Abstract
Recent advances in cell biology, neural injury and repair, and the progress towards development of neurorestorative interventions are the basis for increased optimism. Based on the complexity of the processes of demyelination and remyelination, degeneration and regeneration, damage and repair, functional loss and recovery, it would be expected that effective therapeutic approaches will require a combination of strategies encompassing neuroplasticity, immunomodulation, neuroprotection, neurorepair, neuroreplacement, and neuromodulation. Cell-based restorative treatment has become a new trend, and increasing data worldwide have strongly proven that it has a pivotal therapeutic value in CNS disease. Moreover, functional neurorestoration has been achieved to a certain extent in the CNS clinically. Up to now, the cells successfully used in preclinical experiments and/or clinical trial/treatment include fetal/embryonic brain and spinal cord tissue, stem cells (embryonic stem cells, neural stem/progenitor cells, hematopoietic stem cells, adipose-derived adult stem/precursor cells, skin-derived precursor, induced pluripotent stem cells), glial cells (Schwann cells, oligodendrocyte, olfactory ensheathing cells, astrocytes, microglia, tanycytes), neuronal cells (various phenotypic neurons and Purkinje cells), mesenchymal stromal cells originating from bone marrow, umbilical cord, and umbilical cord blood, epithelial cells derived from the layer of retina and amnion, menstrual blood-derived stem cells, Sertoli cells, and active macrophages, etc. Proof-of-concept indicates that we have now entered a new era in neurorestoratology.
Key words: Cell therapy, Translational medicine, Neurorestoratology, Central nervous system diseases
BRIEF PROFILE OF NEURORESTORATOLOGY
Definition
Neurorestoratology, a distinct discipline within the neurosciences, has been clearly defined by the International Association of Neurorestoratology as one subdiscipline and one new branch of neuroscience, which studies the therapeutic strategies for neural regeneration, repair, and replacement of damaged components of the nervous system, neuroplasticity, neuroprotection, neuromodulation, angiogenesis, immunomodulation, and their mechanisms to cause improvement. The core of neurorestoratology is to restore neurological function in humans. The research field of neurorestoratology covers various neurorestorative treatments including transplantation of tissue and cells, biomaterials and bioengineering, neuromodulation by electrical and/or magnetic stimulation, pharmaceutical or chemical therapies in neurotrauma, neurodegeneration, cerebrovascular anoxia or ischemia, edema, demyelination, sensory and motor disorders, and neuropathic pain, as well as neural damage resulting from toxic, physical, and chemical factors, immune, infectious, inflammatory, hereditary, congenital, developmental, and other intractable neural lesions (376).
Inexorable Law of Neuroscientific Innovation
Thousands of years ago (approx 2500 B.C.), spinal cord injuries were described as “crushed vertebra in his neck” as well as symptoms of neurological deterioration without treatment in the ancient Egyptian medical papyrus known as the Edwin Smith Surgical Papyrus by the physician and architect of the Sakkara pyramids Imhotep (8). Nearly 90 years ago Ramon y Cajal (1926) stated with certainty: “Once the development was ended, the founts of growth and regeneration of the axons and dendrites dried up irrevocably. In the adult centers, the nerve paths are something fixed, ended, and immutable. Everything may die, nothing may be regenerated. It is for the science of the future to change, if possible, this harsh decree” (51). Regeneration and restoration of the central nervous system (CNS) was thought to be almost an impossibility at that time, although scientists still tried to study the special mystery of human life through transplanting brain tissues (374), electrical stimulation (156), nerve growth factor (NGF) administration (204), gene therapy (38), and so forth.
Commonly, physicians of traditional clinical disciplines have believed that sequelae of stroke, CNS trauma, neurodegenerative diseases, and damage lack effective treatments. The majority of the medical community now still think that: “Our knowledge of the pathophysiological processes, both the primary as well as the secondary, has increased tremendously. However, all this knowledge has only led to improved medical care but not to any therapeutic methods to restore, even partially, the neurological function” (8). Unfortunately, the majority of physicians remain ignorant or unaware of the increasing quantity of published evidence concerning CNS functional restoration by neuromodulation, neuroprotection, axon sprouting, neural circuit reconstruction, neurogenesis, neuroregeneration, neurorepair, and neuroreplacement in animal models and patients (109,285,286,303,351,412).
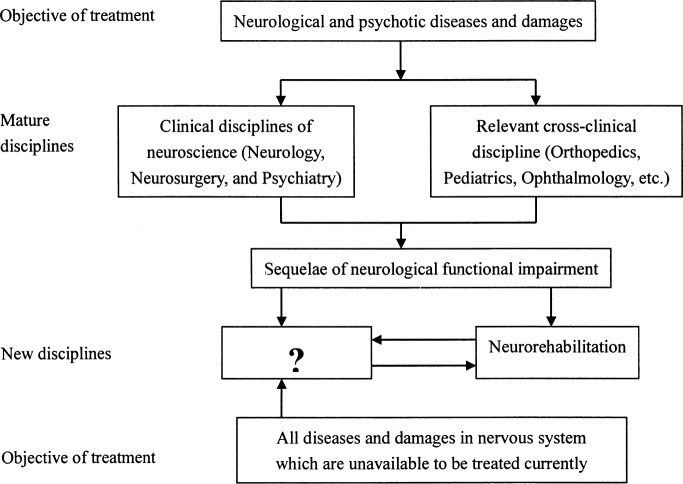
It is the eternal desire for humans to prolong and improve their quality of life, which, with no doubt, it should be the instinct and responsibility of physicians and neuroscientists to search for effective methods. Obviously, it is inappropriate and overly pessimistic for physicians to always say that there is no way to help patients with the sequelae of diseases and damage to the CNS. Therefore, the new discipline, Neurorestoratology, is bound to arise from neuroscientific innovation, filling in the question-marked frame shown in Figure 1, which aims to create effective therapeutic strategies to benefit patients.
Figure 1.
Distribution of disease treated by clinical disciplines of neuroscience and relevant cross-disciplines.
Evolution of Neurorestoratology
Restorative Neurology
Dimitrijevic put forward this term in 1985. It was defined as the branch of the neurological sciences that applied active procedures to improve the functioning of the impaired nervous system through selective structural and functional modification of abnormal neurocontrol according to underlying mechanisms and clinically unrecognized residual functions; its intervention strategies included neurostimulation, neuromodulation, and neuroectomies (83), cell transplants (242), drugs (222), and so on.
Restorative Neurosurgery
This term was put forward by Liberson in 1987 (221). Then restorative neurosurgery, as the frontier of neurosurgery, provided the potential to restore lost function. Intervention strategies include gene therapy, neurotrophic factors, and cell transplantation (195,248,321,377), tissue engineering (402), and neurostimulation (172,369).
Neurorestorative Therapy or Surgery
Intervention strategies include cell-based and pharmacological therapies (423) and electrostimulation (373).
Restorative Neuroscience
Intervention strategies include cell transplantation, stimulation, and medicine (11).
Together, all of the medical terms mentioned above were not considered as distinct disciplines, but instead a branch of neurology, neurosurgery, or a specific kind of therapy. The emergence of the term “neurorestoratology,” however, signals the birth of a new discipline, which is equally important in comparison with neurology and neurosurgery (59). The potential mechanisms of action of neurorestoratology techniques are highlighted below.
NEURORESTORATIVE MECHANISMS OF CELL THERAPY
Neuroprotection by Neurotrophins and Immune or Inflammatory Modulation
Bone marrow mesenchymal stem cells (BM-MSCs) have the capacity to modulate immune/inflammatory responses in Alzheimer disease (AD) mice, ameliorating their pathophysiology, and improving the cognitive decline associated with amyloid-β (Aβ) deposits (199). Neural stem/precursor cells (NSPCs) can be used as an immune regulatory tool for autoimmune encephalomyelitis (300). In parallel, olfactory ensheathing cells (OECs) play a role in neuroprotection through the secretion of neurotrophins or growth factors (350). Studies by Chopp and colleagues have proven that transplanting MSCs into the brain leads to secretion of neurotrophins, growth factors and other supportive substances after brain injury (307), which change the microenvironment in the damaged area and continually facilitate endogenous neurorestorative mechanisms by reducing apoptotic cell death (55). Garbuzova-Davis et al. (114) and Zwart et al. (429) reported that an appropriate dose of mononuclear human umbilical cord blood (MNC hUCB) cells may provide a neuroprotective effect for motor and optic neurons through the active involvement of these cells in modulating the host’s immune inflammatory system response. Neural protection afforded by adipose-derived stromal cells was found to be mostly attributable to activated caspase-3 and Akt-mediated neuroprotective pathway signaling through paracrine support provided by trophic factor secretion (393).
Neurogenesis and Angiogenesis
In early animal studies using neural stem cell treatments, very few cells become neurons (53) and it was believed that there was no evidence that “new” neurons could reinnervate muscle (256). More studies now indicate that mesenchymal stromal cells (137,206) and neural stem cells could survive, migrate, and differentiate into endothelial cells or glia and neurons (173,427), form electrically active and functionally connected neurons (15) that form synapses between host and donor cells (396), and elicit further functional repair following transplantation into the adult CNS (81,100,161,210,252,349,382). Furthermore, evidence shows that bone marrow stromal cells are capable of remodeling the blood vasculature (58).
Neurorepair and Remyelination
Many different cell types have been shown to have potential in the repair or remyelination of CNS diseases (28,92,105,261,265).
Neuroregeneration and Sprouting
Schwann cells (SCs) can induce sprouting of motor and sensory axons in the adult rat spinal cord (214). Accumulated studies show that OECs are capable of aiding axon growth or sprouting following transplantation and continued regeneration of the denervated caudal host tract resulting in the recovery of neurological functions in acute (44,211,312,314,409) as well as chronic spinal injury (267).
Neural Circuit or Network Reconstruction, Neuroplasticity, and Neuroreplacement
In the rat stroke model, a graft of medial ganglionic eminence (MGE) cells may differentiate into multiple neuronal subtypes, establish synaptic contact with host cells, increase the expression of synaptic markers, and enhance axonal reorganization in the injured area. Initial patch-clamp recording demonstrated that the MGE cells received postsynaptic currents from the host cells. Functional recovery could be mediated by neurotrophic support, new synaptic circuit elaboration, and enhancement of the stroke-induced neuroplasticity (74). Recent findings of transplanted embryonic dopamine (DA) neurons into the substantia nigra (SN) indicate that DA neurons could extend neurites towards a desired target through the brain stem and caudal diencephalon to reconstruct the neural circuitry from grafted neurons in the host (315,355). Application of stem cells for neuroreplacement therapy is therefore no longer science fiction—it is science fact (367). OECs can promote neuroplasticity in neurodegenerative diseases (62), while NSCs can suppress abnormal mossy fiber sprouting into the inner molecular layer with subsequent reduction of hippocampal excitability (165).
Neuromodulation or Unmasking and Signaling Repair by Micromilieu Change
Our clinical study showed that patients with chronic spinal cord injury have rapid recovery of some functions following OEC transplantation. The explanation is that they changed the local microenvironment by the secretion of useful chemicals or growth factors, which can promote the nerve cell growth, unmasking the quiescent axons, and therefore restoring some of the lost functions (147). Grafting dental pulp stem/stromal cells (DPSC) can promote proliferation, cell recruitment, and maturation of endogenous stem/progenitor cells by modulating the local microenvironment through enhancing ciliary neurotrophic factor (CNTF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) as stimulators and modulators of the local repair response in the CNS (142) and neuromodulation would likely be necessary to realize the full potential of NSC grafts in restoring function (111). Another study also suggested that the inhibitory neurotransmitters γ-aminobutyric acid (GABA) and glycine secreted by transplanted cells could be an effective clinical tool for treating spinal cord injury (SCI)-associated neuropathic pain (91).
Generally, the patient’s functional restoration originated from some or all of the mechanisms as listed above. But under many conditions, functional recovery is from neuromodulation or unmasking, neuroprotection, sprouting, neural circuit reconstruction, and neuroplasticity by neurotrophins, immune or inflammatory modulation and local microenvironment change, and in a few cases from neurogenesis or neuroregeneration, and neurorepair (378,409). Neuroreplacement may be an important tool for Parkinson’s disease (PD), but may not be a major way for functional neurorestoration in most other CNS diseases or damage. It remains unclear how to explain the exact mechanisms for clinical functional recovery; in the future mechanisms by which cell transplantation enhances functional recovery need to be better understood following further experimental study.
PRECLINICAL STUDIES OF CELL-BASED NEURORESTORATOLOGY IN CNS DISEASES
Fetal/Embryonic Brain Tissue
In 1977, the first evidence was presented that grafts of fetal brain tissue to the adult CNS could counteract an experimentally induced neurological deficit (279). Cell/tissue suspensions, dissociated from selected embryonic brain regions, can mediate considerable reinnervation of a previously denervated brain or spinal cord region, and they can replace neurons intrinsic to a particular target after intracerebral or intraspinal grafting (277,294). Fetal brain tissue, grafted into the CNS of neonatal and adult animals, has been shown to survive and differentiate (318). In brain tissue grafts consisting of undifferentiated matrix cells and few neuroblasts, good development was observed both in the lateral ventricle and inside the parenchyma, 30 and 110 days after transplant. They differentiated into organotypical and histotypical structures and cells similar to those formed in normal development. Nerve and glial cells of these transplants were well differentiated and tightly connected with the surrounding nervous tissue of the host (7). It has been convincingly shown that grafting of fetal/embryonic brain cells/tissue into the brain and/or spinal cord is a useful alternative therapy for a variety of degenerative and traumatic disorders (Table 1). It has been argued that neural transplantation can promote functional recovery by the replacement of damaged nerve cells, the reestablishment of specific nerve pathways lost as a result of injury, the release of specific neurotransmitters, or the production of factors that promote neuronal growth (260,343,360).
Table 1.
Selected Literature in Preclinical Therapeutic Application of Fetal/Embryonic Brain Cells/Tissue
| Disease | Animal Host | Year | Reference | Publication Title |
|---|---|---|---|---|
| Spinal cord trauma | ||||
| Hemitransected cord | cat | 1992 | 401 | In vivo magnetic resonance imaging of fetal cat neural tissue transplants in the adult cat spinal cord |
| Transected cord | rat | 1977 | 279 | Monoaminergic reinnervation of the transected spinal cord by homologous fetal brain grafts |
| rat | 1984 | 68 | Fetal locus coeruleus transplanted into the transected spinal cord of the adult rat: Some observations and implications | |
| Visual deficits | rat | 1985 | 361 | Fetal brain tissue transplants reduce visual deficits in adult rats with bilateral lesions of the occipital cortex |
| Peripheral nerve injury | ||||
| Sciatic nerve crushed | rat | 1983 | 30 | Viability, growth, and maturation of fetal brain and spinal cord in the sciatic nerve of adult rat |
| Parkinson’s disease | rat | 1979 | 294 | Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system |
| Cognitive deficits | rat | 1983 | 193 | Fetal brain transplant: Reduction of cognitive deficits in rats with frontal cortex lesions |
| rat | 1987 | 29 | Fetal brain transplants induce recuperation of taste aversion learning | |
| Brain trauma | ||||
| Injured motor/sensory cortex | rat | 1988 | 121 | Fetal frontal cortex transplanted to injured motor/sensory cortex of adult rats: Reciprocal connections with host thalamus demonstrated with WGA-HRP |
| Fimbria-fornix lesions | rat | 1994 | 162 | The effects of intrahippocampal raphe and/or septal grafts in rats with fimbria-fornix lesions depend on the origin of the grafted tissue and the behavioural task used |
| Kainate lesioned cerebellum | rat | 1989 | 13 | Organization of host afferents to cerebellar grafts implanted into kainate lesioned cerebellum in adult rats |
| Hypoxic hypoxia | rat | 1984 | 302 | Transplantation of embryonic brain tissue into the brain of adult rats after hypoxic hypoxia |
| Stroke | ||||
| MCAo | rat | 1998 | 140 | Neurotrophin-mediated neuroprotection by solid fetal telencephalic graft in middle cerebral artery occlusion: A preventive approach |
| Transient bilateral common carotid artery occlusion | Mongolian gerbils | 2001 | 23 | Transplantation of human fetal brain cells into ischemic lesions of adult gerbil hippocampus |
Fetal/Embryonic Spinal Cord Tissue
The first successful transplantation of fetal spinal cord into adult spinal cord was reported in 1983 (291). Some topographical features of the normal spinal cord may be represented in mature spinal cord transplants (318). Embryonic spinal cord transplants are capable of replacing damaged intraspinal neuronal populations and restoring some degree of anatomical continuity between the isolated rostral and caudal stumps of the injured mammalian spinal cord (317). Improved hind limb behavioral deficits were observed after fetal spinal cord homografts (31). Moreover, the grafted fetal/embryonic tissue may stimulate partial regression of an established glial scar (141), replace missing motoneurons (353), and form myelin (324). Data have shown that spinal cord transplants support regrowth of adult host axons resulting in reconstitution of synaptic complexes within the transplant that in many respects resemble normal synapses. Transplants of fetal spinal cord may also contribute to behavioral recovery by rescuing axotomized host neurons that otherwise would have died. Electrophysiological investigations of functional recovery after intraspinal transplantation have been recorded (32,375).
