IL)-10 (Pearson's r = 0·909, P < 0·01) and (b) tumour necrosis factor (TNF)-α (Pearson's r = 0·769, P < 0·01).
Ileum cytokines
IL-8 was present in all samples infected with Salmonella, but there were no significant differences between the groups (Fig. 3b). IL-10 was not found at all. TNF-α levels were lower (P < 0·01) in pigs di-associated with EcN and LT2 than in the pigs infected with LT2 alone. In contrast, TNF-α levels in the ileum of pigs associated with PR4 and LT2 were similar to these in the pigs infected with S. Typhimurium alone.
Colon cytokines
IL-8 was detected in all samples infected with Salmonella while IL-10 was not found in any sample, as in the ileum (Fig. 3c). The pre-association of pigs with commensal bacteria decreased dramatically (P < 0·01) the levels of IL-8 in Salmonella-infected pigs. TNF-α levels in the colon were lower than those in the ileum and were almost absent in pigs di-associated with EcN and LT2. The same reduction in TNF-α in EcN-di-associated pigs and increase in PR4-di-associated pigs was found as in the ileum, although it was not statistically significant in the colon.
Discussion
Salmonella is one of the major causes of foodborne infections. Serovar Typhimurium is a serious threat in individuals with immune deficiency in some African states [33], but it is also a frequent aetiological agent of salmonellosis in humans and domestic animals in developed countries [34]. The infection in mice represents a model of human systemic typhoid fever caused by serovar Typhi [35,36]. In contrast, serovar Typhimurium causes a similar type of infection in pigs and calves as in humans – i.e. gastroenteritis or systemic disease [19,26]. Therefore, gnotobiotic pigs were chosen as a more appropriate model, in which the results are not affected by background effects of the endogeneous microbiota [1,2].
Autochthonous bacteria and probiotic strains of bacteria can support colonization resistance of the host [3] and can enhance anti-microbial immunity in the gut [4,5]. Both E. coli Nissle 1917 [20,21] and B. choerinum, as an autochthonous pig bifidobacteria [15], have been described as bacteria with suitable probiotic properties in piglets.
The differences between bacterial strains complicate comparisons of their anti-microbial effect. B. choerinum is well adapted to the intestine of pre-weaned piglets [15]. The strain PR4, used in this study, was an autochthonous pig strain. This is important, as it has been demonstrated recently that cytokine responses against Bifidobacteria are strain-specific [24]. A beneficial effect of B. longum against infection with Salmonella Typhimurium has been described in conventional mice [37]. E. coli Nissle (EcN) was isolated originally from the human [17] but spread later to porcine herds [38]. We have reported its ability to colonize [39], and this has also been confirmed by others [40,41]. In spite of this, EcN translocation through the immature gut barrier of gnotobiotic piglets was lower than that of another commensal pig E. coli strain [39]. EcN shows an antagonistic effect against various enteropathogenic bacteria in the pig [42]. We have observed up-regulation of ZO-1 and occludin in ileal enterocytes of gnotobiotic pigs associated with EcN (not published). A combination of these beneficial effects is likely to explain the interference of EcN with translocation of S. Typhimurium.
The distribution of bacteria and their protective effect against subsequent infection with Salmonella correlated with the clinical state of animals (anorexia, somnolence, fever, diarrhoea, vomiting, etc.) and with cytokine expression in the intestine and blood. EcN prevented bacteraemia of Salmonella in gnotobiotic pigs. This important finding was associated with the absence of IL-10 and decreased TNF-α concentrations in plasma after Salmonella infection.
The absence of IL-8, an attractant/activator of neutrophils, in plasma is in accordance with our previous observations [43]. However, IL-8 was found in all intestinal samples from the pigs infected with Salmonella. The flagellin of this bacterial species is its main inducer [44]. As a flagellated bacterium, EcN also induces IL-8 in enterocytes [45,46] and this could be one of the mechanisms by which it protects against Salmonella infection [25,43].
