Abstract
Otitis media is one of the most common and intractable ear diseases, and is the major cause of hearing loss, especially in children. Multiple factors affect the onset or development of otitis media. Prostaglandin D2 is the major prostanoid involved in infection and allergy. However, the role of prostaglandin D2 and prostaglandin D2 receptors on the pathogenesis of otitis media remains to be determined. Recent studies show that D prostanoid receptor (DP) and chemoattractant receptor-homologous molecule expressed on T helper type 2 (Th2) cells (CRTH2) are major prostaglandin D2 receptors. In this study, homozygous DP single gene-deficient (DP–/–) mice, CRTH2 single gene-deficient (CRTH2–/–) mice and DP/CRTH2 double gene-deficient (DP–/– CRTH2–/–) mice were used to investigate the role of prostaglandin D2 and its receptors in otitis media. We demonstrate that prostaglandin D2 is induced by lipopolysaccharide (LPS), a major component of Gram-negative bacteria, and that transtympanic injection of prostaglandin D2 up-regulates macrophage inflammatory protein 2 (MIP-2), interleukin (IL)-1β and IL-6 in the middle ear. We also show that middle ear inflammatory reactions, including infiltration of inflammatory cells and expression of MIP-2, IL-1β and IL-6 induced by LPS, are reduced significantly in DP–/– mice and DP–/– CRTH2–/– mice. CRTH2–/– mice display inflammatory reactions similar to wild-type mice. These findings indicate that prostaglandin D2 may play significant roles in LPS-induced experimental otitis media via DP.
Keywords: animal models/studies – mice/rats, cytokine/interleukin/chemokine receptors, ear immunology/disease, inflammation/inflammatory mediators, eicosanoids, otitis media
Introduction
Otitis media with effusion is a common cause of hearing loss, especially in children. Multiple factors, including Eustachian tube dysfunction and adenoid hypertrophy, affect the onset or development of otitis media with effusions. Lipopolysaccharide (LPS), one of the components of the outer membrane of Gram-negative bacteria, is a powerful mediator of inflammatory reactions, and is frequently detected in the middle ear in patients with otitis media with effusion [1]. Several cytokines and chemokines, including interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 and tumour necrosis factor (TNF)-α, are also considered important factors in the pathogenesis of otitis media with effusions [2–4].
Very early studies detected prostanoids in middle ear effusions in patients with otitis media [5,6]. Cyclooxygenase 2 (COX-2), a rate-determining enzyme that catalyzes the synthesis of prostanoids, plays an important role in otitis media [7]. Cyclooxygenases and specific synthases metabolize arachidonic acid to five primary prostanoids: prostaglandin (PG) D2 (PGD2), PGE2, PGF2α, PGI2 and thromboxane A2 [8,9]. PGD2 (C20H32O5, 1 M = 352 mg/ml) is the major prostanoid produced during infection and acute phase of allergic reactions, and is observed both in middle ear effusions in patients with otitis media and in experimental otitis media in animal studies [10,11]. However, the role of PGD2 in otitis media has not been revealed. Recent studies show that D prostanoid receptor (DP) and the chemoattractant receptor-homologous molecule expressed on T helper type 2 (Th2) cells (CRTH2) are high-affinity receptors for PGD2[12,13]. PGD2 shows various effects by binding and activating these two major PGD2 receptors [9,14]. DP and CRTH2 are G-protein coupled rhodopsin-type receptors with seven transmembrane domains, and the homology among receptor homologues from various species is considerably high. The amino acid and nucleotide sequence homology between human and mouse DP is 73% [15].
To the best of our knowledge, the expression of DP and CRTH2 in the middle ear has not been reported. In this study, we investigate the role of PGD2 and its receptors (DP and CRTH2) in the expression of IL-1β, IL-6 and macrophage inflammatory protein (MIP)-2, which is the murine functional homologue of human IL-8, in LPS-induced otitis media in mice.
Materials and methods
Mice
BALB/c mice (female, 7–12 weeks old) were used in this study. Homozygous DP single gene-deficient (DP–/–) mice and CRTH2 single gene-deficient (CRTH2–/–) mice have been described previously [16,17]. DP/CRTH2 double gene-deficient (DP–/– CRTH2–/–) mice were generated by crossing each of the receptor-deficient mice at Tokyo Medical and Dental University. All gene-deficient mice were derived on a BALB/c background. The mice were housed under specific pathogen-free conditions in the Department of Animal Resources at Okayama University and Tokyo Medical and Dental University. The Animal Research Control Committees of Okayama University and Tokyo Medical and Dental University approved the experimental protocols and procedures performed in this study.
Otoscopic examination was performed for all mice prior to treatment to ensure that tympanic membranes were normal, and that no middle ear effusion was present. The mice were deeply anaesthetized using both ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) via intraperitoneal injection before each procedure.
Establishment of experimental otitis media
Experimental otitis media with effusion was induced by middle ear injection of LPS, as reported previously [18]. Wild-type mice, DP–/– mice, CRTH2–/– mice and DP–/– CRTH2–/– mice were distributed randomly into experimental and control groups. The experimental group received LPS (1·0 mg/ml, Sigma-Aldrich, St Louis, MO, USA) via transtympanic injection. Phosphate-buffered saline (PBS, 0·01 M) was injected into the middle ears of animals in the control group. The middle ears were filled with 20 µl of PBS or LPS.
