Abstract
Commensal bacteria have been shown to modulate the host mucosal immune system. Here, we report that oral treatment of BALB/c mice with components from the commensal, Parabacteroides distasonis, significantly reduces the severity of intestinal inflammation in murine models of acute and chronic colitis induced by dextran sulphate sodium (DSS). The membranous fraction of P. distasonis (mPd) prevented DSS-induced increases in several proinflammatory cytokines, increased mPd-specific serum antibodies and stabilized the intestinal microbial ecology. The anti-colitic effect of oral mPd was not observed in severe combined immunodeficient mice and probably involved induction of specific antibody responses and stabilization of the intestinal microbiota. Our results suggest that specific bacterial components derived from the commensal bacterium, P. distasonis, may be useful in the development of new therapeutic strategies for chronic inflammatory disorders such as inflammatory bowel disease.
Keywords: animal models, cytokines/interleukins, DSS colitis, inflammatory bowel disease, microbiota
Introduction
The current hypothesis for the pathogenesis of Crohn's disease and ulcerative colitis, the two main forms of inflammatory bowel disease (IBD), involves an aberrant host immune response to luminal antigens. Although the precise cause of IBD remains unclear, the pathogenic mechanisms are multi-factorial and additional factors such as increased virulence of commensal bacterial species, disruption of the intestinal mucosal barrier and genetic susceptibility have been proposed [1].
Three lines of evidence suggest a crucial role of the intestinal microbiota in IBD pathogenesis. First, lesions in IBD predominate in areas of highest bacterial exposure [2]. Secondly, manipulation of luminal content using selective antibiotics, or fecal stream diversion, improves inflammation in IBD patients [3,4]. Thirdly, in some models of IBD intestinal inflammation is attenuated, or fails to develop, if the animals are maintained under germ-free conditions [5–9]. It remains to be determined whether IBD can be triggered by the presence of a disbalanced microbiota composition with enhanced proinflammatory capacity. Interestingly, the intestinal microbiota is altered (dysbiosis) in a proportion of patients with IBD, and fecal samples from patients with Crohn's disease exhibit greater temporal instability [10] and decreased number of commensal bacteria with reduction in the Firmicutes phylum [11].
The current treatment of IBD targets the effector phase of the intestinal inflammatory response. In a proportion of patients, however, the disease is refractory to conventional medical treatment, or the effectiveness of the treatment is limited by serious side effects [12]. Probiotics are commensal bacteria with proven health beneficial effects. Thus, several probiotic candidates have been evaluated as an alternate and safe treatment option for IBD [13]. Some randomized, placebo-controlled studies, using Escherichia coli Nissle 1917 and a combination of eight probiotic strains, have demonstrated a beneficial effect in IBD [14–16]. However, others have failed to demonstrate significant therapeutic benefit and therefore the overall efficacy of probiotics in active chronic inflammatory conditions of the gut remains a matter of controversy [17]. More importantly, mechanistic insight linked to a specific potential probiotic strain has been difficult to establish.
Growing evidence indicates that experimental colitis can be mitigated not only with oral administration of live probiotic bacteria, but with bacterial components and by-products of bacteria as well [18–20]. Our previous results suggest that orally administered lysates from anaerobic microbiota decrease the severity of experimental colitis [21]. The aim of this study was to test the effect of oral administration of components of a specific anaerobic strain on experimental colitis, and determine the underlying mechanisms.
Materials and methods
Mice
Female BALB/c mice (6–8 weeks old) or female severe combined immunodeficient (SCID) mice BALB/cJHanHsd-SCID were obtained from a breeding colony at the Institute of Physiology (Academy of Sciences of the Czech Republic, Prague, Czech Republic) or at the Institute of Microbiology (Academy of Sciences of the Czech Republic, Novy Hradek, Czech Republic), respectively. Flow cytometry was used to exclude SCID mice that had detectable T cells. Mice were reared under conventional conditions at the Institute of Microbiology. The studies were approved by the Animal Care and Use Committee of the Institute of Microbiology.
Identification of candidate anaerobic bacteria and preparation of bacterial components
Anaerobic bacteria from mouse intestinal microbiota were grown at 37°C in liquid medium (see Supplementary materials and methods), separated into monocultures, lysed in a French press and tested for anti-inflammatory activity in an acute colitis model. To identify single candidate anaerobic strains for subsequent experiments, groups of mice (n = 5–10/group) were orally treated with isolates of anaerobic bacteria lysates (Parabacteroides distasonis, Bacteroides thetaiotamicron, Veillonella alcalescens, B. ovatus, B. vulgatus and B. stercoris; see supplementary data). Their individual effect on the prevention of acute dextran sulphate sodium (DSS) colitis was evaluated (see Supplementary Tables S4 and S5). Because only the crude lysate of P. distasonis significantly improved clinical parameters of acute DSS colitis, all subsequent experiments in the study were performed using this isolate and its components. After cell disruption with the French press, the lysate was separated by centrifugation into two fractions, membranous (insoluble) and cytoplasmic (soluble). Lipopolysaccharide (LPS) and DNA from P. distasonis were isolated as described previously [22,23].
Evaluation of anti-inflammatory effects of membranous fraction of P. distasonis lysate (mPd) on macrophages in vitro
Because macrophages have been proposed to play a role in acute intestinal inflammation [24,25], we tested the anti-inflammatory effect of bacterial components on the LPS-activated macrophage cell line, RAW 264·7. We cultured the cells in the presence of LPS and different concentrations of P. distasonis lysate or its components (see Supplementary materials and methods) and measured tumour necrosis factor (TNF)-α in supernatants by enzyme-linked immunosorbent assay (ELISA).