Olfactory Ensheathing/Precursor Cells
OECs are cells that display Schwann cell or astrocyte-like properties. They are a source of growth factors and adhesion molecules that play a very important role as a neuronal support enhancing cellular survival (179,292). Transplants of these cells have been shown to have a neuroprotective effect, supporting axonal regeneration, remyelination of demyelinated axons, neuroplasticity, neuromodulation, neurogenesis, angiogenesis, anti-inflammatory response, reducing scar and cavity formation, and/or strong phagocytic activity (107,196,203,233,292,293,311,341,388,397,398,419). The cellular composition of the olfactory tissue and the evidence that equivalent cell types exist in both rodent and human olfactory mucosa suggest that it is potentially a rich source of autologous cells for transplant-mediated repair of the CNS (224). Selected relevant studies are listed in Table 2.
Table 2.
Selected Literature in Preclinical Therapeutic Application of Olfactory Ensheathing/Precursor Cells
| Disease | Animal Host | Year | Reference | Publication Title |
|---|---|---|---|---|
| Spinal cord trauma | ||||
| Rhizotomized dorsal roots | rat | 1994 | 313 | Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants |
| rat | 1999 | 271 | Ensheathing glia transplants promote dorsal root regeneration and spinal reflex restitution after multiple lumbar rhizotomy | |
| Rhizotomized ventral roots | rat | 2002 | 289 | Spinal implants of olfactory ensheathing cells promote axon regeneration and bladder activity after bilateral lumbosacral dorsal rhizotomy in the adult rat |
| Hemitransected cord | rat | 1997 | 211 | Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells |
| rat | 2004 | 301 | Phrenic rehabilitation and diaphragm recovery after cervical injury and transplantation of olfactory ensheathing cells | |
| Transected dorsal column | pig | 2000 | 153 | Xenotransplantation of transgenic pig olfactory ensheathing cells promotes axonal regeneration in rat spinal cord |
| rat | 2003 | 175 | Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats | |
| rat | 2006 | 339 | Protection of corticospinal tract neurons after dorsal spinal cord transection and engraftment of olfactory ensheathing cells | |
| Transected cord | rat | 1998 | 314 | Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants |
| rat | 2000 | 312 | Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia | |
| rat | 2001 | 238 | Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats | |
| rat | 2002 | 239 | Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord | |
| Contusion | rat | 2001 | 146 | Olfactory ensheathing glias transplant improves axonal regeneration and functional recovery in spinal cord contusion injury |
| rat | 2003 | 297 | Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the contused adult rat spinal cord | |
| Electrolytic lesions | rat | 1998 | 212 | Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells |
| Photochemical lesion | rat | 2001 | 384 | Effects of ensheathing cells transplanted into photochemically damaged spinal cord |
| rat | 2004 | 232 | Increased expression of cyclooxygenase 2 and vascular endothelial growth factor in lesioned spinal cord by transplanted olfactory ensheathing cells | |
| X-ray irradiation with focal ethidium bromide injections | rat | 1996 | 106 | Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS |
| rat | 1998 | 155 | Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord | |
| rat | 2000 | 24 | Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons | |
| rat | 2004 | 89 | Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS | |
| monkey | 2004 | 310 | Remyelination of the nonhuman primate spinal cord by transplantation of H-transferase transgenic adult pig olfactory ensheathing cells | |
| rat | 2006 | 338 | Molecular reconstruction of nodes of Ranvier after remyelination by transplanted olfactory ensheathing cells in the demyelinated spinal cord | |
| Sciatic nerve resection | rat | 1999 | 385 | Olfactory bulb ensheathing cells enhance peripheral nerve regeneration |
| rat | 2005 | 391 | Effect of olfactory ensheathing cells transplantation on protecting spinal cord and neurons after peripheral nerve injury | |
| Facial nerve transaction | rat | 2001 | 127 | Transplantation of olfactory ensheathing cells stimulates the collateral sprouting from axotomized adult rat facial motoneurons |
| rat | 2002 | 128 | Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats | |
| Optic nerve injury | ||||
| Optic nerve resection | rat | 2003 | 215 | Transplanted olfactory ensheathing cells promote regeneration of cut adult rat optic nerve axons |
| Glaucoma | rat | 2008 | 213 | Transplanted olfactory ensheathing cells incorporated into the optic nerve head ensheathe retinal ganglion cell axons: Possible relevance to glaucoma |
| Parkinson’s disease | ||||
| 6-OHDA lesions | rat | 2004 | 1 | Olfactory ensheathing cell transplantation restores functional deficits in rat model of Parkinson’s disease: A cotransplantation approach with fetal ventral mesencephalic cells |
| Amyotrophic lateral sclerosis | ||||
| mSOD1 | mouse | 2007 | 250 | Adult olfactory bulb neural precursor cell grafts provide temporary protection from motor neuron degeneration, improve motor function, and extend survival in amyotrophic lateral sclerosis mice |
| Cognitive dysfunction | ||||
| Kainic acid lesions in CA3 | rat | 2009 | 359 | Long-term functional restoration by neural progenitor cell transplantation in rat model of cognitive dysfunction: Eo-transplantation with olfactory ensheathing cells for neurotrophic factor support |
| Stroke | ||||
| MCAo | rat | 2009 | 350 | A long term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat |
Schwann Cells
Schwann cells (SCs) play a pivotal role in the maintenance and regeneration of the axons in the peripheral nervous system (PNS) due to their ability to dedifferentiate, migrate, proliferate, express growth-promoting factors, and myelinate-regenerating axons (197). Further, SCs have been shown to form myelin after transplantation into the demyelinated CNS. They can remyelinate spinal cord lesions after experimental demyelination, leading in some cases to functional recovery in rodent and primate models (154). However, SCs do not normally enter the CNS, and migration of SCs transplanted into the CNS white matter is inhibited by astrocytes. As SC migration and myelination is mediated by interactions between sets of extracellular matrix molecules with cell surface preoteins, genetic engineering of SCs to alter aspects of these interactions is a possible way forward. Efforts are, therefore, focused on enhancing their migration and functional integration into the lesioned CNS. In addition, efforts are under way to use these cells as tissue engineer seeds and gene delivery vehicles for an array of molecules with repair potential (33,282). The SCs ability to promote restorative efforts has led to an increasing interest in using SC grafts for repair of CNS damage (Table 3).
Table 3.
Selected Literature in Preclinical Therapeutic Application of Schwann Cells
| Disease | Animal Host | Year | Reference | Publication Title |
|---|---|---|---|---|
| Spinal cord trauma | ||||
| Hemitransected cord | mouse | 1995 | 254 | Reinnervation of peripheral nerve segments implanted into the hemisected spinal cord estimated by transgenic mice |
| Transected dorsal column | rat | 1994 | 214 | Schwann cells induce sprouting in motor and sensory axons in the adult rat spinal cord |
| Transected cord | mouse and rat | 1981 | 2 | Influences of the glial environment on the elongation of axons after injury: Transplantation studies in adult rodents |
| rat | 1995 | 408 | Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord | |
| Contusion | cat | 1982 | 403 | Reconstruction of the contused cat spinal cord by the delayed nerve graft technique and cultured peripheral non-neuronal cells |
| rat | 1993 | 249 | Syngeneic grafting of adult rat DRG-derived Schwann cells to the injured spinal cord | |
| Photochemical lesion | rat | 1994 | 46 | Transplantation of purified populations of Schwann cells into lesioned adult rat spinal cord |
| Compression injury | rat | 1987 | 389 | Chronic regenerative changes in the spinal cord after cord compression injury in rats |
| Diphtheria toxin injection | cat | 1980 | 133 | Remyelination by cells introduced into a stable demyelinating lesion in the central nervous system |
| Lysolecithin injection | mouse | 1981 | 88 | Transplantation of rat Schwann cells grown in tissue culture into the mouse spinal cord |
| X-irradiation with ethidiumbromide injection | cat | 1985 | 36 | The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system |
| rat | 1987 | 37 | Schwann cell remyelination of CNS axons following injection of cultures of CNS cells into areas of persistent demyelination | |
| Optic nerve injury | rat | 1995 | 135 | Schwann cells and the regrowth of axons in the mammalian CNS: A review of transplantation studies in the rat visual system |
| Monocular deprivation | rat | 1994 | 296 | Schwann cells transplanted in the lateral ventricles prevent the functional and anatomical effects of monocular deprivation in the rat |
| Parkinson’s disease | ||||
| MPTP | mouse | 1990 | 75 | Cografts of adrenal medulla with peripheral nerve enhance the survivability of transplanted adrenal chromaffin cells and recovery of the host nigrostriatal dopaminergic system in MPTP-treated young adult mice |
| monkey | 1994 | 67 | Peripheral nerve-dopamine neuron co-grafts in MPTP-treated monkeys: Augmentation of tyrosine hydroxylase-positive fiber staining and dopamine content in host systems | |
| 6-OHDA-induced hemiparkinsonism | monkey | 2004 | 407 | Therapeutic study of autologous Schwann cells’ bridge graft into the brain of hemiparkinsonian monkey |
| Brain trauma | ||||
| Septal-hippocampal lesions | rat | 1985 | 187 | Transplants of Schwann cell cultures promote axonal regeneration in the adult mammalian brain |
| Unilateral fornix transaction | rat | 1996 | 362 | Reconstruction of transected postcommissural fornix in adult rat by Schwann cell suspension grafts |
| Brain stem injury | rat | 2003 | 390 | Schwann cells transplantation promoted and the repair of brain stem injury in rats |
Oligodendrocyte/Precursor Cells
Remyelination by transplantation of myelin-forming cells is possible in animal models; evidence suggests that both a precursor-type oligodendrocyte as well as an oligodendrocyte that previously formed a myelin sheath is able to remyelinate the CNS (126,386). Oligodendrocytes or precursor cells are much more invasive and have been shown to migrate from the implantation site to the lesion over a distance of several millimeters (72,387). Indeed, a number of studies have demonstrated that transplanted oligodendrocytes survive in the host brain, migrate out of the graft, and synthesize myelin. These cells, therefore, have potential for myelin repair after experimental demyelination and in human diseases, such as multiple sclerosis, though several findings suggest that OECs and SCs might be more effective than oligodendrocytes induced from isolated CNS tissue (87,103,153).
Astrocytes
Cultured astrocytes have been reported to survive and migrate following transplantation. Studies have indicated that there are differences in the ability of immature and mature astrocytes to facilitate plastic changes in the adult brain. Immature astrocytes can synthesize trophic factors to support neuronal survival, produce a permissive environment for neurite extension, and reduce scar formation. In contrast, mature astrocytes produce a non-permissive environment for axon growth and increase scar formation. Purified astrocytes were capable of facilitating behavioral recovery from frontal cortex ablation, demyelinating lesions in spinal cord, and kainic acid (KA) lesions of the striatum (10,104,174,240,392). Implanted cultured immature astrocytes can stimulate axonal regeneration after injury of the postcommissural fornix tract in the adult rat brain (406). In addition, behavior alleviation after astrocyte transplantation was shown in rat models of memory deficit induced by alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid lesions, which is independent of cholinergic recovery. The cultured astrocytes may exert their effects over a short time period (less than 2 weeks) around the lesion site. They can alter the microenvironment and as a result less scar tissue was formed followed by less of a barrier to the regrowth of nerve fibers (43). Furthermore, evidence indicates that astrocytes at an immature stage of differentiation are capable of inducing axon growth from the adult optic nerve (354).
Microglia
Microglia are the principal immune cells in the CNS and are characterized by a highly specific morphology and unusual antigenic phenotype. Evidence from postmortem analysis implicates the involvement of microglia in the neurodegenerative process of several neurode-generative diseases, including AD and PD. Grafting of activated microglia into the lesioned spinal cord may promote hind limb motor function recovery in rats and reduce the size of the liquefaction necrosis area (228,418).
Monocyte/Macrophage
Monocytes/macrophages play an integral role in the inflammatory process and angiogenesis as well as acting as defense mechanisms by exerting microbiocidal and immunomodulatory activity. The recruited monocytes/macrophages are capable of regulating angiogenesis in ischemic tissue, tumors, and chronic inflammation. In terms of neovascularization followed by tissue regeneration, monocytes/macrophages should be highly attractive for cell-based therapy compared to other cells due to their considerable advantages: nononcogenic, nonteratogenic, multiple secretory functions including proangiogenic and growth factors and their straightforward cell harvesting procedure (348). Increasing the presence of activated macrophage/microglial cells at a SCI site can provide an environment beneficial to the promotion of regeneration of sensory axons, possibly by the release of cytokines and interaction with other nonneuronal cells in the immediate vicinity (117,305).
Purkinje Cells
Purkinje cells have a therapeutic value for the replacement and reconstruction of a defective cerebellar circuitry in heredodegenerative ataxia. Insignificant amelioration of motor skills was found in mice after solid cerebellar tissue transplantation, while the cell suspension application had no effect (344,357,358).
Tanycyte
Tanycytes (TAs) are the specialized ependymal glia in the CNS, which are located mostly in the ventral wall of the third ventricle and median eminence (ME). They closely interact with local cerebrospinal fluid, blood, and neurons. TA is a common component of the brain barrier system, the brain–cerebrospinal fluid (CSF) neuro-humoral circuit, and the immune–neuroendocrine network. Recent data indicate that TAs transplanted into the adult rat spinal cord can support the regeneration of lesioned axons and may represent a useful therapeutic tool for CNS diseases (306).
Dopaminergic Neurons
Intracerebral transplantation of dopaminergic (DAergic) neuron/cells is currently performed as a restorative therapy for PD (273). The success of cell therapy in PD greatly relies on the discovery of an abundant source of cells capable of DAergic function in the brain. DAergic neuron/precursor cells derived from human embryonic stem (hES) cells, human-induced pluripotent stem (hiPS) cells, neural stem/progenitor cells, human mesenchymal stem cells, and skin-derived stem cells could be increasingly considered as a pivotal choice for transplant (50,188,227,269,372). It is likely that cell replacement in future will focus on not only ameliorating symptoms of the disease but also try to slow down the progression of the disease by either neuroprotection or restoration of a favorable microenvironment in the midbrain (319).
GABAergic Neurons
Transplantation of predifferentiated GABAergic neurons significantly induces recovery of sensorimotor function in brain injury (25). A deficiency of GABAergic neurons in the neocortex leads to the dysregulation of cortical neuronal circuits, but this can be overcome by cell transplantation. Ventral neural stem cells transfected with neurogenin 1 (Ngn1) are integrated as GABAergic neurons within a few days of transplantation into the adult mouse neocortex, and the transplantation of committed neuronal progenitor cells has been demonstrated to be an effective method for brain repair (268). In addition, transplants of neuronal cells bioengineered to synthesize GABA may alleviate chronic neuropathic pain (90). Fetal GABAergic neurons transplanted into the SN might be an effective means of permanently blocking seizure generalization in kindling epilepsy and probably also other types of epilepsy (101,234).
Cholinergic Neurons
Transplanted cholinergic neurons may reinnervate the host hippocampus, although this reinnervation appears to be different from that seen in the intact hippocampal formation (9). Intraretrosplenial cortical grafts of cholinergic neurons can become functionally incorporated with the host neural circuitry, and the activity of the implanted cholinergic neurons can be modulated by the host brain (216) and it can rectify spatial memory deficits produced by the loss of intrinsic cholinergic afferents from the medial septal nucleus (217). Reconstruction of the septohippocampal pathways by axons extending from embryonic cholinergic neuroblasts grafted into the neuron-depleted septum has been confirmed in the neonatal rat (198). Intrahippocampal septal grafts are able to reinnervate the hippocampal formation and ameliorate spatial learning and memory deficits, which are associated with anatomical and functional incorporation into the circuitry of the host hippocampal formation. Autotransplantation of peripheral cholinergic neurons into the cerebral cortex displayed amelioration of abnormal behavior in AD (160).
Embryonic Stem Cells
ES cells, in particular, possess a nearly unlimited self-renewal capacity and developmental potential to differentiate into virtually any cell type of an organism. They can efficiently differentiate into neural precursors, which can further generate functional neurons, astrocytes, and oligodendrocytes (102,229,231). These cells also have the beneficial properties of secreting neurotrophic and neural growth factors (272). Along with directed differentiation, other current efforts are aimed at efficient enrichment, avoidance of immune rejection, demonstration of functional integration, genetic modification to regulate neurotransmitter and factor release, and directed axon growth with these cells (125).
Neural Stem/Progenitor Cells
Neural stem/progenitor cells (NSPCs) are present during embryonic development and in certain regions of the adult CNS (264). Mobilizing adult NSCs to promote repair of injured or diseased parts of the CNS is a promising approach (3). NSPCs in the adult CNS are capable of generating new neurons, astrocytes, and oligodendrocytes (381). Intraventricular transplantation of neural spheres attenuated brain inflammation in acute and chronic experimental autoimmune encephalomyelitis (EAE), reduced the clinical severity of disease, and reduced demyelination and axonal pathology. Intravenous (IV) NSPCs injection also inhibited EAE and reduced CNS inflammation and tissue injury (27). A recent study showed that adult NSCs transplanted at sites of injury can differentiate into vascular cells (endothelial cells and vascular smooth muscle cells) for vasculogenesis (152). Transplantation of NSCs or their derivatives into a host brain and the proliferation and differentiation of endogenous stem cells by pharmacological manipulations are promising treatments for many neurodegenerative diseases and brain injuries, such as PD, brain ischemia, and SCI (Table 4).
Table 4.