Expression of PGD2 induced by LPS
PBS-treated or LPS-treated wild-type mice were killed at 3 h, 6 h, 12 h, 24 h (1 day), 72 h (3 days) and 168 h (7 days) after injection. Middle ears were washed transtympanically with 150 µl of PBS. The collected PBS from the middle ear wash was transferred to 1·5-ml microcentrifuge tubes (Treff AG, Degersheim, Switzerland) and stored at −30°C until analysis. Concentration of PGD2 was measured with the prostaglandin D2 enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI, USA), according to the manufacturer's instructions.
Detection of DP and CRTH2 by reverse transcription–polymerase chain reaction (RT–PCR)
Middle ear mucosa samples of PBS-treated or LPS-treated wild-type mice were collected 3 h or 24 h after injection, soaked immediately in RNA stabilization reagent (RNAlater; Qiagen, Tokyo, Japan) and stored at −30°C. Total cellular RNA was extracted using the RNeasy mini kit (Qiagen), according to the manufacturer's instructions. The extracted RNA was then treated with amplification grade deoxyribonuclease I (Sigma-Aldrich) for 15 min at room temperature. To perform RT–PCR, cDNA was formed using a first-strand cDNA synthesis kit (Toyobo, Osaka, Japan), according to the manufacturer's instructions, and cDNA was subjected to PCR.
The PCR primer sequences and product sizes were as follows: DP, forward 50-CTTCACCATGTGTTCCCTGC-30 and reverse 50-ATGGCTGCTCCAGTTTCTGTA-30 [226 base pairs (bp)]; and CRTH2, forward 50-TCTCAACCAATCAGCACACC-30 and reverse 50-CCTCCAAGAGTGGACAGAGC-30 (173 bp) (Sigma Genosys, Hokkaido, Japan).
Quantification of MIP-2, IL-1β and IL-6 induced by PGD2 or LPS in middle ear
Wild-type mice were divided into six groups. Four groups received 20 µl of serial concentrations of PGD2 (2 × 10−9 M, 2 × 10−8 M, 2 × 10−7 M or 2 × 10−6 M) by transtympanic injection, respectively. One group was injected with LPS transtympanically. Control group mice were treated by transtympanic injection of PBS. Mutant mice were treated similarly with LPS or PBS. Mice were killed 24 h after injection, and the middle ears were washed transtympanically with 300 µl of PBS. The collected PBS from the middle ear wash was transferred to 1·5-ml microcentrifuge tubes (Treff AG) and stored at −30°C until analysis.
Concentration of MIP-2 in collected PBS from the middle ear wash was measured by enzyme-linked immunosorbent assay (ELISA) using the Mouse DuoSet ELISA Development kit (R&D Systems, Minneapolis, MN, USA). Concentrations of IL-1β and IL-6 in collected PBS from the middle ear wash were measured by ELISA using mouse Opt ELISA sets (BD Biosciences Pharmingen, San Diego, CA, USA). All procedures were performed according to the manufacturer's instructions. All samples were examined in duplicate, and measured values were averaged to determine concentrations of cytokines in each sample.
Preparation of murine polymorphonuclear leucocytes and macrophages
Mice were injected intraperitoneally with 2 ml of 2% sodium caseinate (Sigma-Aldrich) in sterile saline, and were killed by cervical dislocation 6 h or 72 h after injection. Peritoneal lavage was performed with 5 ml of ice-cold sterile PBS containing 2% fetal bovine serum (FBS). Peritoneal exudate cells 6 h after injection of sodium caseinate were polymorphonuclear leucocytes, and collected cells 72 h after injection were macrophages. Polymorphonuclear leucocytes and macrophages were washed with PBS (containing 2% FBS) and resuspended in RPMI-1640 (Sigma-Aldrich) containing 10% FBS supplemented with penicillin and streptomycin. The cell viability and concentration were determined by trypan blue exclusion.
Quantification of MIP-2, IL-1β and IL-6 induced by PGD2 in polymorphonuclear leucocytes and macrophage
Polymorphonuclear leucocytes or macrophages were plated in culture dishes at a density of 2 × 106 cells/ml in RPMI-1640 supplemented with 10% FBS and cultured with PGD2 (2 × 10−6 M) or PBS for 24 h at 37°C, 5% CO2. At the end of the culture, supernatants were harvested and stored at −30°C. Concentrations of MIP-2, IL-1β and IL-6 in supernatants were determined by ELISA, as described above.
Histological examination
Wild-type mice, DP–/– mice, CRTH2–/– mice and DP–/– CRTH2–/– mice were killed 24 h after middle ear administration of PBS or LPS. Temporal bones were removed immediately after killing and processed for histological examination. Bone specimens were placed in 4% paraformaldehyde for 48 h and decalcified in 10% ethylenediamine tetraacetic acid (EDTA) for 3 weeks at 4°C. After dehydration, specimens were embedded in paraffin and sectioned at 4 µm thickness, and then mounted on glass slides for processing with haematoxylin and eosin. The stained slides were examined under a light microscope.
The expression of DP in middle ear mucosa was observed immunohistochemically. Paraffin-embedded temporal bones were sectioned at 4 µm thickness. Sections were deparaffinized, rehydrated and pretreated using autoclave (120°C/10 min) for antigen retrieval. Endogenous peroxidase activity was quenched with 1% hydrogen peroxide (H2O2), and non-specific protein binding was blocked with skimmed milk. The tissue sections were then incubated with anti-PGD2 receptor 1 polyclonal antibody (Cayman Chemical, Ann Arbor, MI, USA) or control immunoglobulin (Ig)G overnight at 4°C. For visualization, N-Histofine Simple Stain MAX PO (R) (Nichirei Biosciences, Tokyo, Japan) and diaminobenzidine substrate (Dako, Tokyo, Japan) were used according to the manufacturer's instructions.