Induction and evaluation of acute and chronic colitis
Acute colitis was induced by 3% (wt/vol) DSS (mol wt = 36–50 kDa; MP Biomedicals, Irvine, CA, USA) dissolved in drinking water for 7 days ad libitum. For chronic colitis, mice received four cycles of DSS as described previously [25]. Each cycle consisted of 3% DSS in drinking water for 7 days, followed by a 7-day interval with normal drinking water. Colitis was evaluated on the last day of the experiment using a disease activity index (DAI) described by Cooper et al. [26], a histological scoring system (see Supplementary materials and methods), and by measuring colon length. The level of acute-phase protein haptoglobin was determined in mouse serum using the modified human haptoglobin ELISA quantitation kit (GenWay Biotech, Inc., San Diego, CA, USA) (see Supplementary materials and methods). Water consumption was measured during DSS administration.
Overall study design
To test whether bacterial components of P. distasonis prevent acute DSS colitis, we administered 1·5 mg of whole lysate, LPS, membranous or cytoplasmic fraction or 200 µg of DNA in 50 µl of sterile phosphate-buffered saline (PBS) to mice by gavage. To reduce proteolytic activity in the gut, the components were co-administered with 1 mg of soybean trypsin inhibitor (Sigma-Aldrich, St Louis, MO, USA) dissolved in 50 µl of 0·15 m sodium bicarbonate buffer (pH 8·0). Control mice were given sterile PBS with soybean trypsin inhibitor in bicarbonate buffer. We repeated the administration every 7 days for a total of four doses (on days 0, 7, 14 and 21). Seven days after the last dose we induced acute DSS colitis, as explained above.
To determine whether a gut-dependent pathway is necessary for bacterial components to modulate acute colitis, additional mice were treated by four intraperitoneal (i.p.) or subcutaneous (s.c.) injections with mPd before acute colitis induction [5 µg of mPd or PBS, together with incomplete Freund's adjuvant (Difco Laboratories, Detroit, MI, USA)]. The dose of mPd was chosen based on preliminary experiments that determined an optimal antibody response when doses of 2·5 to 1500 µg were used.
To investigate mechanisms underlying the anti-colitic effect of mPd, serum transfer experiments from orally treated mice to untreated mice were performed. Specifically, 200 µl of the serum from either PBS or mPd-treated mice were transferred intravenously to untreated mice before acute DSS colitis induction.
To test the possible effect of mPd administration on established and chronic colitis, we administered 21 doses (as described above) of mPd by daily gavage once chronic DSS colitis had been induced, starting after the third cycle of DSS.
Assessment of P. distasonis antibodies by ELISA
We used indirect ELISA assay, optimized in our laboratory, to compare serum antibody [immunoglobulin (Ig)G, IgM and IgA] titres against P. distasonis lysate between PBS and mPd-treated groups (see Supplementary materials and methods).
Gut tissue culture and measurement of cytokines
Five sections of the intestine were obtained (Peyer's patches, jejunum, ileum, caecum and colon), and cultivated for 48 h in complete RPMI-1640 media (see Supplementary materials and methods). The supernatants were collected and frozen at −20°C until analysis for cytokine production. To evaluate changes in cytokine levels induced by DSS treatment and mPd therapy in the colon, we used the RayBio™ Mouse Cytokine Array II (Raybiotech, Inc., Norcross, GA, USA) capable of detecting 32 cytokines, chemokines and growth factors (see Supplementary materials and methods; Table S2). We also used commercial ELISA kits to measure the concentrations of selected cytokines [interleukin (IL)-10, TNF-α, transforming growth factor (TGF)-β, IL-6 and interferon (IFN)-γ] (see Supplementary materials and methods).
Flow cytometry
Single-cell suspensions of spleens, mesenteric lymph nodes and Peyer's patches were prepared and stained for regulatory T cells (Tregs) using forkhead box P3 (FoxP3) staining buffer set (eBioscience, San Diego, CA, USA) with these fluorochrome-labelled anti-mouse monoclonal antibodies (mAbs): CD4-Qdot® 605 (Invitrogen, Carlsbad, CA, USA; clone RM4-5), CD25-allophycocyanin (eBioscience; clone PC61·5) and FoxP3-phycoerythrin (eBioscience; clone FJK-16 s) according to the manufacturer's recommendations. Flow cytometric analysis was performed on LSRII (BD Biosciences, San Jose, CA, USA), and data were analysed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Evaluation of intestinal microbiota
We collected stool samples from five mice chosen randomly from PBS and mPd-treated groups on days 0, 28 (just before DSS administration) and 35 (the last day), and analysed the samples by polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE), as described (see Supplementary materials and methods).
We used quantitative PCR to determine the content of all Eubacteria, Bacteroides-Prevotella group and P. distasonis in mouse faeces (see Supplementary materials and methods).
Statistical analysis
The distributions of variables were tested for normality using the D’Agostino-Pearson omnibus normality test. Differences in colon length, DAI, histological score, haptoglobin levels and TNF-α production of multiple groups were compared to the control group (PBS/DSS) by one-way analysis of variance with Dunnett's multiple comparison test. Differences in specific antibody levels, bacteria numbers, DGGE profiles, cytokine production and Treg numbers between the two groups were evaluated using an unpaired two-tailed Student's t-test. Serum levels of P. distasonis-specific antibodies were compared to the amount of P. distasonis in the stool samples using the Pearson correlation coefficient (r). The values are expressed as means ± standard deviation (s.d.) and differences were considered statistically significant at P ≤ 0·05. GraphPad Prism statistical software (version 5·0; GraphPad Software, Inc., La Jolla, CA, USA) was used for analyses.
Results
Anti-inflammatory properties of P. distasonis in vivo and in vitro
From the anaerobic lysates tested, only the crude lysate obtained from Parabacteroides distasonis, and especially its membranous fraction (mPd), was able to decrease disease severity significantly after induction of acute DSS colitis (Table 1 and Supplementary Table S4).
