Abstract
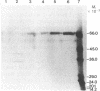
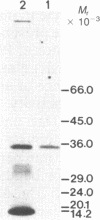
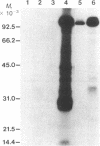
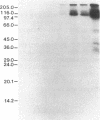
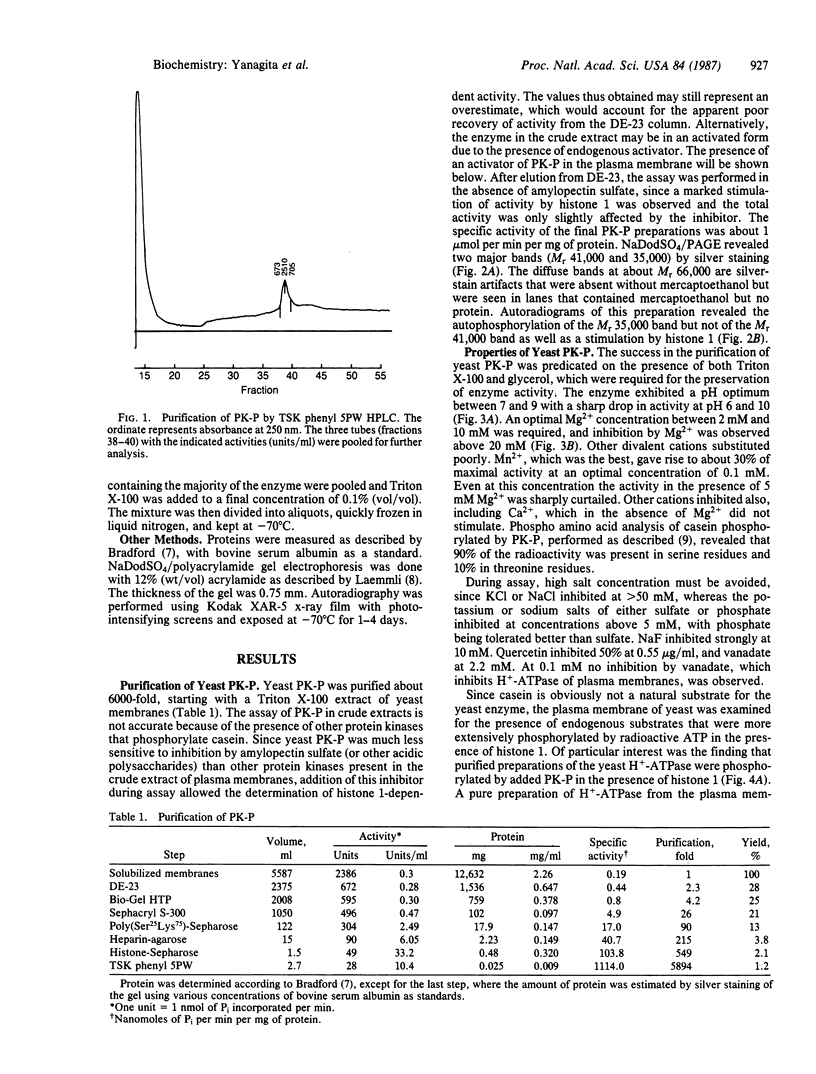
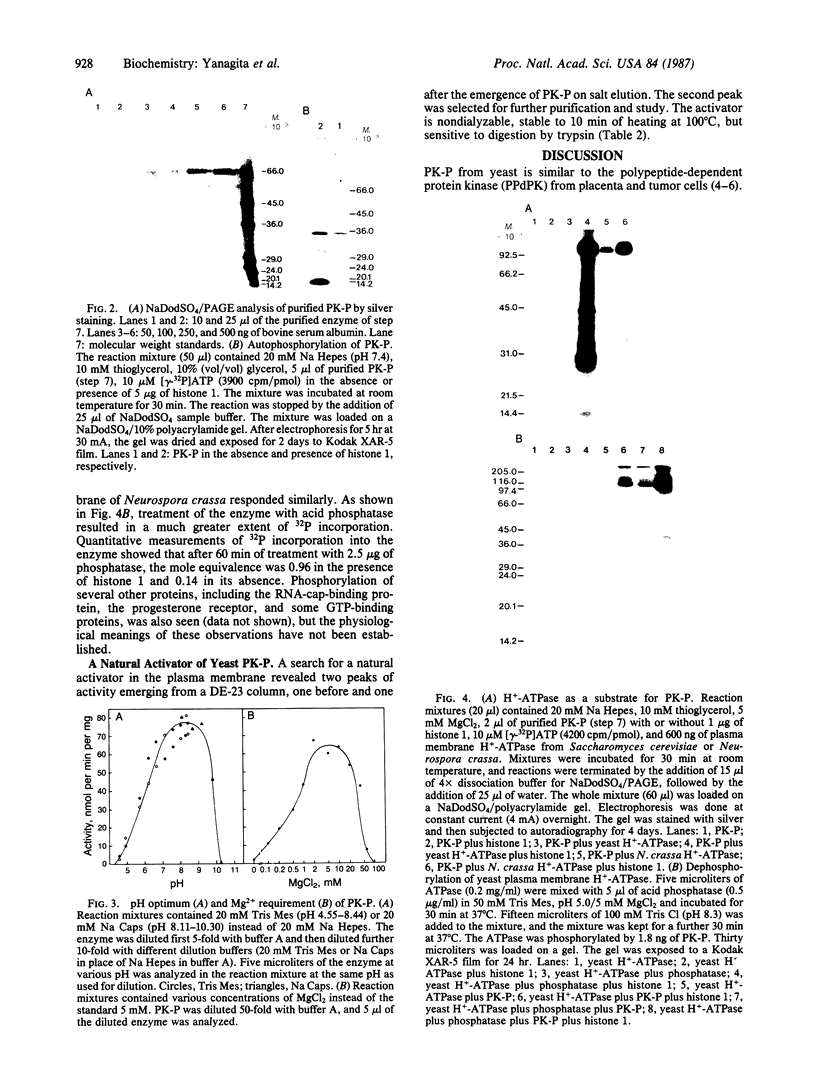
The purification and properties of a protein serine kinase (PK-P) extracted with Triton X-100 from membranes of bakers' yeast are described. The enzyme is virtually inactive unless either a histone or a heat-stable polypeptide from yeast membranes and Mg2+ are added. Other divalent cations substitute for Mg2+ poorly or not at all; most of them, including Mn2+, inhibit when added in the presence of 5 mM Mg2+. The enzyme is unstable but can be stabilized by addition of 0.1% Triton X-100 and 20% glycerol. The final preparation shows, on silver-stained electrophoresis gels, two major bands (Mr 41,000 and 35,000). According to gel filtration the molecular weight of the active protein is about 75,000. Of the two subunits, only the smaller one appears to be autophosphorylated. In addition to casein, the enzyme phosphorylates several proteins including the H+-ATPase (Mr 100,000) in the yeast plasma membrane. In order to demonstrate the phosphorylation of the ATPase (up to 0.9 equivalents), exposure of the latter to an acid phosphatase was required. Other phosphorylated proteins include mRNA cap-binding protein from mammalian erythrocytes and yeast, a glucocorticoid receptor protein, and a preparation of the guanine nucleotide-binding proteins Gi and Go from brain. A partial purification of a natural activator from yeast plasma membranes is described.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., Nakamura S., Navarro J., Racker E. A membrane-bound human placental protein kinase activated by endogenous polypeptides. Biosci Rep. 1983 Mar;3(3):275–282. doi: 10.1007/BF01122460. [DOI] [PubMed] [Google Scholar]
