Abstract
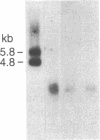
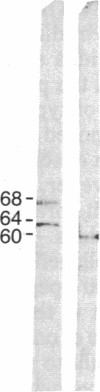
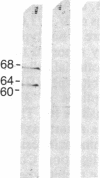
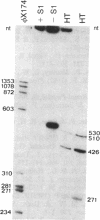
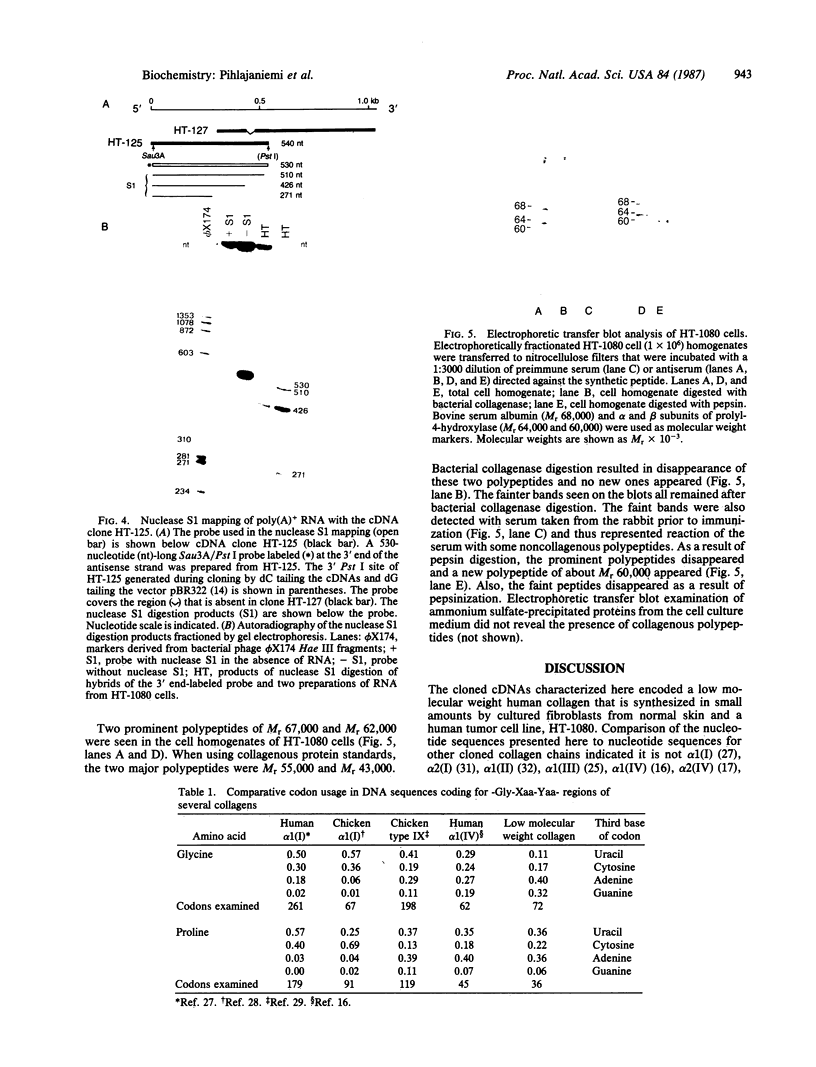
A cDNA library prepared from RNA isolated from a cultured human tumor cell line, HT-1080, was screened with a mouse cDNA clone coding for part of the -Gly-Xaa-Yaa- domain of the alpha 2(IV) collagen chain. Four overlapping cDNA clones were characterized that coded for a low molecular weight human collagen. The cDNA clones did not, however, code for the short-chain collagens, types IX and X. The amino acid sequences derived from the clones resembled type IV collagen in that there were short interruptions in the repeating -Gly-Xaa-Yaa- sequence. The noncollagenous, carboxyl-terminal domain was, however, much shorter and contained only 18 amino acid residues. Interestingly, one of the cDNA clones contained an additional 36 nucleotides not found in an overlapping clone. The 36 nucleotides encoded four -Gly-Xaa-Yaa- repeats without changing the reading frame. Nuclease S1 mapping demonstrated that the difference between the clones was due to existence of two different mRNAs. A synthetic 24-residue peptide corresponding to the last two -Gly-Xaa-Yaa- triplets and the entire carboxyl-terminal domain was used to generate polyclonal antibodies. Electrophoretic transfer blot analysis of HT-1080 cells and normal human skin fibroblasts identified two polypeptides, Mr 67,000 and Mr 62,000, that were sensitive to bacterial collagenase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentz H., Morris N. P., Murray L. W., Sakai L. Y., Hollister D. W., Burgeson R. E. Isolation and partial characterization of a new human collagen with an extended triple-helical structural domain. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3168–3172. doi: 10.1073/pnas.80.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D. EC collagen: biosynthesis by corneal endothelial cells and separation from type IV without pepsin treatment or denaturation. Ren Physiol. 1980;3(1-6):30–35. doi: 10.1159/000172738. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R. Isolation and characterization of type VIII collagen synthesized by cultured rabbit corneal endothelial cells. A conventional structure replaces the interrupted-helix model. J Biol Chem. 1986 Mar 25;261(9):4160–4169. [PubMed] [Google Scholar]
- Bernard M. P., Chu M. L., Myers J. C., Ramirez F., Eikenberry E. F., Prockop D. J. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983 Oct 25;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Cheah K. S. Collagen genes and inherited connective tissue disease. Biochem J. 1985 Jul 15;229(2):287–303. doi: 10.1042/bj2290287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ramirez F. Fine structural analysis of the human pro-alpha 1 (I) collagen gene. Promoter structure, AluI repeats, and polymorphic transcripts. J Biol Chem. 1985 Feb 25;260(4):2315–2320. [PubMed] [Google Scholar]
- Davis A. E., 3rd, Lachmann P. J. Bovine conglutinin is a collagen-like protein. Biochemistry. 1984 May 8;23(10):2139–2144. doi: 10.1021/bi00305a006. [DOI] [PubMed] [Google Scholar]
- Dixit R., Harrison M. W., Dixit S. N. Isolation and partial characterization of a novel basement membrane collagen. Biochem Biophys Res Commun. 1985 Jul 16;130(1):1–8. doi: 10.1016/0006-291x(85)90373-0. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Wiedemann H., Timpl R., Odermatt E., Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983 May 1;211(2):303–311. doi: 10.1042/bj2110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R., Bornstein P., Sage E. H. Type VIII collagen from bovine Descemet's membrane: structural characterization of a triple-helical domain. Biochemistry. 