Table 1.
Evaluation of acute dextran sulphate sodium (DSS)-induced colitis in BALB/c and SCID mice orally treated with Parabacteroides distasonis components.
| Mouse strain | Experimental group | Colon length (cm) | Disease activity index | Histological grade | Serum haptoglobin (g/l) |
|---|---|---|---|---|---|
| BALB/c | PBS (control) | 5·97 ± 0·46 | 3·13 ± 0·74 | 2·05 ± 0·58 | 1·49 ± 0·64 |
| mPd | 6·77 ± 0·40** | 1·77 ± 0·97** | 1·36 ± 0·51* | 0·38 ± 0·35* | |
| cPd | 5·96 ± 0·42 | 3·47 ± 0·53 | 2·14 ± 0·69 | 0·84 ± 0·80 | |
| DNA | 7·32 ± 0·44** | 1·57 ± 0·61** | 1·01 ± 0·36** | 0·05 ± 0·06** | |
| LPS | 6·70 ± 0·36** | 2·07 ± 1·30* | 1·68 ± 0·43 | 0·36 ± 0·08** | |
| mPd without DSS | 8·97 ± 0·40** | 0·00 ± 0·00** | 0·15 ± 0·13** | 0·00 ± 0·01** | |
| PBS without DSS | 9·26 ± 0·41** | 0·00 ± 0·00** | 0·10 ± 0·20** | 0·02 ± 0·01** | |
| SCID | PBS (control) | 6·20 ± 0·55 | 4·00 ± 0·00 | 2·69 ± 0·49 | Not done |
| mPd | 6·13 ± 0·63 | 3·89 ± 0·17 | 2·58 ± 0·46 | Not done |
Data are representative of one experiment. Similar results were obtained from three independent experiments. Values are expressed as means ± standard deviation from six to 10 mice per group. One-way analysis of variance with Dunnett's multiple comparison test (in BALB/c mice) or unpaired Student's t-test [severe combined immunodeficient (SCID) mice] were used to evaluate differences between experimental groups and phosphate-buffered saline (PBS)-treated controls. cPd: cytoplasmic fraction of P. distasonis lysate; mPd: membranous fraction of P. distasonis; LPS: lipopolysaccharide (
P < 0·05;
P < 0·01).
To test whether the anti-colitic activity observed with P. distasonis lysate (Pd) and mPd involved innate immune cells, we treated LPS-activated RAW264·7 macrophage cells with Pd or mPd in vitro. In concentrations above 10 ng/l, Pd and mPd decreased the production of TNF-α, suggesting that they can both directly decrease the inflammatory activity of RAW264·7 macrophages (Supplementary Fig. S5). Neither cytoplasmic fraction of P. distasonis lysate (cPd) nor DNA decreased TNF-α production in vitro (data not shown).
Components of P. distasonis attenuate DSS colitis in BALB/c mice
Both oral mPd and DNA isolated from P. distasonis were effective in preventing acute DSS colitis in BALB/c mice, improving clinical, serological and morphological markers of colitis (Table 1). This effect was seen only with oral administration and was not observed with intraperitoneal or subcutaneous administration of mPd (see Table S6). In contrast to mPd and DNA treatments, oral administration of cPd did not have a protective effect (Table 1). Orally administered mPd did not prevent colitis in SCID mice (Table 1), suggesting that mechanisms of adaptive immunity are necessary for this effect.
Therapeutic administration of mPd improved colonic length and the severity of clinical scores, but did not affect histological scores (Table 2).
Table 2.
Evaluation of chronic dextran sulphate sodium (DSS)-induced colitis in BALB/c mice orally treated with Parabacteroides distasonis components.
| Experimental group | Colon length (cm) | Disease activity index | Histological grade | Serum haptoglobin (g/l) |
|---|---|---|---|---|
| PBS | 6·25 ± 0·37 | 3·08 ± 0·39 | 1·56 ± 0·35 | 0·91 ± 0·60 |
| mPd | 6·79 ± 0·51* | 2·25 ± 0·56* | 1·59 ± 0·28 | 0·20 ± 0·15** |
| cPd | 6·46 ± 0·40 | 3·08 ± 0·39 | 1·63 ± 0·31 | 0·09 ± 0·05** |
| LPS | 6·96 ± 0·34* | 2·82 ± 0·65 | 1·51 ± 0·37 | 0·06 ± 0·02** |
| PBS without DSS | 9·22 ± 0·83** | 0·73 ± 0·26** | 0·14 ± 0·14** | 0·01 ± 0·01** |
| mPd without DSS | 8·89 ± 0·60** | 0·45 ± 0·40** | 0·32 ± 0·20** | 0·01 ± 0·01** |
Data are representative of one experiment. Similar results were obtained from two independent experiments. Values are expressed as means ± standard deviation (10 mice per group). One-way analysis of variance with Dunnett's multiple comparison test was used to evaluate differences between experimental groups and phosphate-buffered saline (PBS)-treated controls (
P < 0·05;
P < 0·01). mPd: membranous fraction of P. distasonis; cPd: cytoplasmic fraction of P. distasonis lysate; LPS: lipopolysaccharide.
Effect of oral mPd on specific antibodies in serum
Serum titres of anti-P. distasonis antibodies were significantly higher (P < 0·001) in mice treated orally with mPd compared to PBS-treated mice (IgA: 0·19 ± 0·07 versus 0·05 ± 0·02; IgM: 0·56 ± 0·19 versus 0·15 ± 0·05 and IgG: 0·46 ± 0·17 versus 0·02 ± 0·02; n = 10). Furthermore, serum antibody titres correlated strongly with the amount of P. distasonis in faeces on day 28 (r = 0·99 for IgA, r = 0·92 for IgG and r = 0·90 for IgM; P < 0·01). The concentration of the specific coproantibodies was below the detection level in all groups.