Immunocytochemistry was performed to detect the expression of DP in polymorphonuclear leucocytes. Plated polymorphonuclear leucocytes were fixed in paraformaldehyde. After blocking the endogenous peroxidase with H2O2 in PBS for 20 min, the cells were incubated with primary antibodies [anti-PGD2 receptor 1 polyclonal antibody (Cayman Chemical) or control IgG]. For visualization, the Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, CA, USA) and diaminobenzidine substrate (Dako) were used according to the manufacturer's instructions. The slides were counterstained with haematoxylin to visualize nuclear morphology.
Statistical analysis
Concentrations of MIP-2, IL-6 and IL-1β represent the mean ± standard error of the mean (s.e.m.). All means are calculated from six to 10 samples. Statistical analyses were performed using the non-parametric Mann–Whitney U-test. A significant difference was established at P< 0·05.
Results
Production of PGD2 in the middle ear by LPS
We first examined PGD2 production by LPS in wild-type mice. PGD2 concentrations in middle ear effusions after LPS injection were quantified by EIA. Significant production of PGD2 was detected 6 h after transtympanic injection with LPS, and the maximum production was seen at 72 h, followed by a decrease in its concentration at 168 h (Fig. 1). The result suggests the possibility of the implication of PGD2 in LPS-mediated otitis media. This hypothesis may be dependent upon the premise that ear tissue cells express PGD2 receptors. RT–PCR examination showed that both DP and CRTH2 were expressed in the middle ear mucosa of wild-type BALB/c mice (Fig. 2). DP was expressed strongly, especially in LPS-injected mice.
Fig. 1.
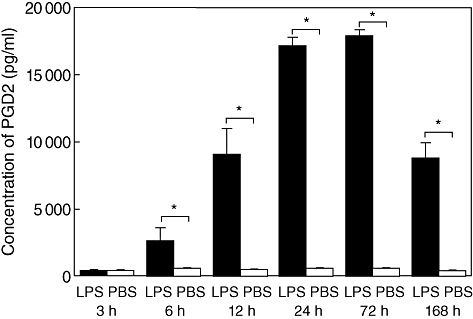
Levels of prostaglandin D2 (PGD2) in middle ear effusions in wild-type mice. Middle ears were washed with phosphate-buffered saline (PBS) at indicated times after lipopolysaccharide (LPS). PGD2 concentrations in PBS were determined and are expressed as mean concentrations ± standard error of the mean (n = 6 per group). *P< 0·05.
Fig. 2.
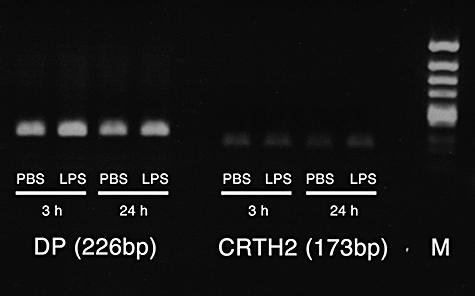
Expression of two prostaglandin D2 (PGD2) receptors in the middle ear. RNA was extracted from middle ear mucosa in wild-type mice 3 h and 24 h after phosphate-buffered saline (PBS) or lipopolysaccharide (LPS) injection. Messenger RNA for D prostanoid receptor (DP) and chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) were detected by reverse transcription–polymerase chain receptor. M, molecular marker.
Production of MIP-2, IL-1β and IL-6 by PGD2
In order to determine whether PGD2 induces MIP-2, IL-1β and IL-6 production in the middle ear, transtympanic administration of PGD2 into the middle ear was performed. Mean concentrations of MIP-2 in middle ear effusions in wild-type mice 24 h after the administration of PGD2 are shown in Fig. 3a. Significant differences were observed between PBS-injected control mice and PGD2-injected mice [PGD2 (2 × 10−8 M), P= 0·002; PGD2 (2 × 10−7 M), P= 0·016; PGD2 (2 × 10-6 M), P= 0·002].
Fig. 3.
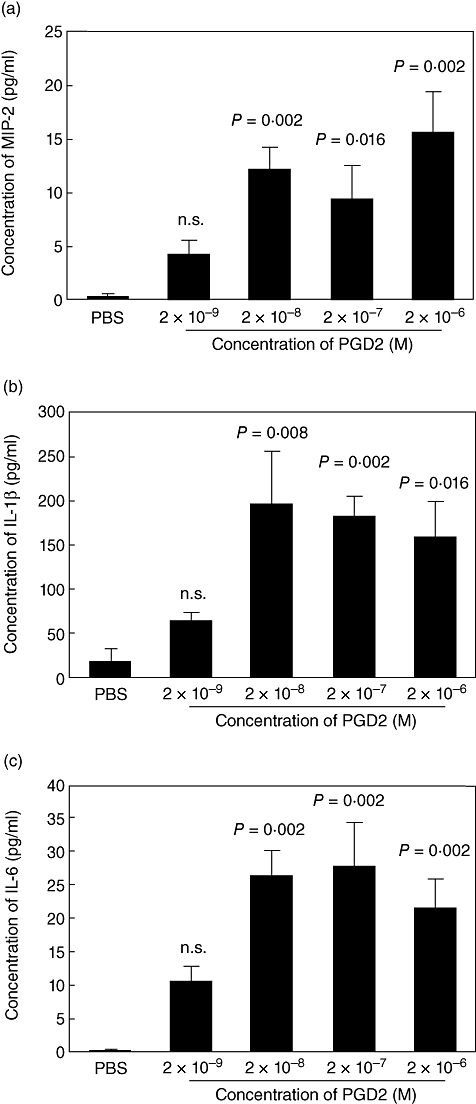
Prostaglandin D2 (PGD2)-mediated production of macrophage inflammatory protein 2 (MIP-2) (a), interleukin (IL)-1β (b) and IL-6 (c) in middle ear effusions. Wild-type mice were injected with phosphate-buffered saline (PBS) or serial concentrations of PGD2. Middle ears were washed with phosphate-buffered saline (PBS) 24 h after injection. The cytokine concentrations in middle ear washes were determined by enzyme-linked immunosorbent assay, and are shown as mean concentrations ± standard error of the mean (n = 6 per group). n.s., not significant.