Animal Model of CNS Diseases Treated Using Neural Stem/Progenitor Cells
| Animal Model | Route of Administration | Reference(s) |
|---|---|---|
| Spinal cord injury | parenchyma | 200,278 |
| intrathecal | 404 | |
| intravenous | 42 | |
| Traumatic brain injury | intravenous | 134 |
| intracerebral | 194 | |
| Stroke | intravenous | 201,356 |
| intracerebral | 263,363 | |
| intracarotid | 129 | |
| Parkinson’s disease | intracerebral | 4,45,93 |
| Lysosomal storage disorders | intracerebral | 176 |
| ALS | into spinal cord | 410 |
| intrathecal | 150 | |
| Hypoxic-ischemic (HI) | intracerebral | 288 |
| Experimental autoimmune encephalomyelitis (EAE) | intravenous and intracerebroventricular | 299 |
| intrathecal | 298 | |
| Huntington’s disease | intracerebral | 22,322 |
| intravenous | 202 | |
| Spinal muscular atrophy | intrathecal | 71 |
Bone Marrow Stromal Cells
Bone marrow stromal (also called “stem”) cells (BMSCs) can be easily amplified in vitro and their transdifferentiation into neural cells has been claimed in vitro and in vivo (63,82,163,171). The possible mechanisms responsible for the beneficial outcome observed after BMSC transplantation into neurodegenerating tissues include cell replacement, trophic factor delivery, immunomodulation, and anti-inflammatory, neuroprotection, and angiogenesis (171,371,379). Transplantation of BMSCs may have a therapeutic role after SCI (64). Adult BMSCs administered intravenously have been shown to migrate into the brain and improve neurological outcome in rats withd traumatic brain injury (236). In parallel, data of intracerebral transplantation suggest that bone marrow could potentially be used to induce plasticity in ischemic brain. Additionally, cotransplantation of BMSCs with ES cell-derived graft cells may be useful for preventing the development of ES cell-derived tumors (253). Results of this field are summarized in Table 5.
Table 5.
Animal Model of CNS Diseases Treated Using Bone Marrow Stromal Cells
| Animal Model | Route of Administration | Reference(s) |
|---|---|---|
| Spinal cord injury | parenchyma | 64 |
| intrathecal | 20,275,342 | |
| intravenous | 79 | |
| Spinal cord ischemia | intrathecal | 415 |
| Traumatic brain injury | intravenous | 236 |
| intracerebral | 245 | |
| Stroke | intravenous | 56 |
| intracerebral | 207,414,425 | |
| intracarotid | 208 | |
| Parkinson’s disease | intracerebral | 120,209 |
| Demyelinated spinal cord | parenchyma | 5,148,340 |
| intravenous | 157 | |
| Lysosomal storage disorders | intracerebral | 164 |
| ALS | intravenous | 70 |
| intrathecal | 131 | |
| Hypoxic-ischemic encephalopathy (HIE) | intraperitoneal | 123 |
| intracerebral | 415 | |
| Experimental autoimmune encephalomyelitis (EAE) | intravenous | 420 |
| Huntington’s disease | intravenous | 86 |
| Peripheral nerve neuropathies | injected into the dorsal root ganglion | 69 |
Umbilical Cord Mesenchymal Stromal Cells
Mesenchymal stromal cells (MSCs) have now been isolated from most tissues, including the umbilical cord (UC) and UC blood (UCB; see below). UC and UCB MSCs are more primitive than those isolated from other tissue sources and do not express the major histocompatibility complex (MHC) class II human leukocyte antigen-D-related (HLA-DR) antigens. Studies have shown that UC MSCs are still viable and are not rejected 4 months after transplantation as xenografts, without the need for immune suppression, suggesting that they are a favorable cell source for transplantation (422). UC including arteries (UCA), veins (UCV), and Wharton’s jelly (UCWJ) is a convenient, efficient source of MSCs that can be expanded easily in vitro for numerous clinical applications for the treatment of nonhematopoietic diseases, and in studies of tissue regeneration and immunosuppression (119,159). UC MSCs have proven to be efficacious in reducing lesion sizes and enhancing behavioral recovery in animal models of ischemic and traumatic CNS injury. Recent findings also suggest that neurons derived from UC-MSC could alleviate movement disorders in hemiparkinsonian animal models. UC-MSC therefore could be a viable alternative to human ES cells or NSCs for transplantation therapy of CNS trauma and neurodegenerative diseases (235) (Table 6).
Table 6.
Animal Model of CNS Diseases Treated Using Umbilical Cord Mesenchymal Stromal Cells
Umbilical Cord Blood Cells
UCB is a rich source of stem cells with great proliferative potential, besides the bone marrow and peripheral blood; it has the advantage of being an easily accessible stem cell source and is less immunogenic compared to other sources for stem cells (334). There are at least three kinds of stem cells in UCB: hematopoietic, mesenchymal, and embryonic-like stem cells, which are capable of differentiating across tissue lineage boundaries into neural, cardiac, epithelial, hepatocytic, and dermal tissue both in vitro and in vivo (132,325,383). Increasing evidence suggests that MSCs from UCB are present within a wide range of tissues and its therapeutic potential extends beyond the hematopoietic component (34). The expanding population of NSPCs can be selected from the human cord blood nonhematopoietic (CD34-negative) mononuclear fraction (49). UCB can be a potential source for autologous or allogeneic monocytes/macrophages. UCB monocytes should be considered as a primary candidate owing to their easy isolation, low immune rejection, and multiple characteristic advantages such as their anti-inflammatory properties by virtue of their unique immune and inflammatory immaturity, and their proangiogenic ability (333). The therapeutic potential of UCB cells may be attributed to the inherent ability of stem cell populations to replace damaged tissues. Alternatively, various cell types within the graft may promote neural repair by delivering neural protection and secretion of neurotrophic factors (284,334). In addition, evidence suggests that delivery of circulating CD34+ human UCB cells can produce functional recovery in an animal stroke model with concurrent angiogenesis and neurogenesis leading to some restoration of cortical tissue (295). UCB cells have been used in preclinical models of brain injury and neurode-generative diseases, directed to differentiate into neural phenotypes, and have been related to functional recovery after engraftment in CNS lesion models (Table 7).
Table 7.
Animal Model of CNS Diseases Treated Using Umbilical Cord Blood Cells
| Animal Model | Route of Administration | Reference(s) |
|---|---|---|
| Spinal cord injury | parenchyma | 189,426 * |
| intravenous | 170,337 | |
| Brain injury | intravenous | 237,270 |
| Stroke | intravenous | 39,57,400 |
| intracerebral | 84,352,399 | |
| intraarterial | 65 | |
| Parkinson’s disease | intravenous | 95 |
| Alzheimer’s disease | intravenous | 96,274 |
| Huntington’s disease | intravenous | 94 |
| Amyotrophic lateral sclerosis | intravenous | 97,114,115 |
| intrathecal | 131 | |
| Hypoxic-ischemic (HI) | intraperitoneal | 258 |
| intravenous | 78,413 | |
| Aged brain | intravenous | 18 |
| Sanfilippo syndrome type B | intravenous | 113 |
Cell subset: CD34+
Hematopoietic Stem Cells
Hematopoietic stem cell transplantation (HSCT) was proposed as a treatment for multiple sclerosis (MS) in 1995 based on favorable results in animal models including EAE (47). Recent studies show that transplantation of HSCs from bone marrow is an effective strategy for SCI after directly transplanting cells into the cord 1 week after injury (185), with a similar potential in comparison with marrow stromal cells (180). An increasing number of studies provide evidence that hematopoietic stem cells, either after stimulation of endogenous stem cell pools or after exogenous hematopoietic stem cell use, improve functional outcome after ischemic brain lesions. Various underlying mechanisms such as transdifferentiation into neural lineages, neuroprotection through trophic support, and cell fusion have been deciphered (130). Furthermore, intracerebral peripheral blood hematopoietic stem cell (CD34+) implantation induces neuroplasticity by enhancing β1 integrin-mediated angiogenesis in chronic cerebral ischemia with significantly increased modulation of neurotrophic factor expression in the ischemic hemisphere (352).
Adipose-Derived Adult Stem/Precursor Cells
Adipose tissue is an abundant, accessible, and replenishable source of adult stem cells that can be isolated from liposuction waste tissue by collagenase digestion and differential centrifugation (118). These adipose-derived adult stem (ADAS) cells, which exhibit characteristics of multipotent adult stem cells, similar to those of MSCs, are multipotent, differentiating along the adipocyte, chondrocyte, myocyte, neuronal, and osteoblast lineages (124). ADAS cells have potential applications for the repair and regeneration of acute and chronically damaged tissues (329). As an alternative stem cell source for CNS therapies, ADAS cells labeled with superparamagnetic iron oxide have been shown using MRI to successfully transplant in vivo in unilateral middle cerebral artery occluded (MCAo) mice (320). The study of Ryu and colleagues indicate that improvement in neurological function by the transplantation of ADAS in dogs with SCI may be partially due to the neural differentiation of the implanted stem cells (326). Furthermore, the transplantation of ADAS can promote the formation of a more robust nerve in rats with a sciatic nerve defect and produce a decrease in muscle atrophy (336).
Skin-Derived Precursors
Skin-derived precursors (SKPs) are a self-renewing, multipotent precursor that are generated during embryo-genesis and persist into adulthood in the dermis, share characteristics with embryonic neural crest stem cells, including their ability to differentiate into neural crest-derived cell types such as peripheral neurons, SCs, astrocytes, and endothelium (26,149). After transplantation, the cells yield healthy cells that migrate to the lesion site, and then differentiate mainly into cells expressing glia and neuronal markers (122). Recent evidence indicates that transplantation of SKP-derived SCs represent a viable alternative strategy for repairing the injured spinal cord, with the neuroanatomical neurorestorative findings including good survival within the injured spinal cord, reduced size of the contusion cavity, myelinated endogenous host axons, recruited endogenous SCs into the injured cord, formation of a bridge across the lesion site, increased size of the spared tissue rim, myelinated spared axons within the tissue rim, reduced reactive gliosis, and an environment that was highly conducive to axonal growth (35). In addition, SKPs transplanted into PD model rats sufficiently differentiated into dopamine neuron-like cells, and partially but significantly corrected their behavior. The generated DA neuron-like cells are expected to serve as donor cells for neuronal repair for PD (188). Thus, this cell line has been identified as novel, accessible, and a potentially autologous source for future nervous system repair (35,192).
Retinal Pigment Epithelial Cells
The retinal pigment epithelium consists of a unicellular layer of neuroepithelial cells, retinal pigment epithelial (RPE) cells, which are essential for the maintenance of the normal function of the retina (139). Cultured human RPE cells have the capacity to synthesize neurotrophins, including NGF, brain-derived growth factor (BDNF), glial cell-derived neurotrophic factor (GDNF), and neurotrophin-3 (NT-3) (158,262). Studies have shown that, as an alternative cell source, RPE cells possess DAergic replacement properties with neurotrophic support on primary cultures of rat striatal (enkephalinergic) and mesencephalic (DAergic) neurons, and therefore could exert a positive effect in parkinsonian animals by intrastriatal transplantation (247,257,365,366). RPE cells can be transduced with high efficiency using an adenoviral vector, making them promising vehicles for local delivery of therapeutic proteins for the treatment of neurodegenerative diseases in a combined cell and gene therapeutic approach (14).
Amniotic Epithelial Cells
Human amniotic epithelial cells (AEC) do not express the HLA-A, -B, -C, or -DR antigens on their surface, which suggests no acute rejection in transplantation (6). The human amnion membrane serves as a bridge for axonal regeneration in vitro and in vivo; cells isolated from the amniotic membrane can differentiate into all three germ layers, have low immunogenicity and anti-inflammatory function (76,417). Given their multipotent differentiation ability, capability of synthesizing catecholamines including DA, and neurotrophic and neuroprotection effect, there is accumulating evidence that suggests that AECs have therapeutic potential for multiple CNS disorders, such as PD, mucopolysaccharidosis, SCI, stroke, brain trauma, etc. (Table 8).
Table 8.
Selected Literatures in Preclinical Therapeutic Application of Amniotic Epithelial Cells
| Disease | Animal Host | Year | Reference | Publication Title |
|---|---|---|---|---|
| Parkinson’s disease | rat | 2000 | 167 | Human amniotic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson’s disease: A potential source of donor for transplantation therapy |
| 6-OHDA lesion | rat | 2003 | 168 | Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions |
| MPTP | mouse | 2008 | 183 | Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice |
| Mucopolysaccharidosis type VII | mouse | 2001 | 186 | Engraftment of genetically engineered amniotic epithelial cells corrects lysosomal storage in multiple areas of the brain in mucopolysaccharidosis type VII mice |
| Brain ischemia | rat | 2001 | 280 | Amniotic epithelial cells transform into neuron-like cells in the ischemic brain |
| mouse | 2007 | 316 | Amniotic fluid derived stem cells ameliorate focal cerebral ischaemia-reperfusion injury induced behavioural deficits in mice | |
| MCAo | rat | 2008 | 230 | Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model |
| Spinal cord trauma | ||||
| Transection | monkey | 2003 | 335 | Role of human amniotic epithelial cell transplantation in spinal cord injury repair research |
| rat | 2006 | 405 | Transplantation of human amniotic epithelial cells improves hind-limb function in rats with spinal cord injury | |
| Peripheral nerve injury | ||||
| Sciatic nerve defects | rat | 2004 | 85 | Bridging rat sciatic nerve defects with the composite nerve-muscle autografts wrapped with human amnion matrix membrane |
| Brain injury | rat | 2006 | 241 | Treatment of traumatic brain injury in rats with transplantation of human amniotic cells |
Menstrual Blood Cells
Endometrial cells supplied as a form of menstrual blood–tissue mixture can be used for cell-based restorative therapy in muscular dystrophy (380); subsequent evidence shows that populations of stromal stem cells derived from menstrual blood are multipotent, being able to differentiate into chondrogenic, adipogenic, osteogenic, neurogenic, and cardiogenic cell lineages (290). The cultured menstrual blood express embryonic like-stem cell phenotypic markers [Octamer-4 (Oct4), stage-specific embryonic antigen (SSEA), Nanog], and when grown in appropriate conditioned media, express neuronal phenotypic markers [nestin, microtubule-associated protein 2 (MAP2)] (40). Transplantation of menstrual blood-derived stem cells, either intracerebrally or intravenously and without immunosuppression, significantly reduces behavioral and histological impairments in adult ischemic stroke rats (40).
Sertoli Cells
Transplanted testis-derived Sertoli cells, which create a localized immune “privileged” site, possess a modulatory function on graft rejection and survival and act as a viable graft source for facilitating the use of xenotransplantation for diabetes, PD, Huntington’s disease, and other neurodegenerative diseases (41,331,332). In addition to producing immunoprotective factors, Sertoli cells also secrete growth and trophic factors that appear to enhance the posttransplantation viability of isolated cells and, likewise, the postthaw viability of isolated, cryopreserved cells (52). Sertoli cells grafted into adult rat brains ameliorated behavioral deficits and enhanced DAergic neuronal survival and outgrowth (331). Cotransplanting of Sertoli cells may be useful as a combination therapy in CNS lesions, a strategy that could enhance the recovery benefits associated with transplantation and decrease the need for, and the risks associated with, long-term systemic immunosuppression (399). Further, recent research has shown that implantation of a Sertoli cell-enriched preparation has a significant neuroprotective benefit to vulnerable motor neurons in a superoxide dismutase 1 (SOD1) transgenic mouse model of amyotrophic lateral sclerosis (ALS) (136).
Induced Pluripotent Stem Cells
Induced pluripotent stem (iPS) cells are derived from somatic cells by ectopic expression of a few transcription factors. iPS cells appear to be able to self-renew indefinitely and to differentiate into all types of cells in the body, and are almost identical to ES cells. The generation of patient-derived pluripotent cells applicable to autologous cell-based therapies has the potential to revolutionize medicine (60). Since the first report from Takahashi and Yamanaka on the reprogramming of mouse fibroblasts into pluripotent stem cells by defined factors in 2006 (370), various new methods have been developed to refine and improve reprogramming technology (281). The current demonstration of DAergic differentiation of human induced pluripotent stem cells (hiPSCs), replacement of segmental losses of interneurons and motorneurons due to gray matter damage and restoration of auditory spiral ganglion neurons suggest a new avenue for highly effective, tumor-free, and immune rejection-free cell therapy for PD, SCI, and hearing disturbance in the near future (151,276,323).
CLINICAL STUDIES OF CELL-BASED NEURORESTORATOLOGY IN CNS DISEASES (TABLE 9)
Table 9.