To investigate the potential protective role of specific antibodies in serum we performed serum transfer experiments. Indeed, serum transfer from mice orally treated with mPd to naive mice decreased the severity of DSS colitis (Table 3).
Table 3.
Evaluation of acute dextran sulphate sodium (DSS)-induced colitis in conventional BALB/c mice, after transfer of serum from mice treated orally with membranous fraction of Parabacteroides distasonis (mPd) or phosphate-buffered saline (PBS).
| Experimental group | Colon length (cm) | Disease activity index | Histological grade |
|---|---|---|---|
| Serum from PBS-treated | 6·10 ± 0·58 | 2·67 ± 1·08 | 1·45 ± 0·36 |
| Serum from mPd-treated | 7·82 ± 0·15** | 0·40 ± 0·37** | 0·35 ± 0·06* |
Data are representative of one experiment. Similar results were obtained from three independent experiments. Values are expressed as means ± standard deviations (five mice per group). An unpaired Student's t-test was used to calculate the significance of differences between the mPd-treated group and the PBS-treated group (
P < 0·05;
P < 0·01).
The production of cytokines in gut tissues
To determine the effect of mPd therapy on cytokine production, colonic cytokine profiles from PBS-treated healthy mice, DSS/PBS-treated mice and DSS/mPd-treated mice were compared using cytokine antibody array (Fig. 1). Treatment with mPd prevented DSS-induced increases in several proinflammatory cytokines, including IFN-γ, IL-12, IL-17 and IL-6. Similarly, an overall decrease in both proinflammatory and anti-inflammatory cytokines was detected in Peyer's patches (IL-6, TGF-β and IFN-γ), caecum (IL-10, TNF-α, TGF-β and IFN-γ) and colon (IL-10, TNF-α, IL-6, TGF-β and IFN-γ) of DSS/mPd-treated mice compared to the DSS/PBS group (Fig. 2), as measured by ELISA. No significant differences in cytokine production were found in the ileum, jejunum mucosa (data not shown) or spleen cells (data not shown). mPd treatment did not change the production of cytokines in SCID mice except for an increase in IL-10 production in the colon (see Fig. S6).
Fig. 1.
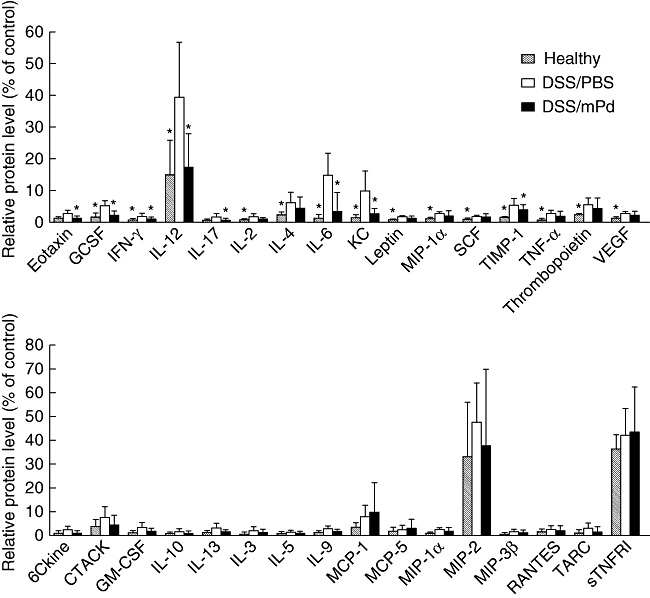
Pretreatment with membranous fraction of Parabacteroides distasonis (mPd) decreases the dextran sulphate sodium (DSS)-related increase in cytokine production in colon tissue as measured by cytokine antibody array. Values represent the percentage of the intensity of positive control. Granulocyte colony-stimulating factor (G-CSF), interleukin (IL)-l–12p40p70, macrophage inflammatory protein-1α (MIP), stem cell factor (SCF), growth-regulated alpha protein precursor (KC), tissue inhibitor of metalloproteinases-1 (TIMP), tumour necrosis factor-α (TNF), thrombopoietin (TPO) and vascular endothelial growth factor (VEGF). Data are means (bars) and standard deviation (whisker) of three samples [*P < 0·05 versus DSS/phosphate-buffered saline (PBS)].
Fig. 2.
