Abstract
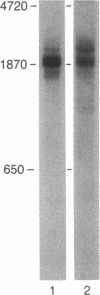
The 25-kDa mRNA cap-binding protein (CBP) involved in translation was purified by affinity chromatography from human erythrocytes and rabbit reticulocytes. The sequences of eight human and seven rabbit tryptic and V8 proteolytic peptides were determined. Based on the peptide sequence data, oligodeoxynucleotide probes were synthesized and used to screen human fibroblast and lymphocyte lambda cDNA libraries. The DNA sequence obtained from recombinant lambda phage inserts was found to code for all but one peptide. A 23-base oligonucleotide was synthesized based on the DNA sequence and used to prime synthesis of cDNA from human placental mRNA to construct a third library in lambda gt10. Screening with a 22-base oligonucleotide, whose sequence was upstream from the 23-base primer, yielded numerous recombinant phages with approximately equal to 250-base inserts. The 1900-base-pair cDNA sequence compiled from all phage inserts appeared to represent the entire primary sequence of CBP (Mr 25,117). Blot analysis of human placental and HeLa mRNA revealed multiple CBP mRNA species ranging from 1925 to 2250 bases. The amino acid sequence of CBP showed homology to the cap-binding PB2 protein of influenza virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam-Markson J., Jaudon C., Krug R. M. Expression of a functional influenza viral cap-recognizing protein by using a bovine papilloma virus vector. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4326–4330. doi: 10.1073/pnas.82.13.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley B., Ehrenfeld E. Two-dimensional gel analyses of the 24-kDa cap binding protein from poliovirus-infected and uninfected HeLa cells. Virology. 1986 Jul 30;152(2):497–501. doi: 10.1016/0042-6822(86)90155-8. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Chen E. Y., Smith D. H., Levinson A. D. Identification and nucleotide sequence of a human locus homologous to the v-myc oncogene of avian myelocytomatosis virus MC29. Nature. 1983 Feb 24;301(5902):722–725. doi: 10.1038/301722a0. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Abundance, synthesis, and turnover rates. J Biol Chem. 1985 May 10;260(9):5486–5492. [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hansen J., Ehrenfeld E. Presence of the cap-binding protein in initiation factor preparations from poliovirus-infected HeLa cells. J Virol. 1981 May;38(2):438–445. doi: 10.1128/jvi.38.2.438-445.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hiremath L. S., Webb N. R., Rhoads R. E. Immunological detection of the messenger RNA cap-binding protein. J Biol Chem. 1985 Jul 5;260(13):7843–7849. [PubMed] [Google Scholar]
- Ishida T., Katsuta M., Inoue M., Yamagata Y., Tomita K. The stacking interactions in 7-methylguanine-tryptophan systems, a model study for the interaction between the 'cap' structure of mRNA and its binding protein. Biochem Biophys Res Commun. 1983 Sep 30;115(3):849–854. doi: 10.1016/s0006-291x(83)80012-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lax S., Fritz W., Browning K., Ravel J. Isolation and characterization of factors from wheat germ that exhibit eukaryotic initiation factor 4B activity and overcome 7-methylguanosine 5'-triphosphate inhibition of polypeptide synthesis. Proc Natl Acad Sci U S A. 1985 Jan;82(2):330–333. doi: 10.1073/pnas.82.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., McMaster G. K., Trachsel H. Cloning of eukaryotic protein synthesis initiation factor genes: isolation and characterization of cDNA clones encoding factor eIF-4A. Nucleic Acids Res. 1985 Oct 11;13(19):6867–6880. doi: 10.1093/nar/13.19.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Panniers R., Stewart E. B., Merrick W. C., Henshaw E. C. Mechanism of inhibition of polypeptide chain initiation in heat-shocked Ehrlich cells involves reduction of eukaryotic initiation factor 4F activity. J Biol Chem. 1985 Aug 15;260(17):9648–9653. [PubMed] [Google Scholar]
- Patzelt E., Blaas D., Kuechler E. CAP binding proteins associated with the nucleus. Nucleic Acids Res. 1983 Sep 10;11(17):5821–5835. doi: 10.1093/nar/11.17.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K., Brendler T. G., Adya S., Daniels-McQueen S., Miller J. K., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc Natl Acad Sci U S A. 1983 Feb;80(3):663–667. doi: 10.1073/pnas.80.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik W., Gardner P. R., Vanaman T. C., Rhoads R. E. Structural analysis of the messenger RNA cap-binding protein. Presence of phosphate, sulfhydryl, and disulfide groups. J Biol Chem. 1986 Jan 5;261(1):71–75. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985 Feb;40(2):223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Guertin D., Cleveland D., Trachsel H. Probing the function of the eucaryotic 5' cap structure by using a monoclonal antibody directed against cap-binding proteins. Cell. 1981 Dec;27(3 Pt 2):563–572. doi: 10.1016/0092-8674(81)90398-6. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Trachsel H. Probing the function of the eukaryotic 5'-cap structure using monoclonal antibodies to cap-binding proteins. Curr Top Cell Regul. 1982;21:65–88. doi: 10.1016/b978-0-12-152821-8.50007-4. [DOI] [PubMed] [Google Scholar]
- Sorge J., West C., Westwood B., Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Zumbé A., Stähli C., Hümbelin M., Sonenberg N. Association of an Mr 50,000 cap binding protein with the cytoskeleton in BHK cells. Biochem Soc Symp. 1982;47:145–161. [PubMed] [Google Scholar]
- Webb N. R., Chari R. V., DePillis G., Kozarich J. W., Rhoads R. E. Purification of the messenger RNA cap-binding protein using a new affinity medium. Biochemistry. 1984 Jan 17;23(2):177–181. doi: 10.1021/bi00297a001. [DOI] [PubMed] [Google Scholar]
- Wood W. M., Wallace J. C., Edmonds M. Sequence content of oligo(uridylic acid)-containing messenger ribonucleic acid from HeLa cells. Biochemistry. 1985 Jul 2;24(14):3686–3693. doi: 10.1021/bi00335a043. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]
- Zumbé A., Stähli C., Trachsel H. Association of a Mr 50,000 cap-binding protein with the cytoskeleton in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2927–2931. doi: 10.1073/pnas.79.9.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]