High plasma levels of IL-10 were observed in piglets infected with Salmonella alone or in piglets colonized with bifidobacteria and infected with Salmonella. IL-10 levels correlated with TNF-α levels and with the presence of Salmonella in blood, suggesting an interplay between both cytokines, or more generally the interplay between pro- and anti-inflammatory reactions. In contrast, IL-10 was absent in the blood of piglets colonized with EcN and subsequently infected with Salmonella. Blood IL-10 levels increase in several septic states, including E. coli sepsis [47] and a swine model of shock caused by heat-killed Neisseria meningitis[48]. The continued presence of IL-10 in blood 24 h after infection of gnotobiotic pigs with S. Typhimurium seems to be a prognostic marker of poor survival in infected animals [43]. Levels of IL-10 also reflect the severity of Salmonella infection in mice [49]. In contrast, increased levels of IL-10 in blood coincided with recovery from experimentally induced swine dysentery [50]. In this study, IL-10 was not found in any intestinal sample. This may be caused by the absence of cells capable of producing it, e.g. by the paucity and immaturity of T lymphocytes in intestinal villi of germ-free pigs.
High levels of TNF-α were found in plasma and ileum of piglets infected with Salmonella alone or in piglets pre-colonized with bifidobacteria before this infection. The statistically significant reduction in TNF-α in pigs di-associated with EcN and LT2 correlated with the ability of EcN to interfere with Salmonella in the ileum and ultimately stop translocation to the mesenteric lymph nodes. The levels of TNF-α are markers of inflammation and high levels are found in bacteraemia. Rapid turnover of TNF-α in blood of pigs challenged by living or heat-killed bacteria or bacterial lipopolysaccharide has been described [47,48]. Prolonged presence of TNF-α in blood circulation was seen in our experimental gnotobiotic piglets which, together with IL-10 levels, correlated with increased lethality. Decreasing levels or neutralization of TNF-α in blood can be one method of protection against the lethal sequelae of bacteraemia [51]. Preliminary association of germ-free piglets with EcN significantly reduced levels of TNF-α in Salmonella-infected piglets compared to animals infected with Salmonella alone.
Unlike conventional animals, the germ-free animals show no resistance to colonization [3], and a single dose of bacteria suffices for the prolonged colonization of their gastrointestinal tract. In our experiments, bifidobacteria reached a density equivalent to that in their conventional counterparts by 24 h after colonization. Contrary to the findings in mice [37,52], the autochthonous pig strain PR4 of B. choerinum did not interfere effectively with Salmonella and was not able to protect gnotobiotic pigs against subsequent infection with S. Typhimurium. Probiotics, including bifidobacteria, were shown to be able to down-regulate expression of genes in the S. Typhimurium pathogenicity islands SPI-1 and SPI-2 [53], and protective bifidobacterial properties after prolonged exposure have been described in conventional mice [54]. We speculate that this microbe needs more time to form an effective biofilm on the intestinal epithelium, as has been shown in gnotobiotic rats [55]. Bifidobacteria are associated more with the colon than ileum, which is the major site of Salmonella translocation, and their beneficial effect is caused rather by their metabolic products and the mechanisms of tolerance they induce [56]. This could be the major reason why the association of gnotobiotic pigs with B. choerinum for 24 h was not protective against a subsequent infection with S. enterica serovar Typhimurium. Further studies of the formation of biofilms by bifidobacteria and their impact on Salmonella pathogenity in gnotobiotic pigs are an interesting target of future study.
Acknowledgments
We thank our colleagues Ms Marie Zahradnickova, Ms Jana Machova, Ms Jarmila Jarkovska and Ms Hana Sychrovska for their technical assistance. We are grateful to Professor M. Bailey (University of Bristol, UK) for his kind help in preparation of the manuscript. This work was supported financially by grant no. 523/07/0572 of the Czech Science Foundation, Ardeypharm GmbH (Herdecke, Germany) and the Institutional Research Concept AV0Z50200510 of the Institute of Microbiology.
Disclosure
U.S. –E. coli Nissle 1917 is the active component of the probiotic preparation Mutaflor® (Ardeypharm GmbH). The other authors have no conflict interests.