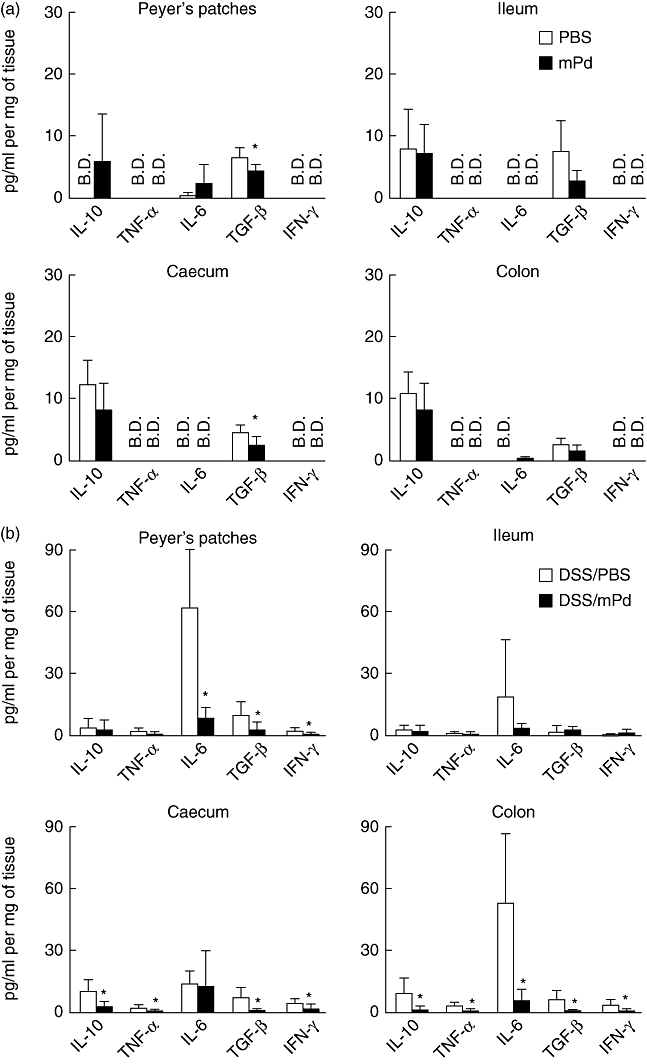
Pretreatment with membranous fraction of Parabacteroides distasonis (mPd) decreases cytokine production (pg/mg of tissue) in different parts of the gut in orally treated BALB/c mice as measured by enzyme-linked immunosorbent assay (ELISA). *P < 0·05 between mPd and phosphate-buffered saline (PBS) (a) or dextran sulphate sodium (DSS)/mPd and DSS/PBS (b)-treated mice. (B.D.) Values below the detection limit; n = 5 mice per group.
Effect of oral mPd on Tregs
We measured the number of Tregs (CD4+CD25+FoxP3+ cells) in the spleen, mesenteric lymph nodes and Peyer's patches of control and mPd-treated mice. We found that after DSS treatment, mice treated with mPd had significantly more CD4+CD25+FoxP3+ cells in their mesenteric lymph nodes (mean ± s.d.; 3·40 ± 0·50 versus 4·81 ± 0·30; P = 0·014) than PBS-treated (control) mice (see Fig. S3). There were no differences between mPd-treated and control groups in Treg numbers in spleen or Peyer's patches (data not shown).
Oral treatment with mPd does not affect the P. distasonis or Bacteroides/Prevotela group stool content but stabilizes the gut microbial ecology
Before colitis induction, the microbiota composition in PBS and mPd-treated groups was similar. In both groups Lactobacillus sp. and Bacteroides acidofaciens were present, as assessed by PCR-DGGE (Fig. 3a). In contrast, marked changes in microbiota composition were observed during DSS administration in the PBS-treated control group, as demonstrated in Fig. 3b.
Fig. 3.
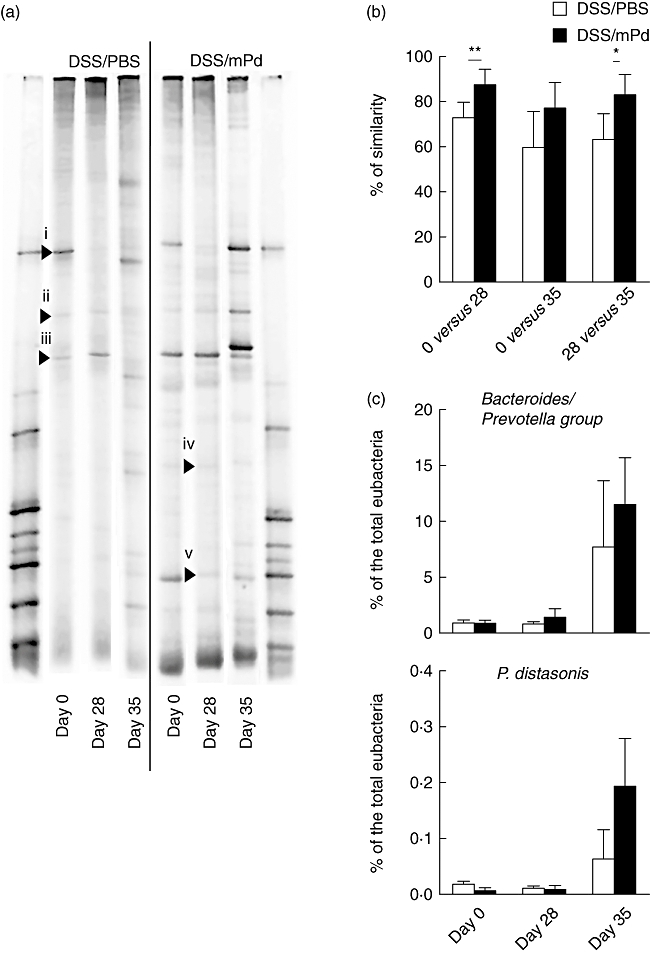
Oral treatment with membranous fraction of Parabacteroides distasonis (mPd) stabilize the intestinal microbiota without changing the P. distasonis or Bacteroides/Prevotela group stool content. (a) Changes in fecal bacterial populations of dextran sulphate sodium/phosphate-buffered saline (DSS/PBS) and DSS/mPd-treated mice were measured by 16S rRNA gene polymerase chain reaction- denaturing gradient gel electrophoresis (PCR-DGGE) before the treatment day 0 (before colitis induction), day 28 and at the end of the experiment (day 35). (b) Comparison of changes in DGGE profiles between PBS and mPd-treated mice at different time-points. (c) Representative quantitative changes in the Bacteroides/Prevotela group or P. distasonis in stool were measured by quantitative PCR. Data are expressed as a percentage of total Eubacteria[bar (mean); whisker (standard deviation)]. Samples from the same mouse at different time-points were arranged together. The bands marked with arrows were identified as B. acidofaciens, 100% (i), uncultured bacterium related to Clostridium (ii), Lactobacillus johnsonii, 98% and L. gasseri, 98% (iii), H. muridarum, 95% (iv) and Lactobacillus sp. 97% (v) by 16S rRNA sequence analysis.