Mean concentrations of IL-1β in middle ear effusions in wild-type mice are shown (Fig. 3b). Compared with PBS, PGD2 up-regulated IL-1β secretion in the middle ear cavity [PGD2 (2 × 10−8 M), P= 0·008; PGD2 (2 × 10−7 M), P= 0·002; PGD2 (2 × 10−6 M), P= 0·016].
Mean concentrations of IL-6 in middle ear effusions in wild-type mice are presented in Fig. 3c. Significant differences were observed between PBS-injected control mice and PGD2-injected mice [PGD2 (2 × 10−8 M), P= 0·002; PGD2 (2 × 10−7 M), P= 0·002; PGD2 (2 × 10−6 M), P= 0·002].
The role of DP and CRTH2 in LPS-induced MIP-2, IL-1β and IL-6 in middle ear secretion
Next we sought to determine the role of DP and CRTH2 in LPS-induced MIP-2, IL-1β and IL-6 secretion in the middle ear. Figure 4a shows mean concentrations of MIP-2 in middle ear effusions in wild-type mice, CRTH2–/– mice, DP–/– mice and DP–/– CRTH2–/– mice 24 h after transtympanic administration of LPS (Fig. 4a). Levels of MIP-2 in PBS-injected wild-type mice, CRTH2–/– mice, DP–/– mice and DP–/– CRTH2–/– mice were low, and no significant difference was observed between them. Mean concentration of MIP-2 in LPS-injected wild-type mice was significantly higher than that in PBS-injected wild-type mice (P< 0·001). No significant difference was observed between LPS-injected wild-type mice and LPS-injected CRTH2–/– mice (P= 0·143). Conversely, concentrations of MIP-2 in LPS-injected DP–/– mice and LPS-injected DP–/– CRTH2–/– mice were significantly lower than that in LPS-injected wild-type mice (DP–/– mice, P= 0·011; DP–/– CRTH2–/– mice, P< 0·001).
Fig. 4.
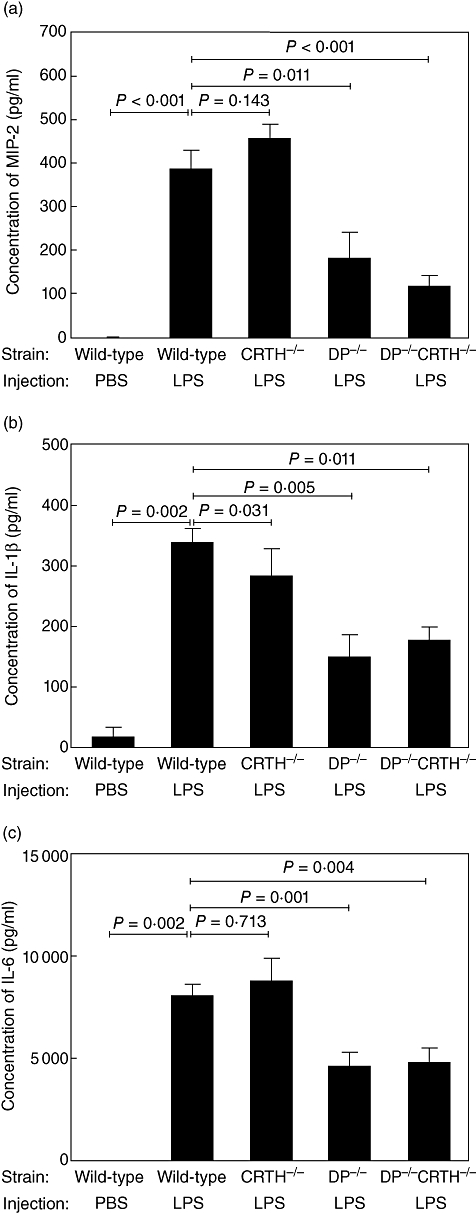
Production of macrophage inflammatory protein 2 (MIP-2) (a), interleukin (IL)-1β (b) and IL-6 (c) in middle ear effusions in wild-type mice and mutant mice. Chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2)–/– mice, D prostanoid receptor (DP)–/– mice and DP–/– CRTH2–/– mice were injected with lipopolysaccharide (1 mg/ml, 20 µl) and cytokine concentrations were determined as shown in Fig. 3 (n = 6–10 per group).
Mean concentrations of IL-1β in middle ear effusions in wild-type mice, CRTH2–/– mice, DP–/– mice and DP–/– CRTH2–/– mice were determined (Fig. 4b). Levels of IL-1β in PBS-injected wild-type mice, CRTH2–/– mice, DP–/– mice and DP–/– CRTH2–/– mice were low, and no significant difference was observed between them. The mean concentration of IL-1β in LPS-injected wild-type mice was significantly higher than that in PBS-injected wild-type mice (P= 0·002). Concentrations of IL-1β in LPS-injected CRTH2–/– mice, LPS-injected DP–/– mice and LPS-injected DP–/– CRTH2–/– mice were significantly lower than those in LPS-injected wild-type mice, respectively (CRTH2–/– mice, P= 0·031; DP–/– mice, P= 0·005; DP–/– CRTH2–/– mice, P= 0·001).