Selected Recent Articles of Clinical Studies Related to Cell-Based CNS Neurorestoratology
| Team/Reference | Country | Publication Year | Case Number | Disease | Results |
|---|---|---|---|---|---|
| Fassas et al. (99) | Greece | 2002 | 85 | multiple sclerosis | effective |
| Huang et al. (143) | China | 2003 | 171 | spinal cord injury | improvement |
| Park et al. (287) | Korea | 2005 | 5 | spinal cord injury | improvement |
| Bang et al. (21) | Korea | 2005 | 5 | stroke | improvement |
| Rabinovich et al. (308) | Russia | 2005 | 10 | stroke | safe & effective |
| Saccardi et al. (328) | Italy | 2006 | 180 | multiple sclerosis | effective |
| Syková et al. (368) | Czech | 2006 | 20 | spinal cord injury | improvement |
| Yoon et al. (416) | Korea | 2007 | 35 | spinal cord injury | improvement |
| Portaccio et al. (304) | Italy | 2007 | 2 | multiple sclerosis | effective |
| Saiz et al. (330) | Spain | 2008 | 14 | multiple sclerosis | safe & effective |
| Huang et al. (144) | China | 2008 | 15 | amyotrophic lateral sclerosis | safe & effective |
| Mazzini et al. (255) | Italy | 2008 | 9 | amyotrophic lateral sclerosis | safe & effective |
| Moviglia et al. (266) | Argentina | 2009 | 8 | spinal cord injury | improvement |
| Pal et al. (283) | India | 2009 | 30 | spinal cord injury | safe |
| Burt et al. (48) | US | 2009 | 21 | multiple sclerosis | effective |
| Farge et al. (96) | France | 2009 | 347 | multiple sclerosis | effective |
| Suárez-Monteagudo et al. (364) | Cuba | 2009 | 5 | stroke | safe & effective |
| Cristante et al. (73) | Brazil | 2009 | 39 | spinal cord injury | safe & improved SSEPs |
| Cicchetti et al. (66) | Canada | 2009 | 3 | Huntington’s disease | effective |
| Huang et al. (145) | China | 2009 | 1255 | spinal cord injury, amyotrophic lateral sclerosis, cerebral palsy, multiple sclerosis, stoke, etc. | improvement & effective |
Adrenal Medullary Tissue, Substantia Nigra, and Dopamine Neurons
Positive findings had initially been observed by Backlund et al. (19) and Lindvall et al. (225) concerning the transplantation of autologous adrenal medullary tissue into the striatum of patients with severe parkinsonism. Subsequently, Hitchcock et al. (138), Lindvall and colleagues (226,395), and Madrazo et al. (244) have separately reported that fetal nigral implants might have provided a modest improvement in motor function and have clinically valuable improvements in most recipients within a period of long-term follow-up after transplantation into the brain of patients with PD. Freed and colleagues randomly assigned patients to receive nerve cell transplants or sham surgery with double-blind follow-up. The result showed that transplanted human embryonic DA neurons survive with clinical benefit in younger, but not in older patients (108). Furthermore, pathologic findings suggest that grafts of fetal mesencephalic DA neurons could survive long term with or without α-synuclein-positive Lewy bodies (184,205,259).
Bachoud-Lévi and coworkers indicated motor and cognitive recovery in patients with Huntington’s disease after neural transplantation (17), which continued during long-term follow-up (16). The therapeutic value of human striatal neuroblasts in Huntington’s disease was identified by Gallina and colleagues (112).
Human Neural Stem/Progenitor Cells or Neuronal Cells
The clinical regimes of intracranial implant of human neuronal cells in stroke patients have proven safe and feasible, though there is a lack of evidence for a significant benefit in motor function (181,182). Data suggest that cell therapy is a safe method and can be effectively used for stroke (308), acute brain injury (346,347), and cerebral palsy (345). In addition, neurological function has been restored after autologous neural stem cell transplantation in patients with brain trauma (428).
Umbilical Cord Mesenchymal Stem Cells
Therapy of UC-MSCs could stabilize the disease course of refractory progressive MS (218).
Umbilical Cord Blood Mesenchymal Stem Cells
Kang and colleagues report that UCB-MSC transplantation may play a role in the treatment of SCI patients (169).
Olfactory Ensheathing Cell and Olfactory Mucosa Autografts
Early OEC/olfactory mucosa autograft transplants for patients with chronic SCI were reported by Huang et al. in 2003 (143), Rabinovich et al. in 2003 (309), and Lima et al. in 2006 (223), and the results were safe, feasible, and positive. Mackay-Sim and colleagues reported autologous OEC transplantation for three patients with chronic SCI with 3-year follow-up was safe, feasible, and one patient showed sensory improvement (243). The therapeutic value of OEC transplantation has been shown in chronic SCI, ALS, cerebral palsy, stroke, MS, and other neurodegenerative diseases and traumatic brain insults in 1,255 patients (144,145).
Schwann Cells
Data from Arjmand and colleagues suggested that autologous SC transplantation is safe for spinal cord injured patients but had no beneficial effects (327).
Microglia/Macrophage
Knoller et al. reported that autologous macrophages were safe for complete SCI (178).
Bone Marrow Stromal Cell/Hematopoietic Stem Cell/Mononuclear Phagocyte
Research of Appel et al. indicated that peripheral cells derived from donor hematopoietic stem cells were able to enter the human CNS primarily at sites of moto-neuron pathology and engrafted as immunomodulatory cells, but they did not provide benefit in sporadic ALS patients (12). On the contrary, autologous anti-human CD133+ mononuclear cell transplantation in the motor cortex delays ALS progression and improves quality of life (251). Furthermore, many studies showed that this kind of cell therapy was feasible, safe, and effective for ALS (78), stroke (21,364), chronic SCI patients (61,77,266,287,368), and there was also improvement in the acute and subacute phase of chronic SCI (416). Different routes of cell transplantation such as by direct injection into spinal cord, intravenous and intrathecal injection have proven to be equally effective in SCI (116) and traumatic brain injury (424). Evidence shows that autol-ogous hematopoietic stem cell transplantation cannot be deemed a curative treatment but instead may give rise to prolonged stabilization or change the aggressive course of diseases (330). Similar results were reported by Farge et al. (98), Fassas et al. (99), Portaccio et al. (304), and Saccardi et al. (328) as well as in studies for malignant or severe MS (54,177,246).
Nonmyeloablative autologous haemopoietic stem cell transplantation in patients with relapsing-remitting MS reverses neurological deficits (48). Also autologous peripheral blood stem cell transplantation has promoted obvious neurologic improvement for patients with poly-neuropathy, organomegaly, endocrinopathy, M-protein, and skin changes syndrome (190,191).
SUMMARY AND PROSPECTS
When entering the 21st century, numerous centers have globally started clinical trials or experimental treatments to investigate the utilization of cells, such as neurons, OECs, bone marrow-derived cells, NSPCs, SCs, etc., for intractable CNS diseases. Despite their diversity in number, clinical status of subjects, route of cell administration, and criteria to evaluate efficacy, the main conclusion drawn from these clinical studies was that such therapies were safe, feasible, and had some neurological functional improvement or restorative effect that improved the patient’s quality of life to a varying extent. These achievements had already answered YES or NO to the question of whether the degeneration and damage in the CNS could be functionally restored.
But from the cellular biology viewpoint, there are several unanswered questions for cell transplantation: what kind of cells would be the best ideal source, the best therapeutic time window, the most suitable selection for patients and diseases of different kinds, and the optimal route. Consequently, emphasis should be placed on solving these questions and evaluating the efficacy of each particular treatment modality in detail.
On the other hand, from a clinical neurorestoratology viewpoint, the current treatment results are far from an effective cure or the miracle effect, as the majority of people expect; however, therapeutic strategies to retard disease progression for neurodegenerative diseases or to improve some functions from acquired damages seem to be a more realistic clinical aim compared with expecting a cure or complete recovery in the present or near future. Patients, scientists, and doctors should value highly the patients’ achievements from effective treatment strategies that are currently still thought by some to not be available.
So far, the pleasurable reality is that landmark advances and the results of preclinical and clinical studies in neurorestoratolgy have been driving our traditional concept from the passive reaction to disease to active attempts to restore the lost functions of the CNS. Now people are more interested in and pay more attention to functional neurorestoration rather than the anatomical structure repair of the CNS.
REFERENCES
- 1. Agrawal A. K.; Shukla S.; Chaturvedi R. K.; Seth K.; Srivastava N.; Ahmad A.; Seth P. K. Olfactory ensheathing cell transplantation restores functional deficits in rat model of Parkinson’s disease: A cotransplantation approach with fetal ventral mesencephalic cells. Neurobiol. Dis. 16(3):516–526; 2004. [DOI] [PubMed] [Google Scholar]
- 2. Aguayo A. J.; David S.; Bray G. M. Influences of the glial environment on the elongation of axons after injury: Transplantation studies in adult rodents. J. Exp. Biol. 95:231–240; 1981. [DOI] [PubMed] [Google Scholar]
- 3. Ahmed S. The culture of neural stem cells. J. Cell. Biochem. 106(1):1–6; 2009. [DOI] [PubMed] [Google Scholar]
- 4. Akerud P.; Canals J. M.; Snyder E. Y.; Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J. Neurosci. 21(20):8108–8118; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akiyama Y.; Radtke C.; Kocsis J. D. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J. Neurosci. 22(15):6623–6630; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akle C. A.; Adinolfi M.; Welsh K. I.; Leibowitz S.; McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 2(8254):1003–1005; 1981. [DOI] [PubMed] [Google Scholar]
- 7. Alexandrova M. A.; Polezhaev L. V. Transplantation of various regions of embryonic brain tissue into the brain of adult rats. J. Hirnforsch. 25(1):89–98; 1984. [PubMed] [Google Scholar]
- 8. Anderberg L.; Aldskogius H.; Holtz A. Spinal cord injury-scientific challenges for the unknown future. Ups. J. Med. Sci. 112(3):259–288; 2007. [DOI] [PubMed] [Google Scholar]
- 9. Anderson K. J.; Gibbs R. B.; Salvaterra P. M.; Cotman C. W. Ultrastructural characterization of identified cholinergic neurons transplanted to the hippocampal formation of the rat. J. Comp. Neurol. 249(2):279–292; 1986. [DOI] [PubMed] [Google Scholar]
- 10. Andersson C.; Tytell M.; Brunso-Bechtold J. Transplantation of cultured type 1 astrocyte cell suspensions into young, adult and aged rat cortex: Cell migration and survival. Int. J. Dev. Neurosci. 11(5):555–568; 1993. [DOI] [PubMed] [Google Scholar]
- 11. Andres R. H.; Meyer M.; Ducray A. D.; Widmer H. R. Restorative neuroscience: Concepts and perspectives. Swiss Med. Wkly. 138(11–12):155–172; 2008. [DOI] [PubMed] [Google Scholar]
- 12. Appel S. H.; Engelhardt J. I.; Henkel J. S.; Siklos L.; Beers D. R.; Yen A. A.; Simpson E. P.; Luo Y.; Carrum G.; Heslop H. E.; Brenner M. K.; Popat U. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology 71(17):1326–1334; 2008. [DOI] [PubMed] [Google Scholar]
- 13. Armengol J. A.; Sotelo C.; Angaut P.; Alvarado-Mallart R. M. Organization of host afferents to cerebellar grafts implanted into kainate lesioned cerebellum in adult rats. Eur. J. Neurosci. 1(1):75–93; 1989. [DOI] [PubMed] [Google Scholar]
- 14. Arnhold S.; Semkova I.; Andressen C.; Lenartz D.; Meissner G.; Sturm V.; Kochanek S.; Addicks K.; Schraermeyer U. Iris pigment epithelial cells: A possible cell source for the future treatment of neurodegenerative diseases. Exp. Neurol. 187(2):410–417; 2004. [DOI] [PubMed] [Google Scholar]
- 15. Auerbach J. M.; Eiden M. V.; McKay R. D. Transplanted CNS stem cells form functional synapses in vivo. Eur. J. Neurosci. 12(5):1696–1704; 2000. [DOI] [PubMed] [Google Scholar]
- 16. Bachoud-Lévi A. C.; Gaura V.; Brugières P.; Lefaucheur J. P.; Boissé M. F.; Maison P.; Baudic S.; Ribeiro M. J.; Bourdet C.; Remy P.; Cesaro P.; Hantraye P.; Peschanski M. Effect of fetal neural transplants in patients with Huntington’s disease 6 years after surgery: A long-term follow-up study. Lancet Neurol. 5(4):303–309; 2006. [DOI] [PubMed] [Google Scholar]
- 17. Bachoud-Lévi A. C.; Rémy P.; Nguyen J. P.; Brugières P.; Lefaucheur J. P.; Bourdet C.; Baudic S.; Gaura V.; Maison P.; Haddad B.; Boissé M. F.; Grandmougin T.; Jény R.; Bartolomeo P.; Dalla Barba G.; Degos J. D.; Lisovoski F.; Ergis A. M.; Pailhous E.; Cesaro P.; Hantraye P.; Peschanski M. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet 356(9246):1975–1979; 2000. [DOI] [PubMed] [Google Scholar]
- 18. Bachstetter A. D.; Pabon M. M.; Cole M. J.; Hudson C. E.; Sanberg P. R.; Willing A. E.; Bickford P. C.; Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 9:22; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Backlund E. O.; Granberg P. O.; Hamberger B.; Knutsson E.; Mårtensson A.; Sedvall G.; Seiger A.; Olson L. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J. Neurosurg. 62(2):169–173; 1985. [DOI] [PubMed] [Google Scholar]
- 20. Bakshi A.; Barshinger A. L.; Swanger S. A.; Madhavani V.; Shumsky J. S.; Neuhuber B.; Fischer I. Lumbar puncture delivery of bone marrow stromal cells in spinal cord contusion: A novel method for minimally invasive cell transplantation. J. Neurotrauma 23(1):55–65; 2006. [DOI] [PubMed] [Google Scholar]
- 21. Bang O. Y.; Lee J. S.; Lee P. H.; Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57(6):874–882; 2005. [DOI] [PubMed] [Google Scholar]
- 22. Bantubungi K.; Blum D.; Cuvelier L.; Wislet-Gendebien S.; Rogister B.; Brouillet E.; Schiffmann S. N. Stem cell factor and mesenchymal and neural stem cell transplantation in a rat model of Huntington’s disease. Mol. Cell. Neurosci. 37(3):454–470; 2008. [DOI] [PubMed] [Google Scholar]
- 23. Barami K.; Hao H. N.; Lotoczky G. A.; Diaz F. G.; Lyman W. D. Transplantation of human fetal brain cells into ischemic lesions of adult gerbil hippocampus. J. Neurosurg. 95(2):308–315; 2001. [DOI] [PubMed] [Google Scholar]
- 24. Barnett S. C.; Alexander C. L.; Iwashita Y.; Gilson J. M.; Crowther J.; Clark L.; Dunn L. T.; Papanastassiou V.; Kennedy P. G.; Franklin R. J. Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain 123(Pt. 8):1581–1588; 2000. [DOI] [PubMed] [Google Scholar]
- 25. Becerra G. D.; Tatko L. M.; Pak E. S.; Murashov A. K.; Hoane M. R. Transplantation of GABAergic neurons but not astrocytes induces recovery of sensorimotor function in the traumatically injured brain. Behav. Brain Res. 179(1):118–125; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belicchi M.; Pisati F.; Lopa R.; Porretti L.; Fortunato F.; Sironi M.; Scalamogna M.; Parati E. A.; Bresolin N.; Torrente Y. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J. Neurosci. Res. 77(4):475–486; 2004. [DOI] [PubMed] [Google Scholar]
- 27. Ben-Hur T. Immunomodulation by neural stem cells. J. Neurol. Sci. 265(1–2):102–104; 2008. [DOI] [PubMed] [Google Scholar]
- 28. Ben-Hur T.; Goldman S. A. Prospects of cell therapy for disorders of myelin. Ann. NY Acad. Sci. 1142:218–249; 2008. [DOI] [PubMed] [Google Scholar]