Quantitative PCR on the Bacteroides/Prevotela group and P. distasonis showed a statistically significant increase of these bacteria during DSS treatment from 0·84 ± 0·39% to 9·58 ± 6·12% (P < 0·05) and from 0·01 ± 0·01% to 0·13 ± 0·11% (P < 0·05) of total Eubacteria, respectively. However, no statistically significant differences in bacteria numbers were found between the mPd-treated and control groups on the same day of the experiment (Fig. 3c).
Discussion
Manipulation of the intestinal microbiota by oral administration of probiotic bacteria or selective antibiotics has emerged as a potentially useful therapeutic strategy for human IBD [4,14–16]. However, the clinical utility of such an approach remains controversial, as the link between specific mechanisms of action and therapeutic effects of specific strains or bacterial components has been difficult to establish. We have shown previously that oral administration of crude lysates from anaerobic bacteria attenuates the severity of experimental colitis [21]. In this study we identified a specific anaerobic lysate of P. distasonis and its cellular components with anti-inflammatory capacity. We investigated the possible mechanisms of action using in vitro techniques and in vivo acute and chronic DSS colitis.
The intestinal microbiota is a complex ecosystem which consists of high levels of obligate anaerobes (making up more than 90% of the microbiota). This system is in constant interaction with the host's immune system. Commensal bacteria play an essential role in mucosal immune system development, and dysregulated immune responses to opportunistic commensals have been suggested to play a role in intestinal inflammation [1,5,27–29]. Moreover, it has been proposed that microbiota composition can be regulated by the host's immune system [29–31].
We found that oral administration of the membranous fraction of P. distasonis (mPd) and its DNA were effective in suppressing acute DSS colitis. mPd also attenuated chronic colitis; however, the effect was less marked. The results are consistent with previous observations that prevention of inflammatory bowel disease is achieved more easily than treatment of ongoing inflammation [19].
Several mechanisms may underlie the protective effect of oral mPd in colitis. We investigated whether oral mPd (a) changes local gut cytokine production, resulting in a non-specific anti-inflammatory milieu (b) stimulates adaptive immune mechanisms (vaccination effect) and/or (c) stabilizes intestinal microbiota composition, thereby rendering the mice less susceptible to DSS colitis. Acute DSS colitis is believed to be driven initially by innate immunity mechanisms and, in particular, the role of macrophages has been suggested [5,24,25,32]. We tested the ability of Pd and mPd to decrease the TNF-α production by LPS-activated macrophages in vitro. We found that at concentrations above 10 ng/l both Pd and mPd decreased TNF-α production by macrophages, suggesting a possible direct effect of Pd and mPd on innate cell immunity. This mechanism may contribute to the attenuation of acute DSS injury observed in vivo by Pd and mPd. Because probiotics and their isolated DNA have been shown to attenuate the DSS colitis via Toll-like receptor (TLR)-dependent pathways [20,33], the involvement of pattern recognition receptors in the initiation of the protective effect by mPd and DNA cannot be ruled out.
Our results show that oral mPd decreases the production of many proinflammatory cytokines, including TNF-α, but also of anti-inflammatory cytokines in the colon of treated mice. The changes were observed in Peyer's patches, caecum and colon of mPd-treated mice compared to control mice, suggesting local mucosal effects of mPd throughout the intestine. This immunomodulatory activity of mPd may interfere with both leucocyte accumulation in intestinal mucosa and with barrier function failure, and contribute to decrease inflammation [34,35]. Our results are consistent with previous work that reported a decrease in proinflammatory, as well as anti-inflammatory, cytokine production in mice treated with DSS and live probiotic E. coli Nissle 1917 [33].
It is known that some components of the indigenous microbiota have immunomodulatory properties and affect cytokine production [20,36–38]. Mazmanian et al. have shown that changes in local cytokine production caused by the common commensal bacterium B. fragilis and its polysaccharide A protected mice from experimental colitis [39]. Unfortunately, changes in microbiota composition or a polysaccharide A-specific immune response were not investigated in that study. Anti-TNF-α-based therapies have been shown to be effective in flare-ups of chronic inflammatory disorders, including IBD and rheumatoid arthritis [40,41].
The presence of serum antibodies directed against commensals in IBD patients suggests that some patients may exhibit systemic priming against microbiota [42]. The pathophysiological significance of this priming remains unclear, but may suggest a role of adaptive immune mechanisms in the luminal containment of microbiota components in patients with mucosal damage. Indeed, a recent study has proposed that systemic immune responses may compensate for innate immune deficiency and constitute a novel homeostatic mechanism in host–microbiota mutualism [43]. Antibodies could occur as a consequence of increased penetration of microbiota components through impaired mucus and epithelial layers. These antibodies may play not only a diagnostic [42], but also a protective role against microbes that could perpetuate intestinal inflammation. Moreover, if bacterial epitopes are shared among several bacterial species (molecular mimicry), generation of antibodies against common commensals could constitute potential therapeutic targets in IBD. After oral administration of mPd, we found increased levels of P. distasonis-specific antibodies in sera compared to controls, suggesting the possibility that specific immune responses to P. distasonis are protective against experimental colitis. The identification of the specific antibody fraction responsible for this effect is being examined currently in our laboratories. Although both oral and parenteral forms of administration of mPd increased the levels of specific serum antibodies, only oral administration had an effect on colitis prevention. This suggests that the gut microenvironment during antigen priming is essential for colitis prevention, but once established it can be transferred with serum. To investigate further the role of adaptive immunity in the anti-colitic effect of mPd, we studied immunodeficient mice lacking T and B lymphocytes. Although the severity of DSS-induced acute inflammation in SCID mice was similar to that in immunocompetent mice [5,32], colitis was not prevented in SCID mice with oral mPd. One limitation of the comparison between BALB/c and SCID mice relates to differences in gut microbiota composition and/or innate immune cell activity between strains [44]. The innate and adaptive immune systems work in synergy to mount appropriate immune responses to the commensal microbiota and maintain homeostasis [43]. As suggested by our in vitro experiments, we cannot rule out the involvement of innate immune mechanisms in the initiation of mPd-induced protection. However, the in vivo experiments show clearly that the adaptive immune response is required for mPd-induced protection.