Figure 4c shows mean concentrations of IL-6 in middle ear effusions in wild-type mice, CRTH2–/– mice, DP–/– mice and DP–/– CRTH2–/– mice (Fig. 4c). Levels of IL-6 in PBS-injected wild-type mice, CRTH2–/– mice, DP–/– mice and DP–/– CRTH2–/– mice were low, and no significant difference was observed between them. The mean concentration of IL-6 in LPS-injected wild-type mice was significantly higher than that in PBS-injected wild-type mice (P= 0·002). No significant difference was observed between LPS-injected wild-type mice and LPS-injected CRTH2–/– mice (P= 0·713). Concentrations of IL-6 in LPS-injected DP–/– mice and LPS-injected DP–/– CRTH2–/– mice were significantly lower than those in LPS-injected wild-type mice (DP–/– mice, P= 0·001; DP–/– CRTH2–/– mice, P= 0·004).
Histological and immunohistochemical findings of LPS-induced otitis media
Histological examination in wild-type mice revealed that LPS-induced severe infiltration of numerous inflammatory cells, including neutrophils and monocytes/macrophages in the middle ear epithelium, and mucosal thickness was observed in the experimental group inoculated with LPS compared with PBS-injected control mice. Middle ear effusion with neutrophils and monocytes/macrophages was observed in the LPS-injected wild-type mice (Fig. 5a). No middle ear effusion was detected in the PBS-injected control mice (Fig. 5b).
Fig. 5.

Histological examination of middle ears of wild-type mice (a) (b), chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2)–/– mice (c), D prostanoid receptor (DP)–/– mice (d) or DP–/– CRTH2–/– mice (e). Wild-type mice received lipopolysaccharide (LPS) (a) or phosphate-buffered saline (PBS) (b). Mutant mice were injected with LPS (c–e). Middle ear tissues were stained with haematoxylin and eosin.
Similar to the wild-type mice, LPS-injected CRTH2–/– mice showed severe infiltration of inflammatory cells in middle ear epithelium and the middle ear cavity. Middle ear mucosa was remarkably thick compared to PBS-injected control mice (Fig. 5c).
Conversely, in both LPS-injected DP–/– mice (Fig. 5d) and in LPS-injected DP–/– CRTH2–/– mice (Fig. 5e), middle ear mucosa was less affected and a small number of infiltrating inflammatory cells were observed in the middle ear cavity.
Using immunohistological staining, DP was highly expressed in infiltrating cells both in middle ear mucosa and in middle ear effusion in LPS-injected wild-type mice (Fig. 6a). In contrast, weak expression of DP was observed around submucosal vessels in PBS-injected control mice (Fig. 6b).
Fig. 6.
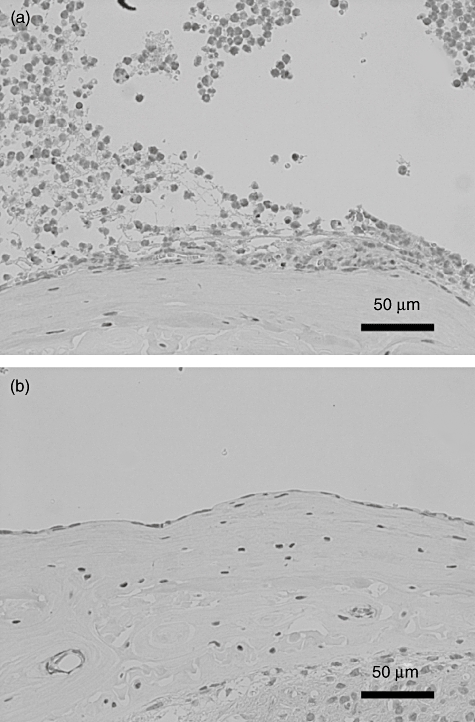
Immunohistological analysis of D prostanoid receptor (DP) expression in wild-type mice. Mice were injected with lipopolysaccharide (a) or phosphate-buffered saline (PBS) (b). Middle ear tissues were treated with anti-prostaglandin D2 (PGD2) receptor 1 antibody.
Cellular origin of MIP-2, IL-1β and IL-6 by PGD2
To identify the cell types responsible for the PGD2-induced cytokine response, we used polymorphonuclear leucocytes and peritoneal macrophage from wild-type mice. Polymorphonuclear leucocytes secreted significant amounts of MIP-2 (Fig. 7a, P= 0·002), IL-1β (Fig. 7b, P= 0·002) and IL-6 (Fig. 7c, P= 0·002). Conversely, no significant difference was observed in the level of MIP-2, IL-1β and IL-6 between PBS-treated and PGD2-treated peritoneal macrophage.
Fig. 7.