- 29. Bermúdez-Rattoni F.; Fernández J.; Sánchez M. A.; Aguilar-Roblero R.; Drucker-Colín R. Fetal brain transplants induce recuperation of taste aversion learning. Brain Res. 416(1):147–152; 1987. [DOI] [PubMed] [Google Scholar]
- 30. Bernstein J. J. Viability, growth, and maturation of fetal brain and spinal cord in the sciatic nerve of adult rat. J. Neurosci. Res. 10(4):343–350; 1983. [DOI] [PubMed] [Google Scholar]
- 31. Bernstein J. J.; Goldberg W. J. Fetal spinal cord homografts ameliorate the severity of lesion-induced hind limb behavioral deficits. Exp. Neurol. 98(3):633–644; 1987. [DOI] [PubMed] [Google Scholar]
- 32. Bernstein-Goral H.; Bregman B. S. Spinal cord transplants support the regeneration of axotomized neurons after spinal cord lesions at birth: A quantitative double-labeling study. Exp. Neurol. 123(1):118–132; 1993. [DOI] [PubMed] [Google Scholar]
- 33. Bhatheja K.; Field J. Schwann cells: Origins and role in axonal maintenance and regeneration. Int. J. Biochem. Cell Biol. 38(12):1995–1999; 2006. [DOI] [PubMed] [Google Scholar]
- 34. Bieback K.; Klüter H. Mesenchymal stromal cells from umbilical cord blood. Curr. Stem Cell Res. Ther. 2(4):310–323; 2007. [DOI] [PubMed] [Google Scholar]
- 35. Biernaskie J.; Sparling J. S.; Liu J.; Shannon C. P.; Plemel J. R.; Xie Y.; Miller F. D.; Tetzlaff W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J. Neurosci. 27(36):9545–9559; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blakemore W. F.; Crang A. J. The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. J. Neurol. Sci. 70(2):207–223; 1985. [DOI] [PubMed] [Google Scholar]
- 37. Blakemore W. F.; Crang A. J.; Patterson R. C. Schwann cell remyelination of CNS axons following injection of cultures of CNS cells into areas of persistent demyelination. Neurosci. Lett. 77(1):20–24; 1987. [DOI] [PubMed] [Google Scholar]
- 38. Blömer U.; Naldini L.; Verma I. M.; Trono D.; Gage F. H. Applications of gene therapy to the CNS. Hum. Mol. Genet. 5:1397–1404; 1996. [DOI] [PubMed] [Google Scholar]
- 39. Borlongan C. V.; Hadman M.; Sanberg C. D.; Sanberg P. R. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 35(10):2385–2389; 2004. [DOI] [PubMed] [Google Scholar]
- 40. Borlongan C. V.; Kaneko Y.; Maki M.; Yu S.; Ali M. M.; Allickson J.; Sanberg C.; Kuzmin-Nichols N.; Sanberg P. R. Menstrual blood cells display stem celllike phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 19(4):439–452; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borlongan C. V.; Stahl C. E.; Cameron D. F.; Saporta S.; Freeman T. B.; Cahill D. W.; Sanberg P. R. CNS immunological modulation of neural graft rejection and survival. Neurol. Res. 18(4):297–304; 1996. [DOI] [PubMed] [Google Scholar]
- 42. Bottai D.; Madaschi L.; Di Giulio A. M.; Gorio A. Viability-dependent promoting action of adult neural precursors in spinal cord injury. Mol. Med. 14(9–10):634–644; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bradbury E. J.; Kershaw T. R.; Marchbanks R. M.; Sinden J. D. Astrocyte transplants alleviate lesion induced memory deficits independently of cholinergic recovery. Neuroscience 65(4):955–972; 1995. [DOI] [PubMed] [Google Scholar]
- 44. Bretzner F.; Liu J.; Currie E.; Roskams A. J.; Tetzlaff W. Undesired effects of a combinatorial treatment for spinal cord injury-transplantation of olfactory ensheathing cells and BDNF infusion to the red nucleus. Eur. J. Neurosci. 28(9):1795–1807; 2008. [DOI] [PubMed] [Google Scholar]
- 45. Brunet J. F.; Redmond D. E. Jr.; Bloch J. Primate adult brain cell autotransplantation, a pilot study in asymptomatic MPTP-treated monkeys. Cell Transplant. 18(7):787–799; 2009. [DOI] [PubMed] [Google Scholar]
- 46. Bunge M. B. Transplantation of purified populations of Schwann cells into lesioned adult rat spinal cord. J. Neurol. 242(1 Suppl. 1):S36–S39; 1994. [DOI] [PubMed] [Google Scholar]
- 47. Burt R. K.; Burns W.; Hess A. Bone marrow transplantation for multiple sclerosis. Bone Marrow Transplant. 16:1–6; 1995. [PubMed] [Google Scholar]
- 48. Burt R. K.; Loh Y.; Cohen B.; Stefoski D.; Balabanov R.; Katsamakis G.; Oyama Y.; Russell E. J.; Stern J.; Muraro P.; Rose J.; Testori A.; Bucha J.; Jovanovic B.; Milanetti F.; Storek J.; Voltarelli J. C.; Burns W. H. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: A phase I/II study. Lancet Neurol. 8(3):244–253; 2009. [DOI] [PubMed] [Google Scholar]
- 49. Buzańska L.; Jurga M.; Domańska-Janik K. Neuronal differentiation of human umbilical cord blood neural stem-like cell line. Neurodegener. Dis. 3(1–2):19–26; 2006. [DOI] [PubMed] [Google Scholar]
- 50. Cai J.; Yang M.; Poremsky E.; Kidd S.; Schneider J. S.; Iacovitti L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA lesioned rats. Stem Cells Dev. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cajal S. R. Y. Degeneration and regeneration of the nervous system (R. M. May, Trans.). London: Oxford University Press; 1928. [Google Scholar]
- 52. Cameron D. F.; Othberg A. I.; Borlongan C. V.; Rashed S.; Anton A.; Saporta S.; Sanberg P. R. Post-thaw viability and functionality of cryopreserved rat fetal brain cells cocultured with Sertoli cells. Cell Transplant. 6(2):185–189; 1997. [DOI] [PubMed] [Google Scholar]
- 53. Cao Q. L.; Zhang Y. P.; Howard R. M.; Walters W. M.; Tsoulfas P.; Whittemore S. R. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 167:48–58; 2001. [DOI] [PubMed] [Google Scholar]
- 54. Capello E.; Saccardi R.; Murialdo A.; Gualandi F.; Pagliai F.; Bacigalupo A.; Marmont A.; Uccelli A.; Inglese M.; Bruzzi P.; Sormani M. P.; Cocco E.; Meucci G.; Massacesi L.; Bertolotto A.; Lugaresi A.; Merelli E.; Solari A.; Filippi M.; Mancardi G. L.; Italian GITMO-Neuro Intergroup on ASCT for Multiple Sclerosis. Intense immunosuppression followed by autologous stem cell transplantation in severe multiple sclerosis. Neurol. Sci. 26(Suppl. 4):S200–S203; 2005. [DOI] [PubMed] [Google Scholar]
- 55. Chen J.; Li Y.; Katakowski M.; Chen X.; Wang L.; Lu D.; Lu M.; Gautam S. C.; Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 73(6):778–786; 2003. [DOI] [PubMed] [Google Scholar]
- 56. Chen J.; Li Y.; Wang L.; Zhang Z.; Lu D.; Lu M.; Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32(4):1005–1011; 2001. [DOI] [PubMed] [Google Scholar]
- 57. Chen J.; Sanberg P. R.; Li Y.; Wang L.; Lu M.; Willing A. E.; Sanchez-Ramos J.; Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32(11):2682–2688; 2001. [DOI] [PubMed] [Google Scholar]
- 58. Chen J.; Zhang Z. G.; Li Y.; Wang L.; Xu Y. X.; Gautam S. C.; Lu M.; Zhu Z.; Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 92:692–699; 2003. [DOI] [PubMed] [Google Scholar]
- 59. Chen L.; Huang H. Neurorestoratology: New concept and bridge from bench to bedside. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 23(3):366–370; 2009. [PubMed] [Google Scholar]
- 60. Chen L.; Liu L. Current progress and prospects of induced pluripotent stem cells. Sci. China C. Life Sci. 52(7):622–636; 2009. [DOI] [PubMed] [Google Scholar]
- 61. Chernykh E. R.; Stupak V. V.; Muradov G. M.; Sizi-kov M. Y.; Shevela E. Y.; Leplina O. Y.; Tikhonova M. A.; Kulagin A. D.; Lisukov I. A.; Ostanin A. A.; Kozlov V. A. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull. Exp. Biol. Med. 143(4):543–547; 2007. [DOI] [PubMed] [Google Scholar]
- 62. Chiu S. C.; Hung H. S.; Lin S. Z.; Chiang E.; Liu D. D. Therapeutic potential of olfactory ensheathing cells in neurodegenerative diseases. J. Mol. Med. 87(12):1179–1189; 2009. [DOI] [PubMed] [Google Scholar]
- 63. Cho S. R.; Kim Y. R.; Kang H. S.; Yim S. H.; Park C. I.; Min Y. H.; Lee B. H.; Shin J. C.; Lim J. B. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone marrow in a rat model of spinal cord injury. Cell Transplant. 18(12):1359–1368; 2009. [DOI] [PubMed] [Google Scholar]
- 64. Chopp M.; Zhang X. H.; Li Y.; Wang L.; Chen J.; Lu D.; Lu M.; Rosenblum M. Spinal cord injury in rat: Treatment with bone marrow stromal cell transplantation. Neuroreport 11(13):3001–3005; 2000. [DOI] [PubMed] [Google Scholar]
- 65. Chung D. J.; Choi C. B.; Lee S. H.; Kang E. H.; Lee J. H.; Hwang S. H.; Han H.; Lee J. H.; Choe B. Y.; Lee S. Y.; Kim H. Y. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J. Neurosci. Res. 87(16):3554–3567; 2009. [DOI] [PubMed] [Google Scholar]
- 66. Cicchetti F.; Saporta S.; Hauser R. A.; Parent M.; Saint-Pierre M.; Sanberg P. R.; Li X. J.; Parker J. R.; Chu Y.; Mufson E. J.; Kordower J. H.; Freeman T. B. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc. Natl. Acad. Sci. USA 106(30):12483–12488; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Collier T. J.; Elsworth J. D.; Taylor J. R.; Sladek J. R. Jr.; Roth R. H.; Redmond D. E. Jr. Peripheral nerve-dopamine neuron co-grafts in MPTP-treated monkeys: Augmentation of tyrosine hydroxylase-positive fiber staining and dopamine content in host systems. Neuroscience 61(4):875–889; 1994. [DOI] [PubMed] [Google Scholar]
- 68. Commissiong J. W. Fetal locus coeruleus transplanted into the transected spinal cord of the adult rat: Some observations and implications. Neuroscience 12(3):839–853; 1984. [DOI] [PubMed] [Google Scholar]
- 69. Coronel M. F.; Musolino P. L.; Villar M. J. Selective migration and engraftment of bone marrow mesenchymal stem cells in rat lumbar dorsal root ganglia after sciatic nerve constriction. Neurosci. Lett. 405(1–2):5–9; 2006. [DOI] [PubMed] [Google Scholar]
- 70. Corti S.; Locatelli F.; Donadoni C.; Guglieri M.; Papadimitriou D.; Strazzer S.; Del Bo R.; Comi G. P. Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain 127(Pt. 11):2518–2532; 2004. [DOI] [PubMed] [Google Scholar]
- 71. Corti S.; Nizzardo M.; Nardini M.; Donadoni C.; Salani S.; Ronchi D.; Saladino F.; Bordoni A.; Fortunato F.; Del Bo R.; Papadimitriou D.; Locatelli F.; Menozzi G.; Strazzer S.; Bresolin N.; Comi G. P. Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. J. Clin. Invest. 118(10):3316–3330; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crang A. J.; Blakemore W. F. Remyelination of demyelinated rat axons by transplanted mouse oligodendrocytes. Glia 4(3):305–313; 1991. [DOI] [PubMed] [Google Scholar]
- 73. Cristante A. F.; Barros-Filho T. E.; Tatsui N.; Mendrone A.; Caldas J. G.; Camargo A.; Alexandre A.; Teixeira W. G.; Oliveira R. P.; Marcon R. M. Stem cells in the treatment of chronic spinal cord injury: Evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord 47(10):733–738; 2009. [DOI] [PubMed] [Google Scholar]
- 74. Daadi M. M.; Lee S. H.; Arac A.; Grueter B. A.; Bhatnagar R.; Maag A. L.; Schaar B.; Malenka R. C.; Palmer T. D.; Steinberg G. K. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant. 18(7):815–826; 2009. [DOI] [PubMed] [Google Scholar]
- 75. Date I.; Felten S. Y.; Felten D. L. Cografts of adrenal medulla with peripheral nerve enhance the survivability of transplanted adrenal chromaffin cells and recovery of the host nigrostriatal dopaminergic system in MPTP-treated young adult mice. Brain Res. 537(1–2):33–39; 1990. [DOI] [PubMed] [Google Scholar]
- 76. Davis G. E.; Blaker S. N.; Engvall E.; Varon S.; Manthorpe M.; Gage F. H. Human amnion membrane serves as a substratum for growing axons in vitro and in vivo. Science 236(4805):1106–1109; 1987. [DOI] [PubMed] [Google Scholar]
- 77. Deda H.; Inci M. C.; Kürekçi A. E.; Kayihan K.; Ozgün E.; Ustünsoy G. E.; Kocabay S. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy 10(6):565–574; 2008. [DOI] [PubMed] [Google Scholar]
- 78. Deda H.; Inci M. C.; Kürekçi A. E.; Sav A.; Kayihan K.; Ozgün E.; Ustünsoy G. E.; Kocabay S. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: A 1-year follow-up. Cytotherapy 11(1):18–25; 2009. [DOI] [PubMed] [Google Scholar]
- 79. de Haro J.; Zurita M.; Ayllón L.; Vaquero J. Detection of 111In-oxine-labeled bone marrow stromal cells after intravenous or intralesional administration in chronic paraplegic rats. Neurosci. Lett. 377(1):7–11; 2005. [DOI] [PubMed] [Google Scholar]
- 80. de Paula S.; Vitola A. S.; Greggio S.; de Paula D.; Mello P. B.; Lubianca J. M.; Xavier L. L.; Fiori H. H.; Dacosta J. C. Hemispheric brain injury and behavioral deficits induced by severe neonatal hypoxia-ischemia in rats are not attenuated by intravenous administration of human umbilical cord blood cells. Pediatr. Res. 65(6):631–635; 2009. [DOI] [PubMed] [Google Scholar]
- 81. Detante O.; Moisan A.; Dimastromatteo J.; Richard M. J.; Riou L.; Grillon E.; Barbier E.; Desruet M. D.; De, Fraipont F.; Segebarth C.; Jaillard A.; Hommel M.; Ghezzi C.; Remy C. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: In vivo imaging and biodistribution. Cell Transplant. 18(12):1369–1379; 2009. [DOI] [PubMed] [Google Scholar]
- 82. Dezawa M. Systematic neuronal and muscle induction systems in bone marrow stromal cells: The potential for tissue reconstruction in neurodegenerative and muscle degenerative diseases. Med. Mol. Morphol. 41(1):14–19; 2008. [DOI] [PubMed] [Google Scholar]
- 83. Dimitrijevic M. R. Recent achievements of restorative neurology. Switzerland: Karger; 1985. [Google Scholar]
- 84. Ding D. C.; Shyu W. C.; Chiang M. F.; Lin S. Z.; Chang Y. C.; Wang H. J.; Su C. Y.; Li H. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol. Dis. 27(3):339–353; 2007. [DOI] [PubMed] [Google Scholar]
- 85. Duan X. L.; Xu Y. Z.; Zeng Z. C. Bridging rat sciatic nerve defects with the composite nerve-muscle autografts wrapped with human amnion matrix membrane. Zhong Nan Da Xue Xue Bao Yi Xue Ban 29(3):279–283; 2004. [PubMed] [Google Scholar]
- 86. Dunbar G. L.; Sandstrom M. I.; Rossignol J.; Lescaudron L. Neurotrophic enhancers as therapy for behavioral deficits in rodent models of Huntington’s disease: Use of gangliosides, substituted pyrimidines, and mesenchymal stem cells. Behav. Cogn. Neurosci. Rev. 5(2):63–79; 2006. [DOI] [PubMed] [Google Scholar]
- 87. Duncan I. D. Glial cell transplantation and remyelination of the central nervous system. Neuropathol. Appl. Neurobiol. 22(2):87–100; 1996. [DOI] [PubMed] [Google Scholar]
- 88. Duncan I. D.; Aguayo A. J.; Bunge R. P.; Wood P. M. Transplantation of rat Schwann cells grown in tissue culture into the mouse spinal cord. J. Neurol. Sci. 49(2):241–252; 1981. [DOI] [PubMed] [Google Scholar]
- 89. Dunning M. D.; Lakatos A.; Loizou L.; Kettunen M.; ffrench-Constant C.; Brindle K. M.; Franklin R. J. Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J. Neurosci. 24(44):9799–9810; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Eaton M. J.; Plunkett J. A.; Martinez M. A.; Lopez T.; Karmally S.; Cejas P.; Whittemore S. R. Transplants of neuronal cells bioengineered to synthesize GABA alleviate chronic neuropathic pain. Cell Transplant. 8(1):87–101; 1999. [DOI] [PubMed] [Google Scholar]
- 91. Eaton M. J.; Wolfe S. Q. Clinical feasibility for cell therapy using human neuronal cell line to treat neuropathic behavioral hypersensitivity following spinal cord injury in rats. J. Rehabil. Res. Dev. 46(1):145–165; 2009. [PubMed] [Google Scholar]
- 92. Einstein O.; Friedman-Levi Y.; Grigoriadis N.; Ben-Hur T. Transplanted neural precursors enhance host brain-derived myelin regeneration. J. Neurosci. 29(50):15694–15702; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Emborg M. E.; Ebert A. D.; Moirano J.; Peng S.; Suzuki M.; Capowski E.; Joers V.; Roitberg B. Z.; Aebischer P.; Svendsen C. N. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 17(4):383–395; 2008. [PubMed] [Google Scholar]
- 94. Ende N.; Chen R. Human umbilical cord blood cells ameliorate Huntington’s disease in transgenic mice. J. Med. 32(3–4):231–240; 2001. [PubMed] [Google Scholar]
- 95. Ende N.; Chen R. Parkinson’s disease mice and human umbilical cord blood. J. Med. 33(1–4):173–180; 2002. [PubMed] [Google Scholar]