Our results also suggest a role for Tregs in this mPd-induced protection. We show that there is an increase in both CD4+FoxP3+ and CD4+FoxP3– T cells in MLN of mPd-treated mice. The increase in CD4+FoxP3+ cells is, however, proportionally higher, therefore the increase in absolute numbers of Tregs cannot be explained solely by the increase in CD4+ cells. The significance of this finding requires confirmation in future experiments. Previous work has proposed that the presence of microbiota and bacterial components in the gut can influence Treg activity [36,45]. Thus, future studies will address the relative importance of induction of protective immunity (vaccination) and of tolerance induction in the prevention and treatment of acute and chronic colitis by mPd.
In accordance with previous work [25], we found that DSS colitis alters the intestinal ecosystem with an increase in P. distasonis. Interestingly, we found that oral treatment with mPd prevents the microbiota changes caused by DSS. The mechanism by which mPd stabilizes the microbiota composition during DSS is not clear, but could be secondary to improvement in inflammation. Alternatively, mPd may directly affect other bacteria, by a direct antimicrobial effect, or indirectly, by improving epithelial barrier function or regulation of the mucosal immune system. The latter has been demonstrated previously for probiotic candidates Propionibacterium freudenreichii and Faecalibacterium prausnitzii[37,38].
In conclusion, oral administration of P. distasonis components (mPd) protects from experimentally induced intestinal inflammation through several innate and adaptive immunomodulatory mechanisms. Oral mPd promotes an increase in the level of mPd-specific antibodies and in the numbers of Tregs. In addition, oral mPd inhibits TNF-α production by macrophages in vitro and stabilizes the intestinal microbiota. These results highlight the importance of individualizing and characterizing the potential capacity of commensal bacteria as immunomodulatory agents. Moreover, oral administration of sterile bacterial components, in contrast to live bacteria, may be safer in severely ill or immunocompromised patients.
Our results support the hypothesis that oral supplements consisting of components from specific commensals may lead to the development of new therapeutic approaches for chronic intestinal inflammation.
Acknowledgments
This work is dedicated to our recently departed friend and colleague Pavel Jelen. This work was supported by grants KJB500200904, S500200572 and A5020205 from the Academy of Sciences of Czech Republic; 310/08/H077, 305/08/0535 and 303/08/0367 from Czech Science Foundation, and Institutional Research Concept Grant AV0Z50200510. E. F. V. is supported by CAG/CIHR and CCFC grants, and an Internal Career Award by the Department of Medicine at McMaster University.
Disclosure
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Supplementary materials and methods.
Fig. S1. Histological examples of different grades of mucosal damage in dextran sulphate sodium (DSS)-treated mice (haematoxylin and eosin-stained colon descendens; magnification, ×40). See Table S1 for detailed description.
Fig. S2. Cytokine production by colon tissue of healthy mice during the first, second and third days of ex vivo cultivation.
Fig. S3. Showing gating strategy in three mice (rows) from dextran sulphate sodium/phosphate-buffered saline (DSS/PBS) and three mice from the DSS/membranous fraction of P. distasonis (mPd)-treated group.
Fig. S4. Cytokine profiling of the supernatants after the cultivation of untreated RAW264.7 cells or cells after treatment with lipopolysaccharide (LPS), Parabacteroides distasonis/lipopolysaccharide (Pd+LPS) or membranous fraction of P.distasonis (mPd)+LPS, as measured by RayBioMouse Cytokine Antibody Array III.
Fig. S5. The effect of Parabacteroides distasonis (Pd) and membranous fraction of P. distasonis (mPd) on tumour necrosis factor (TNF)-α production by lipopolysaccharide (LPS)-activated macrophage cell line RAW 264.7 was measured by enzyme-linked immunosorbent assay (ELISA).
Fig. S6. Pretreatment with membranous fraction of P. distasonis (mPd) decreases cytokine production (pg/mg of tissue) in different parts of the gut in orally treated severe combined immunodeficient (SCID) mice as measured by enzymelinked immunosorbent assay (ELISA).
Table S1. Detailed description of individual histological grades.
Table S2. Layout of the RayBio™ Mouse Cytokine Array II.
Table S3. Poplymerase chain reaction (PCR) primer sets used in the study.
Table S4. Evaluation of acute dextran sulphate sodium (DSS) colitis in orally treated BALB/c mice.
Table S5. Evaluation of acute dextran sulphate sodium (DSS) colitis in orally treated BALB/c mice.