References
- 1.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature. 2007;449:811–18. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Waaij D. Colonization resistance of the digestive tract: clinical consequences and implications. J Antimicrob Chemother. 1982;10:263–70. doi: 10.1093/jac/10.4.263. [DOI] [PubMed] [Google Scholar]
- 4.Senok AC, Ismaeel AY, Botta GA. Probiotics: facts and myths. Clin Microbiol Infect. 2005;11:958–66. doi: 10.1111/j.1469-0691.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 5.Guarner F. Enteric flora in health and disease. Digestion. 2006;73(Suppl 1):5–12. doi: 10.1159/000089775. [DOI] [PubMed] [Google Scholar]
- 6.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86(Suppl 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen LL, Jensen BB. Effect of fructo-oligosaccharides and transgalacto-oligosaccharides on microbial populations and microbial activity in the gastrointestinal tract of piglets post-weaning. Anim Feed Sci Technol. 2004;117:107–19. [Google Scholar]
- 8.Reuter G. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol. 2001;2:43–53. [PubMed] [Google Scholar]
- 9.Vlkova E, Trojanova I, Rada V. Distribution of bifidobacteria in the gastrointestinal tract of calves. Folia Microbiol (Praha) 2006;51:325–8. doi: 10.1007/BF02931825. [DOI] [PubMed] [Google Scholar]
- 10.Huurre A, Kalliomaki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery – effects on gut microbiota and humoral immunity. Neonatology. 2008;93:236–40. doi: 10.1159/000111102. [DOI] [PubMed] [Google Scholar]
- 11.Shim SB, Verstegen MW, Kim IH, Kwon OS, Verdonk JM. Effects of feeding antibiotic-free creep feed supplemented with oligofructose, probiotics or synbiotics to suckling piglets increases the preweaning weight gain and composition of intestinal microbiota. Arch Anim Nutr. 2005;59:419–27. doi: 10.1080/17450390500353234. [DOI] [PubMed] [Google Scholar]
- 12.Fava F, Makivuokko H, Siljander-Rasi H, et al. Effect of polydextrose on intestinal microbes and immune functions in pigs. Br J Nutr. 2007;98:123–33. doi: 10.1017/S0007114507691818. [DOI] [PubMed] [Google Scholar]
- 13.Franklin MA, Mathew AG, Vickers JR, Clift RA. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J Anim Sci. 2002;80:2904–10. doi: 10.2527/2002.80112904x. [DOI] [PubMed] [Google Scholar]
- 14.Letellier A, Messier S, Lessard L, Quessy S. Assessment of various treatments to reduce carriage of Salmonella in swine. Can J Vet Res. 2000;6:27–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell FJ, Duncan SH, Hold G, Stewart CS. Isolation, growth on prebiotics and probiotic potential of novel bifidobacteria from pigs. Anaerobe. 2004;10:33–9. doi: 10.1016/j.anaerobe.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Schultz M. In: Escherichia coli Therapeutic microbiology: probiotics and related strategies. Versalovic J, Wilson M, editors. Washington, DC: ASM Press; 2008. pp. 83–96. [Google Scholar]
- 17.Nissle A. Antagonistic treatment of chronic intestinal diseases with colibacteria. Med Klinik. 1918;2:29–30. [Google Scholar]
- 18.Henker J, Laass M, Blokhin BM, et al. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur J Pediatr. 2007;166:311–18. doi: 10.1007/s00431-007-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Buenau R, Jaekel L, Schubotz E, Schwarz S, Stroff T, Krueger M. Escherichia coli strain Nissle 1917: significant reduction of neonatal calf diarrhea. J Dairy Sci. 2005;88:317–23. doi: 10.3168/jds.S0022-0302(05)72690-4. [DOI] [PubMed] [Google Scholar]
- 20.Barth S, Duncker S, Hempe J, Breves G, Baljer G, Bauerfeind R. Escherichia coli Nissle 1917 for probiotic use in piglets: evidence for intestinal colonization. J Appl Microbiol. 2009;107:1697–710. doi: 10.1111/j.1365-2672.2009.04361.x. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder B, Duncker S, Barth S, et al. Preventive effects of the probiotic Escherichia coli strain Nissle 1917 on acute secretory diarrhea in a pig model of intestinal infection. Dig Dis Sci. 2006;51:724–31. doi: 10.1007/s10620-006-3198-8. [DOI] [PubMed] [Google Scholar]