- 96. Ende N.; Chen R.; Ende-Harris D. Human umbilical cord blood cells ameliorate Alzheimer’s disease in transgenic mice. J. Med. 32(3–4):241–247; 2001. [PubMed] [Google Scholar]
- 97. Ende N.; Weinstein F.; Chen R.; Ende M. Human umbilical cord blood effect on sod mice (amyotrophic lateral sclerosis). Life Sci. 67(1):53–59; 2000. [DOI] [PubMed] [Google Scholar]
- 98. Farge D.; Labopin M.; Tyndall A.; Fassas A.; Mancardi G. L.; Van Laar J.; Ouyang J.; Kozak T.; Moore J.; Kötter I.; Chesnel V.; Marmont A.; Gratwohl A.; Saccardi R. Autologous hematopoietic stem cell transplantation (HSCT) for autoimmune diseases: An observational study on 12 years of experience from the European Group for Blood and Marrow Transplantation (EBMT) Working Party on Autoimmune Diseases. Haematologica 95(2):284–292; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fassas A.; Passweg J. R.; Anagnostopoulos A.; Kazis A.; Kozak T.; Havrdova E.; Carreras E.; Graus F.; Kashyap A.; Openshaw H.; Schipperus M.; Deconinck E.; Mancardi G.; Marmont A.; Hansz J.; Rabusin M.; Zuazu, Nagore F. J.; Besalduch J.; Dentamaro T.; Fouillard L.; Hertenstein B.; La, Nasa G.; Musso M.; Papineschi F.; Rowe J. M.; Saccardi R.; Steck A.; Kappos L.; Gratwohl A.; Tyndall A.; Samijn J.; Autoimmune Disease Working Party of the EBMT (European Group for Blood and Marrow Transplantation). Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J. Neurol. 249(8):1088–1097; 2002. [DOI] [PubMed] [Google Scholar]
- 100. Ferrari D.; Sanchez-Pernaute R.; Lee H.; Studer L.; Isacson O. Transplanted dopamine neurons derived from primate ES cells preferentially innervate DARPP-32 striatal progenitors within the graft. Eur. J. Neurosci. 24(7):1885–1896; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fine A.; Meldrum B. S.; Patel S. Modulation of experimentally induced epilepsy by intracerebral grafts of fetal GABAergic neurons. Neuropsychologia 28(6):627–634; 1990. [DOI] [PubMed] [Google Scholar]
- 102. Fluckiger A. C.; Dehay C.; Savatier P. Embryonic stem cells and cell replacement therapies in the nervous system. Med. Sci (Paris) 19(6–7):699–708; 2003. [DOI] [PubMed] [Google Scholar]
- 103. Franklin R. J.; Bayley S. A.; Milner R.; Ffrench-Constant C.; Blakemore W. F. Differentiation of the O-2A progenitor cell line CG-4 into oligodendrocytes and astrocytes following transplantation into glia-deficient areas of CNS white matter. Glia 13(1):39–44; 1995. [DOI] [PubMed] [Google Scholar]
- 104. Franklin R. J.; Crang A. J.; Blakemore W. F. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. J. Neurocytol. 20(5):420–430; 1991. [DOI] [PubMed] [Google Scholar]
- 105. Franklin R. J.; Ffrench-Constant C. Remyelination in the CNS: From biology to therapy. Nat. Rev. Neurosci. 9(11):839–855; 2008. [DOI] [PubMed] [Google Scholar]
- 106. Franklin R. J.; Gilson J. M.; Franceschini I. A.; Barnett S. C. Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia 17(3):217–224; 1996. [DOI] [PubMed] [Google Scholar]
- 107. Franssen E. H.; De Bree F. M.; Essing A. H.; Ramon-Cueto A.; Verhaagen J. Comparative gene expression profiling of olfactory ensheathing glia and Schwann cells indicates distinct tissue repair characteristics of olfactory ensheathing glia. Glia 56(12):1285–1298; 2008. [DOI] [PubMed] [Google Scholar]
- 108. Freed C. R.; Greene P. E.; Breeze R. E.; Tsai W. Y.; DuMouchel W.; Kao R.; Dillon S.; Winfield H.; Culver S.; Trojanowski J. Q.; Eidelberg D.; Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 344(10):710–719; 2001. [DOI] [PubMed] [Google Scholar]
- 109. Freedman M. S.; Bar-Or A.; Atkins H. L.; Karussis D.; Frassoni F.; Lazarus H.; Scolding N.; Slavin S.; Le Blanc K.; Uccelli A. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: Consensus report of the International MSCT Study Group. Mult. Scler. 16(4):503–510; 2010. [DOI] [PubMed] [Google Scholar]
- 110. Fu Y. S.; Cheng Y. C.; Lin M. Y.; Cheng H.; Chu P. M.; Chou S. C.; Shih Y. H.; Ko M. H.; Sung M. S. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: Potential therapeutic application for parkinsonism. Stem Cells 24(1):115–124; 2006. [DOI] [PubMed] [Google Scholar]
- 111. Furmanski O.; Gajavelli S.; Lee J. W.; Collado M. E.; Jergova S.; Sagen J. Combined extrinsic and intrinsic manipulations exert complementary neuronal enrichment in embryonic rat neural precursor cultures: An in vitro and in vivo analysis. J. Comp. Neurol. 515(1):56–71; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gallina P.; Paganini M.; Lombardini L.; Mascalchi M.; Porfirio B.; Gadda D.; Marini M.; Pinzani P.; Salvianti F.; Crescioli C.; Bucciantini S.; Mechi C.; Sarchielli E.; Romoli A. M.; Bertini E.; Urbani S.; Bartolozzi B.; De Cristofaro M. T.; Piacentini S.; Saccardi R.; Pupi A.; Vannelli G. B.; Di Lorenzo N. Human striatal neuroblasts develop and build a striatal-like structure into the brain of Huntington’s disease patients after transplantation. Exp. Neurol. 222(1):30–41; 2010. [DOI] [PubMed] [Google Scholar]
- 113. Garbuzova-Davis S.; Klasko S. K.; Sanberg P. R. Intravenous administration of human umbilical cord blood cells in an animal model of MPS III B. J. Comp. Neurol. 515(1):93–101; 2009. [DOI] [PubMed] [Google Scholar]
- 114. Garbuzova-Davis S.; Sanberg C. D.; Kuzmin-Nichols N.; Willing A. E.; Gemma C.; Bickford P. C.; Miller C.; Rossi R.; Sanberg P. R. Human umbilical cord blood treatment in a mouse model of ALS: Optimization of cell dose. PLoS ONE 3(6):e2494; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Garbuzova-Davis S.; Willing A. E.; Zigova T.; Saporta S.; Justen E. B.; Lane J. C.; Hudson J. E.; Chen N.; Davis C. D.; Sanberg P. R. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: Distribution, migration, and differentiation. J. Hematother. Stem Cell Res. 12(3):255–270; 2003. [DOI] [PubMed] [Google Scholar]
- 116. Geffner L. F.; Santacruz P.; Izurieta M.; Flor L.; Maldonado B.; Auad A. H.; Montenegro X.; Gonzalez R.; Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transplant. 17(12):1277–1293; 2008. [DOI] [PubMed] [Google Scholar]
- 117. Gensel J. C.; Nakamura S.; Guan Z.; van, Rooijen N.; Ankeny D. P.; Popovich P. G. Macrophages promote axon regeneration with concurrent neurotoxicity. J. Neurosci. 29(12):3956–3968; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gimble J.; Guilak F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 5(5):362–369; 2003. [DOI] [PubMed] [Google Scholar]
- 119. Girdlestone J.; Limbani V. A.; Cutler A. J.; Navarrete C. V. Efficient expansion of mesenchymal stromal cells from umbilical cord under low serum conditions. Cytotherapy 11(6):738–748; 2009. [DOI] [PubMed] [Google Scholar]
- 120. Glavaski-Joksimovic A.; Virag T.; Chang Q. A.; West N. C.; Mangatu T. A.; McGrogan M. P.; Dugich-Djordjevic M.; Bohn M. C. Reversal of dopaminergic degeneration in a parkinsonian rat following micrografting of human bone marrow-derived neural progenitors. Cell Transplant. 18(7):801–814; 2009. [DOI] [PubMed] [Google Scholar]
- 121. Gonzalez M. F.; Sharp F. R.; Loken J. E. Fetal frontal cortex transplanted to injured motor/sensory cortex of adult rats: Reciprocal connections with host thalamus demonstrated with WGA-HRP. Exp. Neurol. 99(1):154–165; 1988. [DOI] [PubMed] [Google Scholar]
- 122. Gorio A.; Torrente Y.; Madaschi L.; Di Stefano A. B.; Pisati F.; Marchesi C.; Belicchi M.; Di Giulio A. M.; Bresolin N. Fate of autologous dermal stem cells transplanted into the spinal cord after traumatic injury (TSCI). Neuroscience 125(1):179–189; 2004. [DOI] [PubMed] [Google Scholar]
- 123. Guan X. Q.; Yu J. L.; Li L. Q.; Liu G. X. Study on mesenchymal stem cells entering the brain through the blood–brain barrier. Zhonghua Er Ke Za Zhi 42(12):920–923; 2004. [PubMed] [Google Scholar]
- 124. Guilak F.; Awad H. A.; Fermor B.; Leddy H. A.; Gimble J. M. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology 41(3–4):389–399; 2004. [PubMed] [Google Scholar]
- 125. Guillaume D. J.; Zhang S. C. Human embryonic stem cells: A potential source of transplantable neural progenitor cells. Neurosurg. Focus 24(3–4):E3; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gumpel M.; Gout O.; Lubetzki C.; Gansmuller A.; Baumann N. Myelination and remyelination in the central nervous system by transplanted oligodendrocytes using the shiverer model. Discussion on the remyelinating cell population in adult mammals. Dev. Neurosci. 11(2):132–139; 1989. [DOI] [PubMed] [Google Scholar]
- 127. Guntinas-Lichius O.; Angelov D. N.; Tomov T. L.; Dramiga J.; Neiss W. F.; Wewetzer K. Transplantation of olfactory ensheathing cells stimulates the collateral sprouting from axotomized adult rat facial motoneurons. Exp. Neurol. 172(1):70–80; 2001. [DOI] [PubMed] [Google Scholar]
- 128. Guntinas-Lichius O.; Wewetzer K.; Tomov T. L.; Azzolin N.; Kazemi S.; Streppel M.; Neiss W. F.; Angelov D. N. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J. Neurosci. 22(16):7121–7131; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Guzman R.; De Los, Angeles A.; Cheshier S.; Choi R.; Hoang S.; Liauw J.; Schaar B.; Steinberg G. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke 39(4):1300–1306; 2008. [DOI] [PubMed] [Google Scholar]
- 130. Haas S.; Weidner N.; Winkler J. Adult stem cell therapy in stroke. Curr. Opin. Neurol. 18(1):59–64; 2005. [DOI] [PubMed] [Google Scholar]
- 131. Habisch H. J.; Janowski M.; Binder D.; Kuzma-Kozakiewicz M.; Widmann A.; Habich A.; Schwalenstöcker B.; Hermann A.; Brenner R.; Lukomska B.; Domanska-Janik K.; Ludolph A. C.; Storch A. Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: Limited intraparenchymal migration and survival narrows therapeutic effects. J. Neural Transm. 114(11):1395–1406; 2007. [DOI] [PubMed] [Google Scholar]
- 132. Harris D. T.; Rogers I. Umbilical cord blood: A unique source of pluripotent stem cells for regenerative medicine. Curr. Stem Cell Res. Ther. 2(4):301–309; 2007. [DOI] [PubMed] [Google Scholar]
- 133. Harrison B. M. Remyelination by cells introduced into a stable demyelinating lesion in the central nervous system. J. Neurol. Sci. 46(1):63–81; 1980. [DOI] [PubMed] [Google Scholar]
- 134. Harting M. T.; Sloan L. E.; Jimenez F.; Baumgartner J.; Cox C. S. Jr. Subacute neural stem cell therapy for traumatic brain injury. J. Surg. Res. 153(2):188–194; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Harvey A. R.; Plant G. W.; Tan M. M. Schwann cells and the regrowth of axons in the mammalian CNS: A review of transplantation studies in the rat visual system. Clin. Exp. Pharmacol. Physiol. 22(8):569–579; 1995. [DOI] [PubMed] [Google Scholar]
- 136. Hemendinger R.; Wang J.; Malik S.; Persinski R.; Copeland J.; Emerich D.; Gores P.; Halberstadt C.; Rosenfeld J. Sertoli cells improve survival of motor neurons in SOD1 transgenic mice, a model of amyotrophic lateral sclerosis. Exp. Neurol. 196(2):235–243; 2005. [DOI] [PubMed] [Google Scholar]
- 137. Hess D. C.; Hill W. D.; Martin-Studdard A.; Carroll J.; Brailer J.; Carothers J. Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke 33(5):1362–1368; 2002. [DOI] [PubMed] [Google Scholar]
- 138. Hitchcock E. R.; Kenny B. G.; Clough C. G.; Hughes R. C.; Henderson B. T.; Detta A. Stereotactic implantation of fetal mesencephalon. Stereotact. Funct. Neurosurg. 54–55:282–289; 1990. [DOI] [PubMed] [Google Scholar]
- 139. Hooks J. J.; Detrick B.; Percopo C.; Hamel C.; Siraganian R. P. Development and characterization of monoclonal antibodies directed against the retinal pigment epithelial cell. Invest. Ophthalmol. Vis. Sci. 30(10):2106–2113; 1989. [PubMed] [Google Scholar]
- 140. Hortobágyi T.; Harkany T.; Reisch R.; Urbanics R.; Kálmán M.; Nyakas C.; Nagy Z. Neurotrophin-mediated neuroprotection by solid fetal telencephalic graft in middle cerebral artery occlusion: A preventive approach. Brain Res. Bull. 47(2):185–191; 1998. [DOI] [PubMed] [Google Scholar]
- 141. Houlé J. D.; Reier P. J. Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J. Comp. Neurol. 269(4):535–547; 1988. [DOI] [PubMed] [Google Scholar]
- 142. Huang A. H.; Snyder B. R.; Cheng P. H.; Chan A. W. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells 26(10):2654–2663; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Huang H.; Chen L.; Wang H.; Xiu B.; Li B.; Wang R.; Zhang J.; Zhang F.; Gu Z.; Li Y.; Song Y.; Hao W.; Pang S.; Sun J. Influence of patients’ age on functional recovery after transplantation of olfactory ensheathing cells into injured spinal cord injury. Chin. Med. J. (Engl.) 116(10):1488–1491; 2003. [PubMed] [Google Scholar]
- 144. Huang H.; Chen L.; Xi H.; Wang H.; Zhang J.; Zhang F.; Liu Y. Fetal olfactory ensheathing cells transplantation in amyotrophic lateral sclerosis patients: A controlled pilot study. Clin. Transplant. 22(6):710–718; 2008. [DOI] [PubMed] [Google Scholar]
- 145. Huang H.; Chen L.; Xi H.; Wang Q.; Zhang J.; Liu Y.; Zhang F. Olfactory ensheathing cells transplantation for central nervous system diseases in 1,255 patients. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 23(1):14–20; 2009. [PubMed] [Google Scholar]
- 146. Huang H.; Liu K.; Huang W.; Liu Z.; Young W. Olfactory ensheathing glias transplant improves axonal regeneration and functional recovery in spinal cord contusion injury. J. Naval Gen. Hosp. 14(2):65–67; 2001. [Google Scholar]
- 147. Huang H.; Wang H.; Chen L.; Gu Z.; Zhang J.; Zhang F.; Song Y.; Li Y.; Tan K.; Liu Y.; Xi H. Influence factors for functional improvement after olfactory ensheathing cell transplantation for chronic spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 20(4):434–438; 2006. [PubMed] [Google Scholar]
- 148. Hunt D. P.; Irvine K. A.; Webber D. J.; Compston D. A.; Blakemore W. F.; Chandran S. Effects of direct transplantation of multipotent mesenchymal stromal/stem cells into the demyelinated spinal cord. Cell Transplant. 17(7):865–873; 2008. [DOI] [PubMed] [Google Scholar]
- 149. Hunt D. P.; Jahoda C.; Chandran S. Multipotent skin-derived precursors: From biology to clinical translation. Curr. Opin. Biotechnol. 20(5):522–530; 2009. [DOI] [PubMed] [Google Scholar]
- 150. Hwang D. H.; Lee H. J.; Park I. H.; Seok J. I.; Kim B. G.; Joo I. S.; Kim S. U. Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Ther. 16(10):1234–1244; 2009. [DOI] [PubMed] [Google Scholar]
- 151. Hwang D. Y.; Kim D. S.; Kim D. W. Human ES and iPS cells as cell sources for the treatment of Parkinson’s disease: Current state and problems. J. Cell. Biochem. 109(2):292–301; 2010. [DOI] [PubMed] [Google Scholar]
- 152. Ii M.; Nishimura H.; Sekiguchi H.; Kamei N.; Yokoyama A.; Horii M.; Asahara T. Concurrent vasculogenesis and neurogenesis from adult neural stem cells. Circ. Res. 105(9):860–868; 2009. [DOI] [PubMed] [Google Scholar]
- 153. Imaizumi T.; Lankford K. L.; Burton W. V.; Fodor W. L.; Kocsis J. D. Xenotransplantation of transgenic pig olfactory ensheathing cells promotes axonal regeneration in rat spinal cord. Nat. Biotechnol. 18(9):949–953; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Imaizumi T.; Lankford K. L.; Kocsis J. D.; Sasaki M.; Akiyama Y.; Hashi K. Comparison of myelin-forming cells as candidates for therapeutic transplantation in demyelinated CNS axons. No To Shinkei 52(7):609–615; 2000. [PubMed] [Google Scholar]
- 155. Imaizumi T.; Lankford K. L.; Waxman S. G.; Greer C. A.; Kocsis J. D. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J. Neurosci. 18(16):6176–6185; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ingvar S. Reactions of cells to the galvanic current in tissue cultures. Proc. Soc. Exp. Biol. Med. 17:198–199; 1920. [Google Scholar]