- Abdel-Ghany M., Riegler C., Racker E. A placental polypeptide activator of a membranous protein kinase and its relation to histone 1. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7388–7391. doi: 10.1073/pnas.81.23.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed K., Goueli S. A., Williams-Ashman H. G. Characteristics of polyamine stimulation of cyclic nucleotide-independent protein kinase reactions. Biochem J. 1985 Dec 15;232(3):767–771. doi: 10.1042/bj2320767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986 Sep 12;233(4769):1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlicki W., Szyszka R., Gasior E. A cytoplasmic, cyclic nucleotide-independent casein kinase II from Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Jan 31;784(2-3):102–107. doi: 10.1016/0167-4838(84)90115-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerch K., Muir L. W., Fischer E. H. Purification and properties of a yeast protein kinase. Biochemistry. 1975 May 6;14(9):2015–2023. doi: 10.1021/bi00680a032. [DOI] [PubMed] [Google Scholar]
- Meggio F., Grankowski N., Kudlicki W., Szyszka R., Gasior E., Pinna L. A. Structure and properties of casein kinase-2 from Saccharomyces cerevisiae. A comparison with the liver enzyme. Eur J Biochem. 1986 Aug 15;159(1):31–38. doi: 10.1111/j.1432-1033.1986.tb09829.x. [DOI] [PubMed] [Google Scholar]
- Racker E., Abdel-Ghany M., Sherrill K., Riegler C., Blair E. A. New protein kinase from plasma membrane of Ehrlich ascites tumor cells activated by natural polypeptides. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4250–4254. doi: 10.1073/pnas.81.14.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik W., Gardner P. R., Vanaman T. C., Rhoads R. E. Structural analysis of the messenger RNA cap-binding protein. Presence of phosphate, sulfhydryl, and disulfide groups. J Biol Chem. 1986 Jan 5;261(1):71–75. [PubMed] [Google Scholar]