Table S6. Evaluation of acute dextran sulphate sodium (DSS) colitis in parenterally treated BALB/c mice.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–94. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Seksik P, Sokol H, Lepage P, et al. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(Suppl 3):11–18. doi: 10.1111/j.1365-2036.2006.03053.x. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991;338:771–4. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- 4.Prantera C, Scribano ML. Antibiotics and probiotics in inflammatory bowel disease: why, when, and how. Curr Opin Gastroenterol. 2009;25:329–33. doi: 10.1097/MOG.0b013e32832b20bf. [DOI] [PubMed] [Google Scholar]
- 5.Hudcovic T, Stepankova R, Cebra J, Tlaskalova-Hogenova H. The role of microflora in the development of intestinal inflammation: acute and chronic colitis induced by dextran sulfate in germ-free and conventionally reared immunocompetent and immunodeficient mice. Folia Microbiol (Praha) 2001;46:565–72. doi: 10.1007/BF02818004. [DOI] [PubMed] [Google Scholar]
- 6.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–53. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 8.Stepankova R, Powrie F, Kofronova O, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis. 2007;13:1202–11. doi: 10.1002/ibd.20221. [DOI] [PubMed] [Google Scholar]
- 9.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seksik P, Rigottier-Gois L, Gramet G, et al. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003;52:237–42. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scanlan PD, Shanahan F, O’Mahony C, Marchesi JR. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn's disease. J Clin Microbiol. 2006;44:3980–8. doi: 10.1128/JCM.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Assche G, Vermeire S, Rutgeerts P. Safety issues with biological therapies for inflammatory bowel disease. Curr Opin Gastroenterol. 2006;22:370–6. doi: 10.1097/01.mog.0000231810.87901.e8. [DOI] [PubMed] [Google Scholar]
- 13.Mahida YR, Rolfe VE. Host–bacterial interactions in inflammatory bowel disease. Clin Sci (Lond) 2004;107:331–41. doi: 10.1042/CS20040136. [DOI] [PubMed] [Google Scholar]
- 14.Bibiloni R, Fedorak RN, Tannock GW, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–46. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 15.Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–23. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–14. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seksik P, Dray X, Sokol H, Marteau P. Is there any place for alimentary probiotics, prebiotics or synbiotics, for patients with inflammatory bowel disease? Mol Nutr Food Res. 2008;52:906–12. doi: 10.1002/mnfr.200700147. [DOI] [PubMed] [Google Scholar]
- 18.Kokesova A, Frolova L, Kverka M, et al. Oral administration of probiotic bacteria (E. coli Nissle, E. coli O83, Lactobacillus casei) influences the severity of dextran sodium sulfate-induced colitis in BALB/c mice. Folia Microbiol (Praha) 2006;51:478–84. doi: 10.1007/BF02931595. [DOI] [PubMed] [Google Scholar]
- 19.Obermeier F, Dunger N, Strauch UG, et al. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217–24. doi: 10.1046/j.1365-2249.2003.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rachmilewitz D, Katakura K, Karmeli F, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–8. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Verdu EF, Bercik P, Cukrowska B, et al. Oral administration of antigens from intestinal flora anaerobic bacteria reduces the severity of experimental acute colitis in BALB/c mice. Clin Exp Immunol. 2000;120:46–50. doi: 10.1046/j.1365-2249.2000.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westphal O, Lüderitz O, Bister F. Extraction of bacteria with phenol/water. Z Naturforsch. 1952;7b:148–55. [Google Scholar]
- 23.Ali IK, Zaki M, Clark CG. Use of PCR amplification of tRNA gene-linked short tandem repeats for genotyping Entamoeba histolytica. J Clin Microbiol. 2005;43:5842–7. doi: 10.1128/JCM.43.12.5842-5847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murano M, Maemura K, Hirata I, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–8. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 26.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 27.Monteleone G, Peluso I, Fina D, et al. Bacteria and mucosal immunity. Dig Liver Dis. 2006;38(Suppl 2):S256–60. doi: 10.1016/S1590-8658(07)60005-X. [DOI] [PubMed] [Google Scholar]
- 28.Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Williams AM, Probert CS, Stepankova R, Tlaskalova-Hogenova H, Phillips A, Bland PW. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology. 2006;119:470–8. doi: 10.1111/j.1365-2567.2006.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petnicki-Ocwieja T, Hrncir T, Liu YJ, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA. 2009;106:15813–18. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 33.Grabig A, Paclik D, Guzy C, et al. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via Toll-like receptor 2- and Toll-like receptor 4-dependent pathways. Infect Immun. 2006;74:4075–82. doi: 10.1128/IAI.01449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–19. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hrncir T, Stepankova R, Kozakova H, Hudcovic T, Tlaskalova-Hogenova H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: studies in germ-free mice. BMC Immunol. 2008;9:65. doi: 10.1186/1471-2172-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada Y, Tsuzuki Y, Miyazaki J, et al. Propionibacterium freudenreichii component 1.4-dihydroxy-2-naphthoic acid (DHNA) attenuates dextran sodium sulphate induced colitis by modulation of bacterial flora and lymphocyte homing. Gut. 2006;55:681–8. doi: 10.1136/gut.2005.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 40.Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology. 2000;119:1148–57. doi: 10.1053/gast.2000.18160. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui MA, Scott LJ. Infliximab: a review of its use in Crohn's disease and rheumatoid arthritis. Drugs. 2005;65:2179–208. doi: 10.2165/00003495-200565150-00014. [DOI] [PubMed] [Google Scholar]
- 42.Adams RJ, Heazlewood SP, Gilshenan KS, O’Brien M, McGuckin MA, Florin TH. IgG antibodies against common gut bacteria are more diagnostic for Crohn's disease than IgG against mannan or flagellin. Am J Gastroenterol. 2008;103:386–96. doi: 10.1111/j.1572-0241.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- 43.Slack E, Hapfelmeier S, Stecher B, et al. Innate and adaptive immunity cooperate flexibly to maintain host–microbiota mutualism. Science. 2009;325:617–20. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keilbaugh SA, Shin ME, Banchereau RF, et al. Activation of RegIIIbeta/gamma and interferon gamma expression in the intestinal tract of SCID mice: an innate response to bacterial colonisation of the gut. Gut. 2005;54:623–9. doi: 10.1136/gut.2004.056028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