- 157. Inoue M.; Honmou O.; Oka S.; Houkin K.; Hashi K.; Kocsis J. D. Comparative analysis of remyelinating potential of focal and intravenous administration of autologous bone marrow cells into the rat demyelinated spinal cord. Glia 44(2):111–118; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ishida K.; Yoshimura N.; Yoshida M.; Honda Y.; Murase K.; Hayashi K. Expression of neurotrophic factors in cultured human retinal pigment epithelial cells. Curr. Eye Res. 16(2):96–101; 1997. [DOI] [PubMed] [Google Scholar]
- 159. Ishige I.; Nagamura-Inoue T.; Honda M. J.; Harnprasopwat R.; Kido M.; Sugimoto M.; Nakauchi H.; Tojo A. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton’s jelly explants of human umbilical cord. Int. J. Hematol. 90(2):261–269; 2009. [DOI] [PubMed] [Google Scholar]
- 160. Itakura T.; Yokote H.; Yukawa S.; Nakai M.; Komai N.; Umemoto M. Transplantation of peripheral cholinergic neurons into Alzheimer model rat brain. Stereotact. Funct. Neurosurg. 54–55:368–372; 1990. [DOI] [PubMed] [Google Scholar]
- 161. Jacquet B. V.; Patel M.; Iyengar M.; Liang H.; Therit B.; Salinas-Mondragon R.; Lai C.; Olsen J. C.; Anton E. S.; Ghashghaei H. T. Analysis of neuronal proliferation, migration and differentiation in the postnatal brain using equine infectious anemia virus-based lentiviral vectors. Gene Ther. 16(8):1021–1033; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Jeltsch H.; Cassel J. C.; Neufang B.; Kelche C.; Hertting G.; Jackisch R.; Will B. The effects of intrahippocampal raphe and/or septal grafts in rats with fimbria-fornix lesions depend on the origin of the grafted tissue and the behavioural task used. Neuroscience 63(1):19–39; 1994. [DOI] [PubMed] [Google Scholar]
- 163. Jiang Y.; Jahagirdar B. N.; Reinhardt R. L.; Schwartz R. E.; Keene C. D.; Ortiz-Gonzalez X. R.; Reyes M.; Lenvik T.; Lund T.; Blackstad M.; Du J.; Aldrich S.; Lisberg A.; Low W. C.; Largaespada D. A.; Verfaillie C. M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418(6893):41–49; 2002. [DOI] [PubMed] [Google Scholar]
- 164. Jin H. K.; Carter J. E.; Huntley G. W.; Schuchman E. H. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. J. Clin. Invest. 109(9):1183–1191; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jing M.; Shingo T.; Yasuhara T.; Kondo A.; Morimoto T.; Wang F.; Baba T.; Yuan W. J.; Tajiri N.; Uozumi T.; Murakami M.; Tanabe M.; Miyoshi Y.; Zhao S.; Date I. The combined therapy of intrahippocampal transplantation of adult neural stem cells and intraventricular erythropoietin-infusion ameliorates spontaneous recurrent seizures by suppression of abnormal mossy fiber sprouting. Brain Res. 1295:203–217; 2009. [DOI] [PubMed] [Google Scholar]
- 166. Jomura S.; Uy M.; Mitchell K.; Dallasen R.; Bode C. J.; Xu Y. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells 25(1):98–106; 2007. [DOI] [PubMed] [Google Scholar]
- 167. Kakishita K.; Elwan M. A.; Nakao N.; Itakura T.; Sakuragawa N. Human amniotic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson’s disease: A potential source of donor for transplantation therapy. Exp. Neurol. 165(1):27–34; 2000. [DOI] [PubMed] [Google Scholar]
- 168. Kakishita K.; Nakao N.; Sakuragawa N.; Itakura T. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 980(1):48–56; 2003. [DOI] [PubMed] [Google Scholar]
- 169. Kang K. S.; Kim S. W.; Oh Y. H.; Yu J. W.; Kim K. Y.; Park H. K.; Song C. H.; Han H. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: A case study. Cytotherapy 7(4):368–373; 2005. [DOI] [PubMed] [Google Scholar]
- 170. Kao C. H.; Chen S. H.; Chio C. C.; Lin M. T. Human umbilical cord blood-derived CD34+ cells may attenuate spinal cord injury by stimulating vascular endothelial and neurotrophic factors. Shock 29(1):49–55; 2008. [DOI] [PubMed] [Google Scholar]
- 171. Karussis D.; Kassis I.; Kurkalli B. G.; Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): A proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J. Neurol. Sci. 265(1–2):131–135; 2008. [DOI] [PubMed] [Google Scholar]
- 172. Katayama Y. Deep brain stimulation therapy: Control of human brain function by chronically implanted electrodes. No To Shinkei 52(4):297–305; 2000. [PubMed] [Google Scholar]
- 173. Kelly S.; Bliss T. M.; Shah A. K.; Sun G. H.; Ma M.; Foo W. C.; Masel J.; Yenari M. A.; Weissman I. L.; Uchida N.; Palmer T.; Steinberg G. K. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA 101(32):11839–11844; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kesslak J. P.; Nieto-Sampedro M.; Globus J.; Cotman C. W. Transplants of purified astrocytes promote behavioral recovery after frontal cortex ablation. Exp. Neurol. 92(2):377–390; 1986. [DOI] [PubMed] [Google Scholar]
- 175. Keyvan-Fouladi N.; Raisman G.; Li Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. J. Neurosci. 23(28):9428–9434; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kim S. U. Genetically engineered human neural stem cells for brain repair in neurological diseases. Brain Dev. 29(4):193–201; 2007. [DOI] [PubMed] [Google Scholar]
- 177. Kimiskidis V.; Sakellari I.; Tsimourtou V.; Kapina V.; Papagiannopoulos S.; Kazis D.; Vlaikidis N.; Anagnostopoulos A.; Fassas A. Autologous stem-cell transplantation in malignant multiple sclerosis: A case with a favorable long-term outcome. Mult. Scler. 14(2):278–283; 2008. [DOI] [PubMed] [Google Scholar]
- 178. Knoller N.; Auerbach G.; Fulga V.; Zelig G.; Attias J.; Bakimer R.; Marder J. B.; Yoles E.; Belkin M.; Schwartz M.; Hadani M. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: Phase I study results. J. Neurosurg. Spine 3(3):173–181; 2005. [DOI] [PubMed] [Google Scholar]
- 179. Kocsis J. D.; Lankford K. L.; Sasaki M.; Radtke C. Unique in vivo properties of olfactory ensheathing cells that may contribute to neural repair and protection following spinal cord injury. Neurosci. Lett. 456(3):137–142; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Koda M.; Okada S.; Nakayama T.; Koshizuka S.; Kamada T.; Nishio Y.; Someya Y.; Yoshinaga K.; Okawa A.; Moriya H.; Yamazaki M. Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport 16(16):1763–1767; 2005. [DOI] [PubMed] [Google Scholar]
- 181. Kondziolka D.; Steinberg G. K.; Wechsler L.; Meltzer C. C.; Elder E.; Gebel J.; Decesare S.; Jovin T.; Zafonte R.; Lebowitz J.; Flickinger J. C.; Tong D.; Marks M. P.; Jamieson C.; Luu D.; Bell-Stephens T.; Teraoka J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J. Neurosurg. 103(1):38–45; 2005. [DOI] [PubMed] [Google Scholar]
- 182. Kondziolka D.; Wechsler L.; Goldstein S.; Meltzer C.; Thulborn K. R.; Gebel J.; Jannetta P.; DeCesare S.; Elder E. M.; McGrogan M.; Reitman M. A.; Bynum L. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55(4):565–569; 2000. [DOI] [PubMed] [Google Scholar]
- 183. Kong X. Y.; Cai Z.; Pan L.; Zhang L.; Shu J.; Dong Y. L.; Yang N.; Li Q.; Huang X. J.; Zuo P. P. Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice. Brain Res. 1205:108–115; 2008. [DOI] [PubMed] [Google Scholar]
- 184. Kordower J. H.; Chu Y.; Hauser R. A.; Freeman T. B.; Olanow C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14(5):504–506; 2008. [DOI] [PubMed] [Google Scholar]
- 185. Koshizuka S.; Okada S.; Okawa A.; Koda M.; Murasawa M.; Hashimoto M.; Kamada T.; Yoshinaga K.; Murakami M.; Moriya H.; Yamazaki M. Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J. Neuropathol. Exp. Neurol. 263(1):64–67; 2004. [DOI] [PubMed] [Google Scholar]
- 186. Kosuga M.; Sasaki K.; Tanabe A.; Li X. K.; Okawa H.; Ogino I.; Okuda O.; Arai H.; Sakuragawa N.; Kamata Y.; Azuma N.; Suzuki S.; Yamada M.; Okuyama T. Engraftment of genetically engineered amniotic epithelial cells corrects lysosomal storage in multiple areas of the brain in mucopolysaccharidosis type VII mice. Mol. Ther. 3(2):139–148; 2001. [DOI] [PubMed] [Google Scholar]
- 187. Kromer L. F.; Cornbrooks C. J. Transplants of Schwann cell cultures promote axonal regeneration in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 82(18):6330–6334; 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kubo A.; Yoshida T.; Kobayashi N.; Yokoyama T.; Mimura T.; Nishiguchi T.; Higashida T.; Yamamoto I.; Kanno H. Efficient generation of dopamine neuron-like cells from skin-derived precursors with a synthetic peptide derived from von Hippel-Lindau protein. Stem Cells Dev. 18(10):1523–1532; 2009. [DOI] [PubMed] [Google Scholar]
- 189. Kuh S. U.; Cho Y. E.; Yoon D. H.; Kim K. N.; Ha Y. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir. (Wien) 147(9):985–992; 2005. [DOI] [PubMed] [Google Scholar]
- 190. Kuwabara S.; Misawa S.; Kanai K.; Kikkawa Y.; Nishimura M.; Nakaseko C.; Cho R. K.; Hattori T. Autologous peripheral blood stem cell transplantation for POEMS syndrome. Neurology 66(1):105–107; 2006. [DOI] [PubMed] [Google Scholar]
- 191. Kuwabara S.; Misawa S.; Kanai K.; Suzuki Y.; Kikkawa Y.; Sawai S.; Hattori T.; Nishimura M.; Nakaseko C. Neurologic improvement after peripheral blood stem cell transplantation in POEMS syndrome. Neurology 71(21):1691–1695; 2008. [DOI] [PubMed] [Google Scholar]
- 192. Kyung K. S.; Ho C. W.; Kwan C. B. Potential therapeutic clue of skin-derived progenitor cells following cytokine-mediated signal overexpressed in injured spinal cord. Tissue Eng. 13(6):1247–1258; 2007. [DOI] [PubMed] [Google Scholar]
- 193. Labbe R.; Firl A. Jr.; Mufson E. J.; Stein D. G. Fetal brain transplant: Reduction of cognitive deficits in rats with frontal cortex lesions. Science 221(4609):470–472; 1983. [DOI] [PubMed] [Google Scholar]
- 194. Lacza Z.; Horváth E. M.; Komjáti K.; Hortobágyi T.; Szabó C.; Busija D. W. PARP inhibition improves the effectiveness of neural stem cell transplantation in experimental brain trauma. Int. J. Mol. Med. 12(2):153–159; 2003. [PubMed] [Google Scholar]
- 195. Langmoen I. A.; Ohlsson M.; Westerlund U.; Svensson M. A new tool in restorative neurosurgery: creating niches for neuronal stem cells. Neurosurgery 52(5):1150–1153; 2003. [PubMed] [Google Scholar]
- 196. Lankford K. L.; Sasaki M.; Radtke C.; Kocsis J. D. Olfactory ensheathing cells exhibit unique migratory, phagocytic, and myelinating properties in the X-irradiated spinal cord not shared by Schwann cells. Glia 56(15):1664–1678; 2008. [DOI] [PubMed] [Google Scholar]
- 197. Lavdas A. A.; Papastefanaki F.; Thomaidou D.; Matsas R. Schwann cell transplantation for CNS repair. Curr. Med. Chem. 15(2):151–160; 2008. [DOI] [PubMed] [Google Scholar]
- 198. Leanza G.; Nikkhah G.; Nilsson O. G.; Wiley R. G.; Björklund A. Extensive reinnervation of the hippocampus by embryonic basal forebrain cholinergic neurons grafted into the septum of neonatal rats with selective cholinergic lesions. J. Comp. Neurol. 373(3):355–357; 1996. [DOI] [PubMed] [Google Scholar]
- 199. Lee J. K.; Jin H. K.; Endo S.; Schuchman E. H.; Carter J. E.; Bae J. S. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells 28(2):329–343; 2010. [DOI] [PubMed] [Google Scholar]
- 200. Lee S. I.; Kim B. G.; Hwang D. H.; Kim H. M.; Kim S. U. Overexpression of Bcl-XL in human neural stem cells promotes graft survival and functional recovery following transplantation in spinal cord injury. J. Neurosci. Res. 87(14):3186–3197; 2009. [DOI] [PubMed] [Google Scholar]
- 201. Lee S. T.; Chu K.; Jung K. H.; Kim S. J.; Kim D. H.; Kang K. M.; Hong N. H.; Kim J. H.; Ban J. J.; Park H. K.; Kim S. U.; Park C. G.; Lee S. K.; Kim M.; Roh J. K. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 131(Pt. 3):616–629; 2008. [DOI] [PubMed] [Google Scholar]
- 202. Lee S. T.; Chu K.; Park J. E.; Lee K.; Kang L.; Kim S. U.; Kim M. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci. Res. 52(3):243–249; 2005. [DOI] [PubMed] [Google Scholar]
- 203. Leung J. Y.; Chapman J. A.; Harris J. A.; Hale D.; Chung R. S.; West A. K.; Chuah M. I. Olfactory ensheathing cells are attracted to, and can endocytose, bacteria. Cell. Mol. Life Sci. 65(17):2732–2739; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Levi-Montalcini R.; Hamburger V. Selective growth-stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zool. 116:321–361; 1951. [DOI] [PubMed] [Google Scholar]
- 205. Li J. Y.; Englund E.; Holton J. L.; Soulet D.; Hagell P.; Lees A. J.; Lashley T.; Quinn N. P.; Rehncrona S.; Björklund A.; Widner H.; Revesz T.; Lindvall O.; Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14(5):501–503; 2008. [DOI] [PubMed] [Google Scholar]
- 206. Li W. Y.; Choi Y. J.; Lee P. H.; Huh K.; Kang Y. M.; Kim H. S.; Ahn Y. H.; Lee G.; Bang O. Y. Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 17(9):1045–1059; 2008. [DOI] [PubMed] [Google Scholar]
- 207. Li Y.; Chen J.; Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 10(1):31–40; 2001. [PubMed] [Google Scholar]
- 208. Li Y.; Chen J.; Wang L.; Lu M.; Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56(12):1666–1672; 2001. [DOI] [PubMed] [Google Scholar]
- 209. Li Y.; Chen J.; Wang L.; Zhang L.; Lu M.; Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci. Lett. 316(2):67–70; 2001. [DOI] [PubMed] [Google Scholar]
- 210. Li Y.; Chen J.; Zhang C. L.; Wang L.; Lu D.; Katakowski M.; Gao Q.; Shen L. H.; Zhang J.; Lu M.; Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49(3):407–417; 2005. [DOI] [PubMed] [Google Scholar]
- 211. Li Y.; Field P. M.; Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277(5334):2000–2002; 1997. [DOI] [PubMed] [Google Scholar]
- 212. Li Y.; Field P. M.; Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J. Neurosci. 18(24):10514–10524; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Li Y.; Li D.; Khaw P. T.; Raisman G. Transplanted olfactory ensheathing cells incorporated into the optic nerve head ensheathe retinal ganglion cell axons: Possible relevance to glaucoma. Neurosci. Lett. 440(3):251–254; 2008. [DOI] [PubMed] [Google Scholar]
- 214. Li Y.; Raisman G. Schwann cells induce sprouting in motor and sensory axons in the adult rat spinal cord. J. Neurosci. 14(7):4050–4063; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Li Y.; Sauve Y.; Li D.; Lund R. D.; Raisman G. Transplanted olfactory ensheathing cells promote regeneration of cut adult rat optic nerve axons. J. Neurosci. 23(21):7783–7788; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Li Y. J.; Low W. C. Intra-retrosplenial cortical grafts of cholinergic neurons: Functional incorporation and restoration of high affinity choline uptake. Neurochem. Res. 22(5):589–595; 1997. [DOI] [PubMed] [Google Scholar]
- 217. Li Y. J.; Low W. C. Intraretrosplenial cortical grafts of fetal cholinergic neurons and the restoration of spatial memory function. Cell Transplant. 6(1):85–93; 1997. [DOI] [PubMed] [Google Scholar]
- 218. Liang J.; Zhang H.; Hua B.; Wang H.; Wang J.; Han Z.; Sun L. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult. Scler. 15(5):644–646; 2009. [DOI] [PubMed] [Google Scholar]
- 219. Liao W.; Xie J.; Zhong J.; Liu Y.; Du L.; Zhou B.; Xu J.; Liu P.; Yang S.; Wang J.; Han Z.; Han Z. C. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation 87(3):350–359; 2009. [DOI] [PubMed] [Google Scholar]
- 220. Liao W.; Zhong J.; Yu J.; Xie J.; Liu Y.; Du L.; Yang S.; Liu P.; Xu J.; Wang J.; Han Z.; Han Z. C. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: Implications of anti-inflammation and angiogenesis. Cell. Physiol. Biochem. 24(3–4):307–316; 2009. [DOI] [PubMed] [Google Scholar]
- 221. Liberson W. T. More on restorative neurosurgery. Electromyogr. Clin. Neurophysiol. 27(6–7):323–325; 1987. [PubMed] [Google Scholar]
- 222. Liepert J. Pharmacotherapy in restorative neurology. Curr. Opin. Neurol. 21(6):639–643; 2008. [DOI] [PubMed] [Google Scholar]
- 223. Lima C.; Pratas-Vital J.; Escada P.; Hasse-Ferreira A.; Capucho C.; Peduzzi J. D. Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. J. Spinal Cord Med. 29(3):191–203; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Lindsay S. L.; Riddell J. S.; Barnett S. C. Olfactory mucosa for transplant-mediated repair: A complex tissue for a complex injury? Glia 58(2):125–134; 2010. [DOI] [PubMed] [Google Scholar]