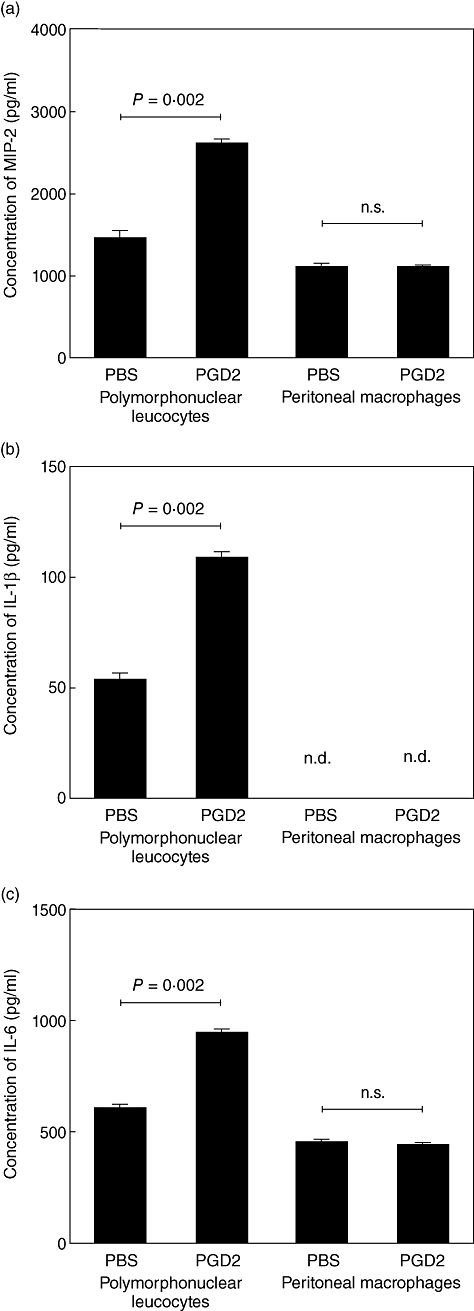
Concentrations of macrophage inflammatory protein 2 (MIP-2) (a), interleukin (IL)-1β (b) and IL-6 (c) in supernatants of polymorphonuclear leucocytes and peritoneal macrophage treated with either phosphate-buffered saline (PBS) or PGD2 for 24 h. The cytokine levels were determined by enzyme-linked immunosorbent assay (n = 6 per group). n.s., not significant; n.d., not detected.
Expression of DP in polymorphonuclear leucocytes
Immunocytochemistry revealed strong expression of DP in intraperitoneal polymorphonuclear leucocytes induced by sodium caseinate (Fig. 8a). No significant finding was observed in control IgG-treated polymorphonuclear leucocytes (Fig. 8b). Infiltrating inflammatory cells in LPS-induced otitis media and intraperitoneal polymorphonuclear leucocytes showed similar expression of DP.
Fig. 8.
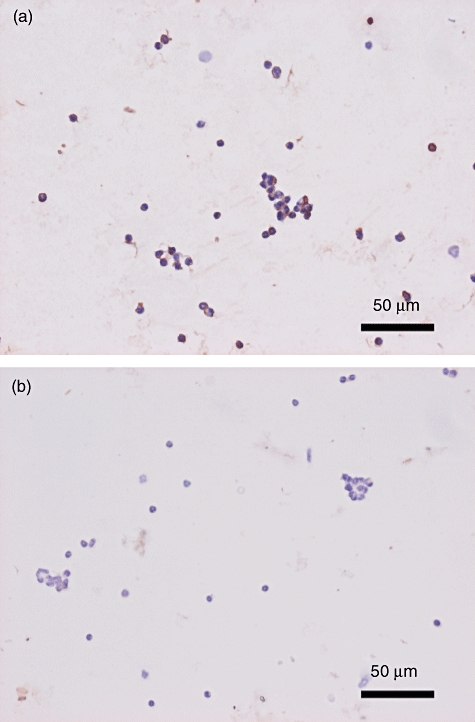
Immunocytochemical findings of D prostanoid receptor (DP) in polymorphonuclear leucocytes. Polymorphonuclear leucocytes were treated with anti-PGD2 receptor 1 polyclonal antibody (a) or control immunoglobulin G (b).
Discussion
In this study, we show that LPS is responsible for inducing the secretion of PGD2 in the middle ear in mice. LPS-induced production of inflammatory cytokines and chemokines (IL-1β, IL-6, MIP-2) and the histological inflammation observed were reduced in DP-deficient but not CRTH2-deficient mice. Together with the finding that direct administration of PGD2 also induced IL-1β, IL-6 and MIP-2 production, this suggests that DP plays an essential and selective role in the pathogenesis of LPS-induced otitis media in mice.
DP is expressed in various cells and tissues, including granulocytes [19], intestinal epithelium [20] and the brain [21]. DP is also observed in nasal epithelial goblet cells in normal human volunteers [22]. In addition, we have reported recently that DP is expressed widely in nasal polyps, including infiltrating inflammatory cells and constitutive cells such as vascular endothelial cells and epithelial cells [23]. Conversely, DP is not detected in the human pulmonary mucoepidermoid carcinoma cell line (NCI-H 292) or normal human bronchial epithelial (NHBE) cells by RT–PCR and flow cytometry [24]. Boie et al. also reported from Northern blot analysis that DP is not expressed in the human lung [20]. In an experimental animal study, only slight expression of DP was detected in the lung of non-immunized mice [16]. We demonstrate for the first time that DP is present in the vessel wall in the middle ear mucosa, and not in the normal middle ear epithelium. Middle ear epithelium consists of ciliated cells, goblet cells and supporting cells, and similarities of immunological response have been reported between middle ear mucosa and lower respiratory tract mucosa [25,26]. Our results suggest that DP expression in the middle ear is similar to that in the lung.