- 22.Vassiliadis G, Destoumieux-Garzon D, Lombard C, Rebuffat S, Peduzzi J. Isolation and characterization of two members of the siderophore–microcin family, microcins M and H47. Antimicrob Agents Chemother. 2010;54:288–97. doi: 10.1128/AAC.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altenhoefer A, Oswald S, Sonnenborn U, et al. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol Med Microbiol. 2004;40:223–9. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- 24.Menard O, Butel MJ, Gaboriau-Routhiau V, Waligora-Dupriet AJ. Gnotobiotic mouse immune response induced by Bifidobacterium sp. strains isolated from infants. Appl Environ Microbiol. 2008;74:660–6. doi: 10.1128/AEM.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster N, Lovell MA, Marston KL, et al. Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leukocytes by avirulent Salmonella enterica. Infect Immun. 2003;71:2182–91. doi: 10.1128/IAI.71.4.2182-2191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trebichavsky I, Schulze J, Dlabac V, Cukrowska B, Tlaskalova-Hogenova H, Rehakova Z. Salmonellosis: lessons drawn from a germ-free pig model. Folia Microbiol (Praha) 1998;43:697–701. doi: 10.1007/BF02816393. [DOI] [PubMed] [Google Scholar]
- 27.Inman CF, Haverson K, Konstantinov SR, et al. Rearing environment affects development of the immune system in neonates. Clin Exp Immunol. 2010;160:431–9. doi: 10.1111/j.1365-2249.2010.04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandel L, Travnicek J. The minipig as a model in gnotobiology. Nahrung. 1987;31:613–18. doi: 10.1002/food.19870310580. [DOI] [PubMed] [Google Scholar]
- 29.Dlabac V. Report from a meeting of the committee for standardization of control of germfree state. Folia Microbiol (Praha) 1980;25:354–8. doi: 10.1007/BF02876619. [DOI] [PubMed] [Google Scholar]
- 30.Rada V, Petr J. A new selective medium for the isolation of glucose non-fermenting bifidobacteria from hen caeca. J Microbiol Methods. 2000;43:127–32. doi: 10.1016/s0167-7012(00)00205-0. [DOI] [PubMed] [Google Scholar]
- 31.Sakata S, Kitahara M, Sakamoto M, Hayashi H, Fukuyama M, Benno Y. Unification of Bifidobacterium infantis and Bifidobacterium suis as Bifidobacterium longum. Int J Syst Evol Microbiol. 2002;52:1945–51. doi: 10.1099/00207713-52-6-1945. [DOI] [PubMed] [Google Scholar]
- 32.Splichal I, Muneta Y, Mori Y, Takahashi E. Development and application of a pig IL-8 ELISA detection system. J Immunoassay Immunochem. 2003;24:219–32. doi: 10.1081/IAS-120020086. [DOI] [PubMed] [Google Scholar]
- 33.Kankwatira AM, Mwafulirwa GA, Gordon MA. Non-typhoidal salmonella bacteraemia – an under-recognized feature of AIDS in African adults. Trop Doct. 2004;34:198–200. doi: 10.1177/004947550403400404. [DOI] [PubMed] [Google Scholar]
- 34.Nyachuba DG. Foodborne illness: is it on the rise? Nutr Rev. 2010;68:257–69. doi: 10.1111/j.1753-4887.2010.00286.x. [DOI] [PubMed] [Google Scholar]
- 35.Hapfelmeier S, Hardt WD. A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 2005;13:497–503. doi: 10.1016/j.tim.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Mastroeni P, Sheppard M. Salmonella infections in the mouse model: host resistance factors and in vivo dynamics of bacterial spread and distribution in the tissues. Microbes Infect. 2004;6:398–405. doi: 10.1016/j.micinf.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Silva AM, Barbosa FH, Duarte R, Vieira LQ, Arantes RM, Nicoli JR. Effect of Bifidobacterium longum ingestion on experimental salmonellosis in mice. J Appl Microbiol. 2004;97:29–37. doi: 10.1111/j.1365-2672.2004.02265.x. [DOI] [PubMed] [Google Scholar]
- 38.Kleta S, Steinruck H, Breves G, et al. Detection and distribution of probiotic Escherichia coli Nissle 1917 clones in swine herds in Germany. J Appl Microbiol. 2006;101:1357–66. doi: 10.1111/j.1365-2672.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 39.Splichal I, Fagerhol MK, Trebichavsky I, Splichalova A, Schulze J. The effect of intestinal colonization of germ-free pigs with Escherichia coli on calprotectin levels in plasma, intestinal and bronchoalveolar lavages. Immunobiology. 2005;209:681–7. doi: 10.1016/j.imbio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Hancock V, Dahl M, Klemm P. Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J Med Microbiol. 2010;59:392–9. doi: 10.1099/jmm.0.008672-0. [DOI] [PubMed] [Google Scholar]