1986 Jul 1;25(13):3930–3937. doi: 10.1021/bi00361a029. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Kwan A. P., Holmes D. F., Schor S. L., Grant M. E. Type X collagen, a product of hypertrophic chondrocytes. Biochem J. 1985 Apr 15;227(2):545–554. doi: 10.1042/bj2270545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkinen M., Bernard M. P., Barlow D. P., Chow L. T. Characterization of 64-, 123- and 182-base-pair exons in the mouse alpha 2(IV) collagen gene. Nature. 1985 Sep 12;317(6033):177–179. doi: 10.1038/317177a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loidl H. R., Brinker J. M., May M., Pihlajaniemi T., Morrow S., Rosenbloom J., Myers J. C. Molecular cloning and carboxyl-propeptide analysis of human type III procollagen. Nucleic Acids Res. 1984 Dec 21;12(24):9383–9394. doi: 10.1093/nar/12.24.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Bornstein P. Heparin regulates the collagen phenotype of vascular smooth muscle cells: induced synthesis of an Mr 60,000 collagen. J Cell Biol. 1985 Feb;100(2):613–619. doi: 10.1083/jcb.100.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Loidl H. R., Seyer J. M., Dion A. S. Complete primary structure of the human alpha 2 type V procollagen COOH-terminal propeptide. J Biol Chem. 1985 Sep 15;260(20):11216–11222. [PubMed] [Google Scholar]
- Ninomiya Y., Gordon M., van der Rest M., Schmid T., Linsenmayer T., Olsen B. R. The developmentally regulated type X collagen gene contains a long open reading frame without introns. J Biol Chem. 1986 Apr 15;261(11):5041–5050. [PubMed] [Google Scholar]
- Olsen B. R., Ninomiya Y., Lozano G., Konomi H., Gordon M., Green G., Parsons J., Seyer J., Thompson H., Vasios G. Short-chain collagen genes and their expression in cartilage. Ann N Y Acad Sci. 1985;460:141–153. doi: 10.1111/j.1749-6632.1985.tb51162.x. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Tryggvason K., Myers J. C., Kurkinen M., Lebo R., Cheung M. C., Prockop D. J., Boyd C. D. cDNA clones coding for the pro-alpha1(IV) chain of human type IV procollagen reveal an unusual homology of amino acid sequences in two halves of the carboxyl-terminal domain. J Biol Chem. 1985 Jun 25;260(12):7681–7687. [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Sage H. Low molecular weight fibroblast collagen: structure, secretion, and differential expression as a function of fetal and cellular age. Biochemistry. 1985 Dec 3;24(25):7430–7440. doi: 10.1021/bi00346a060. [DOI] [PubMed] [Google Scholar]
- Sage H., Trüeb B., Bornstein P. Biosynthetic and structural properties of endothelial cell type VIII collagen. J Biol Chem. 1983 Nov 10;258(21):13391–13401. [PubMed] [Google Scholar]
- Sangiorgi F. O., Benson-Chanda V., de Wet W. J., Sobel M. E., Tsipouras P., Ramirez F. Isolation and partial characterization of the entire human pro alpha 1(II) collagen gene. Nucleic Acids Res. 1985 Apr 11;13(7):2207–2225. doi: 10.1093/nar/13.7.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate V., Finer M., Boedtker H., Doty P. Procollagen genes: further sequence studies and interspecies comparisons. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1039–1049. doi: 10.1101/sqb.1983.047.01.117. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Fukushima H., Dewji N. N., Wilcox E., O'Brien J. S., Helinski D. R. Chromogenic immunodetection of human serum albumin and alpha-L-fucosidase clones in a human hepatoma cDNA expression library. DNA. 1984 Dec;3(6):437–447. doi: 10.1089/dna.1.1984.3.437. [DOI] [PubMed] [Google Scholar]
- de Wet W. J., Pihlajaniemi T., Myers J., Kelly T. E., Prockop D. J. Synthesis of a shortened pro-alpha 2(I) chain and decreased synthesis of pro-alpha 2(I) chains in a proband with osteogenesis imperfecta. J Biol Chem. 1983 Jun 25;258(12):7721–7728. [PubMed] [Google Scholar]