LPS has been shown to induce the production of IL-1β, IL-6, TNF-α, COX-1 and COX-2 in the middle ear, and significant levels of these inflammatory mediators have been found in middle ear effusions in otitis media [18,27,28]. Numerous studies have demonstrated the significance of LPS in the pathogenesis of otitis media. Controlling LPS-induced inflammatory signalling is considered a major therapeutic approach in the management of otitis media. IL-1β activates neutrophils and lymphocytes in middle ear inflammation and promotes inflammatory cell infiltration into the middle ear [3]. IL-6 has multiple functions, including mucin secretion in the middle ear, and is thought to be a potential candidate for the detection of the severity or chronicity of otitis media [27,29]. MIP-2 is regarded as the murine functional homologue of human IL-8. MIP-2 is reportedly a very potent neutrophil attractant and activator, and a critical proinflammatory CXC chemokine in mice [30]. Neutrophils are the major inflammatory cells in otitis media, and neutrophil counts and IL-8 concentration in middle ear effusion correlate positively in patients with otitis media with effusion [31]. This study shows that LPS-induced infiltration of inflammatory cells occurs less in both DP–/– mice and DP–/– CRTH2–/– mice. Our findings suggest that the DP receptor has a significant role in otitis media by promoting these cytokines and chemokines.
The effect of PGD2 on cytokine and chemokine expression has received controversial reports. Peeraully et al. showed that PGD2 induced a marked stimulation of IL-6 and monocyte chemoattractant protein 1 (MCP-1) expression in 3T3-L1 adipocyte cell lines [32]. However, a recent study reported that 10 µM of PGD2 did not produce any cytokines or chemokines in THP-1 cells, although the activation of DP on THP-1 cells enhanced TNF-α-induced MCP-1 and IL-8 production via the cyclic adenosine monophosphate/cAMP-dependent protein kinase (cAMP/PKA) signalling pathway [33]. Józefowski et al. reported that BW245C, a selective agonist of the murine DP receptor, suppressed LPS-stimulated IL-6 and IL-12 p40 release and enhanced IL-10 release from murine bone marrow-derived dendritic cells [34]. From our in vivo studies in mice, we show clearly that the DP receptor has a critical role in LPS-induced otitis media.
In patients with asthma, the DP receptor is expressed on bronchial epithelium and induces airway inflammation and hyperreactivity by producing chemokines and cytokines that recruit inflammatory lymphocytes and eosinophils [35]. Antigen-challenged mice also show significant expression of DP on bronchiole epithelial cells [16]. Recent studies demonstrate that PGD2-DP signalling mediates allergic reactions and that DP receptor antagonists might be useful in the treatment of allergic diseases [15]. Even though the role of DP in pulmonary inflammation induced by LPS has not been revealed, we show in this study that LPS induces severe inflammatory cell infiltration in the middle ear, and that infiltrated granulocytes and monocytes strongly express DP. Down-regulation of inflammatory cytokines and chemokines in DP–/– mice and DP–/– CRTH2–/– mice used in this study suggests that DP receptor antagonists might be novel therapeutic options in the future management of otitis media.
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosure
All authors declare that they have no competing financial or other interest in relation to their work.
References
- 1.Kariya S, Okano M, Aoji K, et al. Role of macrophage migration inhibitory factor in otitis media with effusion in adults. Clin Diagn Lab Immunol. 2003;10:417–22. doi: 10.1128/CDLI.10.3.417-422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skotnicka B, Hassmann E. Cytokines in children with otitis media with effusion. Eur Arch Otorhinolaryngol. 2000;257:323–6. doi: 10.1007/s004059900218. [DOI] [PubMed] [Google Scholar]
- 3.Smirnova MG, Kiselev SL, Gnuchev NV, Birchall JP, Pearson JP. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur Cytokine Netw. 2002;13:161–72. [PubMed] [Google Scholar]
- 4.Kariya S, Okano M, Hattori H, et al. TH1/TH2 and regulatory cytokines in adults with otitis media with effusion. Otol Neurotol. 2006;27:1089–93. doi: 10.1097/01.mao.0000224087.93096.4d. [DOI] [PubMed] [Google Scholar]
- 5.Jackson RT, Waitzman MB, Pickford L, Nathanson SE. Prostaglandins in human middle ear effusions. Prostaglandins. 1975;10:365–71. doi: 10.1016/0090-6980(75)90055-6. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein JM, Okazaki T, Reisman RE. Prostaglandins in middle ear effusions. Arch Otolaryngol. 1976;102:257–8. doi: 10.1001/archotol.1976.00780100043001. [DOI] [PubMed] [Google Scholar]
- 7.Kakiuchi M, Tsujigiwa H, Orita Y, et al. Cyclooxygenase 2 expression in otitis media with effusion. Am J Otolaryngol. 2006;27:81–5. doi: 10.1016/j.amjoto.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 9.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Kawata R, Mizukoshi O, Kuriyama K, Urade Y. Prostaglandin content in human middle ear effusions. Arch Otorhinolaryngol. 1989;246:133–6. doi: 10.1007/BF00456653. [DOI] [PubMed] [Google Scholar]
- 11.Harada T, Juhn SK, Kim Y, Sakakura Y. Arachidonic acid metabolism by isolated and cultured middle ear epithelial cells from the chinchilla. Eur Arch Otorhinolaryngol. 1993;250:220–3. doi: 10.1007/BF00171528. [DOI] [PubMed] [Google Scholar]