- 41.Hudcovic T, Stepankova R, Kozakova H, Hrncir T, Tlaskalova-Hogenova H. Effects of monocolonization with Escherichia coli strains O6K13 and Nissle 1917 on the development of experimentally induced acute and chronic intestinal inflammation in germ-free immunocompetent and immunodeficient mice. Folia Microbiol (Praha) 2007;52:618–26. doi: 10.1007/BF02932191. [DOI] [PubMed] [Google Scholar]
- 42.Mandel L, Trebichavsky I, Splichal I, Schulze J. Stimulation of intestinal immune cells by E. coli in gnotobiotic piglets. In: Mestecky J, editor. Advances in mucosal immunology. New York: Plenum Press; 1995. pp. 463–4. [DOI] [PubMed] [Google Scholar]
- 43.Splichal I, Trebichavsky I, Splichalova A, Barrow PA. Protection of gnotobiotic pigs against Salmonella enterica serotype Typhimurium by rough mutant of the same serotype is accompanied by the change of local and systemic cytokine response. Vet Immunol Immunopathol. 2005;103:155–61. doi: 10.1016/j.vetimm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Zeng H, Carlson AQ, Guo Y, et al. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171:3668–74. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 45.Hafez M, Hayes K, Goldrick M, Warhurst G, Grencis R, Roberts IS. The K5 capsule of Escherichia coli strain Nissle 1917 is important in mediating interactions with intestinal epithelial cells and chemokine induction. Infect Immun. 2009;77:2995–3003. doi: 10.1128/IAI.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007;75:2399–407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorgersen EB, Hellerud BC, Nielsen EW, et al. CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs. FASEB J. 2010;24:712–22. doi: 10.1096/fj.09-140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen EW, Hellerud BC, Thorgersen EB, et al. A new dynamic porcine model of meningococcal shock. Shock. 2009;32:302–9. doi: 10.1097/SHK.0b013e31819c37be. [DOI] [PubMed] [Google Scholar]
- 49.Pie S, Matsiota-Bernard P, Truffa-Bachi P, Nauciel C. Gamma interferon and interleukin-10 gene expression in innately susceptible and resistant mice during the early phase of Salmonella typhimurium infection. Infect Immun. 1996;64:849–54. doi: 10.1128/iai.64.3.849-854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kruse R, Essen-Gustavsson B, Fossum C, Jensen-Waern M. Blood concentrations of the cytokines IL-1beta, IL-6, IL-10, TNF-alpha and IFN-gamma during experimentally induced swine dysentery. Acta Vet Scand. 2008;50:32. doi: 10.1186/1751-0147-50-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jesmok G, Lindsey C, Duerr M, Fournel M, Emerson T., Jr Efficacy of monoclonal antibody against human recombinant tumor necrosis factor in E. coli-challenged swine. Am J Pathol. 1992;141:1197–207. [PMC free article] [PubMed] [Google Scholar]
- 52.Searle LE, Best A, Nunez A, et al. A mixture containing galactooligosaccharide, produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium infection in mice. J Med Microbiol. 2009;58:37–48. doi: 10.1099/jmm.0.004390-0. [DOI] [PubMed] [Google Scholar]
- 53.Bayoumi MA, Griffiths MW. Probiotics down-regulate genes in Salmonella enterica serovar typhimurium pathogenicity islands 1 and 2. J Food Prot. 2010;73:452–60. doi: 10.4315/0362-028x-73.3.452. [DOI] [PubMed] [Google Scholar]
- 54.O'Mahony C, Scully P, O'Mahony D, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleessen B, Blaut M. Modulation of gut mucosal biofilms. Br J Nutr. 2005;93(Suppl 1):S35–40. doi: 10.1079/bjn20041346. [DOI] [PubMed] [Google Scholar]
- 56.Trebichavsky I, Rada V, Splichalova A, Splichal I. Cross-talk of human gut with bifidobacteria. Nutr Rev. 2009;67:77–82. doi: 10.1111/j.1753-4887.2008.00141.x. [DOI] [PubMed] [Google Scholar]