- 12.Narumiya S. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med. 2009;87:1015–22. doi: 10.1007/s00109-009-0500-1. [DOI] [PubMed] [Google Scholar]
- 13.Oguma T, Asano K, Ishizaka A. Role of prostaglandin D(2) and its receptors in the pathophysiology of asthma. Allergol Int. 2008;57:307–12. doi: 10.2332/allergolint.08-RAI-0033. [DOI] [PubMed] [Google Scholar]
- 14.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka T, Narumiya S. Prostaglandin receptor signaling in disease. Sci World J. 2007;7:1329–47. doi: 10.1100/tsw.2007.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuoka T, Hirata M, Tanaka H, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–17. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 17.Nomiya R, Okano M, Fujiwara T, et al. CRTH2 plays an essential role in the pathophysiology of Cry j 1-induced pollinosis in mice. J Immunol. 2008;180:5680–8. doi: 10.4049/jimmunol.180.8.5680. [DOI] [PubMed] [Google Scholar]
- 18.Kariya S, Schachern PA, Cureoglu S, et al. Up-regulation of macrophage migration inhibitory factor induced by endotoxin in experimental otitis media with effusion in mice. Acta Otolaryngol. 2008;128:750–5. doi: 10.1080/00016480701714228. [DOI] [PubMed] [Google Scholar]
- 19.Virgolini I, Li S, Sillaber C, et al. Characterization of prostaglandin-binding sites expressed on human basophils. Evidence for a prostaglandin E1, I2, and a D2 receptor. J Biol Chem. 1992;267:12700–8. [PubMed] [Google Scholar]
- 20.Boie Y, Sawyer N, Slipetz DM, Metters KM, Abramovitz M. Molecular cloning and characterization of the human prostanoid DP receptor. J Biol Chem. 1995;270:18910–16. doi: 10.1074/jbc.270.32.18910. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T, Yamashita A, Hayaishi O. Specific binding of prostaglandin D2 to rat brain synaptic membrane. Occurrence, properties, and distribution. J Biol Chem. 1982;257:13570–5. [PubMed] [Google Scholar]
- 22.Nantel F, Fong C, Lamontagne S, et al. Expression of prostaglandin D synthase and the prostaglandin D2 receptors DP and CRTH2 in human nasal mucosa. Prostaglandins Other Lipid Mediat. 2004;73:87–101. doi: 10.1016/j.prostaglandins.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Okano M, Fujiwara T, et al. Expression and characterization of PGD2 receptors in chronic rhinosinusitis: modulation of DP and CRTH2 by PGD2. Int Arch Allergy Immunol. 2009;148:127–36. doi: 10.1159/000155743. [DOI] [PubMed] [Google Scholar]
- 24.Chiba T, Kanda A, Ueki S, et al. Prostaglandin D2 induces IL-8 and GM-CSF by bronchial epithelial cells in a CRTH2-independent pathway. Int Arch Allergy Immunol. 2006;141:300–7. doi: 10.1159/000095436. [DOI] [PubMed] [Google Scholar]
- 25.Schuknecht HF. Anatomy. In: Schuknecht HF, editor. Pathology of the ear. 2nd edn. Philadelphia, PA: Lea & Febiger; 1993. pp. 30–7. [Google Scholar]
- 26.Cripps AW, Kyd JM. Comparison of mucosal and parenteral immunisation in two animal models of pneumococcal infection: otitis media and acute pneumonia. Vaccine. 2007;25:2471–7. doi: 10.1016/j.vaccine.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Juhn SK, Jung MK, Hoffman MD, et al. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008;1:117–38. doi: 10.3342/ceo.2008.1.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho JG, Lee ES, Woo JS, et al. Expressions of cyclooxygenase 1 and 2 in endotoxin-induced otitis media with effusion in the rat. Int J Pediatr Otorhinolaryngol. 2007;71:101–6. doi: 10.1016/j.ijporl.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Kerschner JE, Meyer TK, Yang C, Burrows A. Middle ear epithelial mucin production in response to interleukin-6 exposure in vitro. Cytokine. 2004;26:30–6. doi: 10.1016/j.cyto.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Kariya S, Okano M, Fukushima K, et al. Expression of inflammatory mediators in the otitis media induced by Helicobacter pylori antigen in mice. Clin Exp Immunol. 2008;154:134–40. doi: 10.1111/j.1365-2249.2008.03740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassif PS, Simpson SQ, Izzo AA, Nicklaus PJ. Interleukin-8 concentration predicts the neutrophil count in middle ear effusion. Laryngoscope. 1997;107:1223–7. doi: 10.1097/00005537-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Peeraully MR, Sievert H, Bulló M, Wang B, Trayhurn P. Prostaglandin D2 and J2-series (PGJ2, Delta12-PGJ2) prostaglandins stimulate IL-6 and MCP-1, but inhibit leptin, expression and secretion by 3T3-L1 adipocytes. Pflugers Arch. 2006;453:177–87. doi: 10.1007/s00424-006-0118-x. [DOI] [PubMed] [Google Scholar]
- 33.Hirano Y, Shichijo M, Deguchi M, et al. Synergistic effect of PGD2 via prostanoid DP receptor on TNF-alpha-induced production of MCP-1 and IL-8 in human monocytic THP-1 cells. Eur J Pharmacol. 2007;560:81–8. doi: 10.1016/j.ejphar.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Józefowski S, Bobek M, Marcinkiewicz J. Exogenous but not endogenous prostanoids regulate cytokine secretion from murine bone marrow dendritic cells: EP2, DP, and IP but not EP1, EP3, and FP prostanoid receptors are involved. Int Immunopharmacol. 2003;3:865–78. doi: 10.1016/S1567-5769(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 35.Kabashima K, Narumiya S. The DP receptor, allergic inflammation and asthma. Prostaglandins Leukot Essent Fatty Acids. 2003;69:187–94. doi: 10.1016/s0952-3278(03)00080-2. [DOI] [PubMed] [Google Scholar]