- 225. Lindvall O.; Backlund E. O.; Farde L.; Sedvall G.; Freedman R.; Hoffer B.; Nobin A.; Seiger A.; Olson L. Transplantation in Parkinson’s disease: Two cases of adrenal medullary grafts to the putamen. Ann. Neurol. 22(4):457–468; 1987. [DOI] [PubMed] [Google Scholar]
- 226. Lindvall O.; Rehncrona S.; Brundin P.; Gustavii B.; Astedt B.; Widner H.; Lindholm T.; Björklund A.; Leenders K. L.; Rothwell J. C. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch. Neurol. 46(6):615–631; 1989. [DOI] [PubMed] [Google Scholar]
- 227. Liste I.; García-García E.; Martínez-Serrano A. The generation of dopaminergic neurons by human neural stem cells is enhanced by Bcl-XL, both in vitro and in vivo. J. Neurosci. 24(48):10786–10795; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Liu B.; Hong J. S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 304(1):1–7; 2003. [DOI] [PubMed] [Google Scholar]
- 229. Liu S.; Qu Y.; Stewart T. J.; Howard M. J.; Chakrabortty S.; Holekamp T. F.; McDonald J. W. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc. Natl. Acad. Sci. USA 97(11):6126–6131; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Liu T.; Wu J.; Huang Q.; Hou Y.; Jiang Z.; Zang S.; Guo L. Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock 29(5):603–611; 2008. [DOI] [PubMed] [Google Scholar]
- 231. López-González R.; Kunckles P.; Velasco I. Transient recovery in a rat model of familial amyotrophic lateral sclerosis after transplantation of motor neurons derived from mouse embryonic stem cells. Cell Transplant. 18(10–11):1171–1181; 2009. [DOI] [PubMed] [Google Scholar]
- 232. Lopez-Vales R.; Garcia-Alias G.; Fores J.; Navarro X.; Verdu E. Increased expression of cyclo-oxygenase 2 and vascular endothelial growth factor in lesioned spinal cord by transplanted olfactory ensheathing cells. J. Neurotrauma 21(8):1031–1043; 2004. [DOI] [PubMed] [Google Scholar]
- 233. López-Vales R.; García-Alías G.; Forés J.; Vela J. M.; Navarro X.; Verdú E. Transplanted olfactory ensheathing cells modulate the inflammatory response in the injured spinal cord. Neuron Glia Biol. 1(3):201–209; 2004. [DOI] [PubMed] [Google Scholar]
- 234. Löscher W.; Ebert U.; Lehmann H.; Rosenthal C.; Nikkhah G. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J. Neurosci. Res. 51(2):196–209; 1998. [DOI] [PubMed] [Google Scholar]
- 235. Low C. B.; Liou Y. C.; Tang B. L. Neural differentiation and potential use of stem cells from the human umbilical cord for central nervous system transplantation therapy. J. Neurosci. Res. 86(8):1670–1679; 2008. [DOI] [PubMed] [Google Scholar]
- 236. Lu D.; Mahmood A.; Wang L.; Li Y.; Lu M.; Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport 12(3):559–563; 2001. [DOI] [PubMed] [Google Scholar]
- 237. Lu D.; Sanberg P. R.; Mahmood A.; Li Y.; Wang L.; Sanchez-Ramos J.; Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 11(3):275–281; 2002. [PubMed] [Google Scholar]
- 238. Lu J.; Feron F.; Ho S. M.; Mackay-Sim A.; Waite P. M. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. 889(1–2):344–357; 2001. [DOI] [PubMed] [Google Scholar]
- 239. Lu J.; Feron F.; Mackay-Sim A.; Waite P. M. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain 125(Pt. 1):14–21; 2002. [DOI] [PubMed] [Google Scholar]
- 240. Lu S. Y.; Pixley S. K.; Emerich D. F.; Lehman M. N.; Norman A. B. Effect of fetal striatal and astrocyte transplants into unilateral excitotoxin-lesioned striatum. J. Neural Transplant. Plast. 4(4):279–287; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lu Y.; Hui G. Z.; Wu Z. Y.; Guo L. H.; Ji X. H.; Wu X. Treatment of traumatic brain injury in rats with transplantation of human amniotic cells. Chin. Med. J. (Engl.). 119(21):1843–1845; 2006. [PubMed] [Google Scholar]
- 242. Luque J. M.; Giménez y Ribotta M. Neural stem cells and the quest for restorative neurology. Histol. Histopathol. 19(1):271–280; 2004. [DOI] [PubMed] [Google Scholar]
- 243. Mackay-Sim A.; Féron F.; Cochrane J.; Bassingthwaighte L.; Bayliss C.; Davies W.; Fronek P.; Gray C.; Kerr G.; Licina P.; Nowitzke A.; Perry C.; Silburn P. A.; Urquhart S.; Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain 131(Pt. 9):2376–2386; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Madrazo I.; León V.; Torres C.; Aguilera M. C.; Varela G.; Alvarez F.; Fraga A.; Drucker-Colín R.; Ostrosky F.; Skurovich M. Transplantation of fetal substantia nigra and adrenal medulla to the caudate nucleus in two patients with Parkinson’s disease. N. Engl. J. Med. 318(1):51; 1988. [DOI] [PubMed] [Google Scholar]
- 245. Mahmood A.; Lu D.; Wang L.; Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J. Neurotrauma 19(12):1609–1617; 2002. [DOI] [PubMed] [Google Scholar]
- 246. Mancardi G. L.; Murialdo A.; Rossi P.; Gualandi F.; Martino G.; Marmont A.; Ciceri F.; Schenone A.; Parodi R. C.; Capello E.; Comi G.; Uccelli A. Autologous stem cell transplantation as rescue therapy in malignant forms of multiple sclerosis. Mult. Scler. 11(3):367–371; 2005. [DOI] [PubMed] [Google Scholar]
- 247. Marchionini D. M.; Subramanian T.; Bumette B. Dopaminergic properties of retinal pigmented epithelial cells attached to microcarriers (RPE-M) transplanted into parkinsonian animals. Exp. Neurol. 3:559–608; 1999. [Google Scholar]
- 248. Marquez Y. D.; Wang M. Y.; Liu C. Y. Cellular signaling in neural stem cells: Implications for restorative neurosurgery. Neurosurg. Focus 19(3):E2; 2005. [DOI] [PubMed] [Google Scholar]
- 249. Martin D.; Schoenen J.; Delree P.; Rigo J. M.; Rogister B.; Leprince P.; Moonen G. Syngeneic grafting of adult rat DRG-derived Schwann cells to the injured spinal cord. Brain Res. Bull. 30(3–4):507–514; 1993. [DOI] [PubMed] [Google Scholar]
- 250. Martin L. J.; Liu Z. Adult olfactory bulb neural precursor cell grafts provide temporary protection from motor neuron degeneration, improve motor function, and extend survival in amyotrophic lateral sclerosis mice. J. Neuropathol. Exp. Neurol. 66(11):1002–1018; 2007. [DOI] [PubMed] [Google Scholar]
- 251. Martinez H. R.; Gonzalez-Garza M. T.; Moreno-Cuevas J. E.; Caro E.; Gutierrez-Jimenez E.; Segura J. J. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy 11(1):26–34; 2009. [DOI] [PubMed] [Google Scholar]
- 252. Marutle A.; Ohmitsu M.; Nilbratt M.; Greig N. H.; Nordberg A.; Sugaya K. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc. Natl. Acad. Sci. USA 104(30):12506–12511; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Matsuda R.; Yoshikawa M.; Kimura H.; Ouji Y.; Nakase H.; Nishimura F.; Nonaka J.; Toriumi H.; Yamada S.; Nishiofuku M.; Moriya K.; Ishizaka S.; Nakamura M.; Sakaki T. Cotransplantation of mouse embryonic stem cells and bone marrow stromal cells following spinal cord injury suppresses tumor development. Cell Transplant. 18(1):39–54; 2009. [PubMed] [Google Scholar]
- 254. Matsuyama Y.; Mimatsu K.; Sugimura T.; Kondou S.; Iwata H.; Isobe K. Reinnervation of peripheral nerve segments implanted into the hemisected spinal cord estimated by transgenic mice. Paraplegia 33(7):381–386; 1995. [DOI] [PubMed] [Google Scholar]
- 255. Mazzini L.; Mareschi K.; Ferrero I.; Vassallo E.; Oliveri G.; Nasuelli N.; Oggioni G. D.; Testa L.; Fagioli F. Stem cell treatment in amyotrophic lateral sclerosis. J. Neurol. Sci. 265(1–2):78–83; 2008. [DOI] [PubMed] [Google Scholar]
- 256. McDonald J. W.; Liu X. Z.; Qu Y.; Liu S.; Mickey S. K.; Turetsky D.; Gottlieb D. I.; Choi D. W. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 5:1410–1412; 1999. [DOI] [PubMed] [Google Scholar]
- 257. McKay B. S.; Goodman B.; Falk T.; Sherman S. J. Retinal pigment epithelial cell transplantation could provide trophic support in Parkinson’s disease: Results from an in vitro model system. Exp. Neurol. 201(1):234–243; 2006. [DOI] [PubMed] [Google Scholar]
- 258. Meier C.; Middelanis J.; Wasielewski B.; Neuhoff S.; Roth-Haerer A.; Gantert M.; Dinse H. R.; Dermietzel R.; Jensen A. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr. Res. 59(2):244–249; 2006. [DOI] [PubMed] [Google Scholar]
- 259. Mendez I.; Viñuela A.; Astradsson A.; Mukhida K.; Hallett P.; Robertson H.; Tierney T.; Holness R.; Dagher A.; Trojanowski J. Q.; Isacson O. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat. Med. 14(5):507–509; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Miljan E. A.; Hines S. J.; Pande P.; Corteling R. L.; Hicks C.; Zbarsky V.; Umachandran M.; Sowinski P.; Richardson S.; Tang E.; Wieruszew M.; Patel S.; Stroemer P.; Sinden J. D. Implantation of c-mycER TAM immortalized human mesencephalic-derived clonal cell lines ameliorates behavior dysfunction in a rat model of Parkinson’s disease. Stem Cells Dev. 18(2):307–319; 2009. [DOI] [PubMed] [Google Scholar]
- 261. Miller R. H.; Bai L. Cellular approaches for stimulating CNS remyelination. Regen. Med. 2(5):817–829; 2007. [DOI] [PubMed] [Google Scholar]
- 262. Ming M.; Li X.; Fan X.; Yang D.; Li L.; Chen S.; Gu Q.; Le W. Retinal pigment epithelial cells secrete neurotrophic factors and synthesize dopamine: Possible contribution to therapeutic effects of RPE cell transplantation in Parkinson’s disease. J. Transl. Med. 7:53; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Modo M.; Rezaie P.; Heuschling P.; Patel S.; Male D. K.; Hodges H. Transplantation of neural stem cells in a rat model of stroke: assessment of short-term graft survival and acute host immunological response. Brain Res. 958(1):70–82; 2002. [DOI] [PubMed] [Google Scholar]
- 264. Mothe A. J.; Kulbatski I.; Parr A.; Mohareb M.; Tator C. H. Adult spinal cord stem/progenitor cells transplanted as neurospheres preferentially differentiate into oligodendrocytes in the adult rat spinal cord. Cell Transplant. 17(7):735–751; 2008. [DOI] [PubMed] [Google Scholar]
- 265. Mothe A. J.; Tator C. H. Transplanted neural stem/progenitor cells generate myelinating oligodendrocytes and Schwann cells in spinal cord demyelination and dysmyelination. Exp. Neurol. 213(1):176–190; 2008. [DOI] [PubMed] [Google Scholar]
- 266. Moviglia G. A.; Varela G.; Brizuela J. A.; Moviglia Brandolino M. T.; Farina P.; Etchegaray G.; Piccone S.; Hirsch J.; Martinez G.; Marino S.; Deffain S.; Coria N.; Gonz?les A.; Sztanko M.; Salas-Zamora P.; Previgliano I.; Aingel V.; Farias J.; Gaeta C. A.; Saslavsky J.; Blasseti N. Case report on the clinical results of a combined cellular therapy for chronic spinal cord injured patients. Spinal Cord 47(6):499–503; 2009. [DOI] [PubMed] [Google Scholar]
- 267. Muñoz-Quiles C.; Santos-Benito F. F.; Llamusí M. B.; Ramón-Cueto A. Chronic spinal injury repair by olfactory bulb ensheathing glia and feasibility for autologous therapy. J. Neuropathol. Exp. Neurol. 68(12):1294–1308; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Muramatsu D.; Sato Y.; Hishiyama S.; Miyamoto Y.; Hisatsune T. Transplantation of GABAergic neurons into adult mouse neocortex. Exp. Neurol. 194(1):1–11; 2005. [DOI] [PubMed] [Google Scholar]
- 269. Nadri S.; Soleimani M.; Mobarra Z.; Amini S. Expression of dopamine-associated genes on conjunctiva stromal-derived human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 377(2):423–428; 2008. [DOI] [PubMed] [Google Scholar]
- 270. Nan Z.; Grande A.; Sanberg C. D.; Sanberg P. R.; Low W. C. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann. NY Acad. Sci. 1049:84–96; 2005. [DOI] [PubMed] [Google Scholar]
- 271. Navarro X.; Valero A.; Gudino G.; Fores J.; Rodriguez F. J.; Verdu E.; Pascual R.; Cuadras J.; Nieto-Sampedro M. Ensheathing glia transplants promote dorsal root regeneration and spinal reflex restitution after multiple lumbar rhizotomy. Ann. Neurol. 45(2):207–215; 1999. [DOI] [PubMed] [Google Scholar]
- 272. Newman M. B.; Bakay R. A. Therapeutic potentials of human embryonic stem cells in Parkinson’s disease. Neurotherapeutics 5(2):237–251; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Nikkhah G.; Rosenthal C.; Falkenstein G.; Roedter A.; Papazoglou A.; Brandis A. Microtransplantation of dopaminergic cell suspensions: Further characterization and optimization of grafting parameters. Cell Transplant. 18(2):119–133; 2009. [DOI] [PubMed] [Google Scholar]
- 274. Nikolic W. V.; Hou H.; Town T.; Zhu Y.; Giunta B.; Sanberg C. D.; Zeng J.; Luo D.; Ehrhart J.; Mori T.; Sanberg P. R.; Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 17(3):423–439; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Nishida K.; Tanaka N.; Nakanishi K.; Kamei N.; Hamasaki T.; Yanada S.; Mochizuki Y.; Ochi M. Magnetic targeting of bone marrow stromal cells into spinal cord: Through cerebrospinal fluid. Neuroreport 17(12):1269–1272; 2006. [DOI] [PubMed] [Google Scholar]
- 276. Nishimura K.; Nakagawa T.; Ono K.; Ogita H.; Sakamoto T.; Yamamoto N.; Okita K.; Yamanaka S.; Ito J. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport 20(14):1250–1254; 2009. [DOI] [PubMed] [Google Scholar]
- 277. Nornes H.; Björklund A.; Stenevi U. Reinnervation of the denervated adult spinal cord of rats by intraspinal transplants of embryonic brain stem neurons. Cell Tissue Res. 230(1):15–35; 1983. [DOI] [PubMed] [Google Scholar]
- 278. Numano F.; Inoue A.; Enomoto M.; Shinomiya K.; Okawa A.; Okabe S. Critical involvement of Rho GTPase activity in the efficient transplantation of neural stem cells into the injured spinal cord. Mol. Brain 2(1):37; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Nygren L. G.; Olson L.; Seiger A. Monoaminergic re-innervation of the transected spinal cord by homologous fetal brain grafts. Brain Res. 129(2):227–235; 1977. [DOI] [PubMed] [Google Scholar]
- 280. Okawa H.; Okuda O.; Arai H.; Sakuragawa N.; Sato K. Amniotic epithelial cells transform into neuron-like cells in the ischemic brain. Neuroreport 12(18):4003–4007; 2001. [DOI] [PubMed] [Google Scholar]
- 281. O’Malley J.; Woltjen K.; Kaji K. New strategies to generate induced pluripotent stem cells. Curr. Opin. Biotechnol. 20(5):516–521; 2009. [DOI] [PubMed] [Google Scholar]
- 282. Oudega M.; Xu X. M. Schwann cell transplantation for repair of the adult spinal cord. J. Neurotrauma 23(3–4):453–467; 2006. [DOI] [PubMed] [Google Scholar]
- 283. Pal R.; Venkataramana N. K.; Bansal A.; Balaraju S.; Jan M.; Chandra R.; Dixit A.; Rauthan A.; Murgod U.; Totey S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 11(7):897–911; 2009. [DOI] [PubMed] [Google Scholar]
- 284. Park D. H.; Borlongan C. V.; Willing A. E.; Eve D. J.; Cruz L. E.; Sanberg C. D.; Chung Y. G.; Sanberg P. R. Human umbilical cord blood cell grafts for brain ischemia. Cell Transplant. 18(9):985–998; 2009. [DOI] [PubMed] [Google Scholar]
- 285. Park D. H.; Eve D. J.; Musso J. 3rd; Klasko S. K.; Cruz E.; Borlongan C. V.; Sanberg P. R. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 18(5):693–702; 2009. [DOI] [PubMed] [Google Scholar]
- 286. Park D. H.; Eve D. J.; Sanberg P. R.; Musso J. 3rd; Bachstetter A. D.; Wolfson A.; Schlunk A.; Baradez M. O.; Sinden J. D.; Bickford P. C.; Gemma C. Increased neuronal proliferation in the dentate gyrus of aged rats following neural stem cell implantation. Stem Cells Dev. 19(2):175–180; 2010. [DOI] [PubMed] [Google Scholar]
- 287. Park H. C.; Shim Y. S.; Ha Y.; Yoon S. H.; Park S. R.; Choi B. H.; Park H. S. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 11(5–6):913–922; 2005. [DOI] [PubMed] [Google Scholar]
- 288. Park K. I. Transplantation of neural stem cells: Cellular & gene therapy for hypoxicischemic brain injury. Yonsei Med. J. 41(6):825–835; 2000. [DOI] [PubMed] [Google Scholar]
- 289. Pascual J. I.; Gudino-Cabrera G.; Insausti R.; Nieto-Sampedro M. Spinal implants of olfactory ensheathing cells promote axon regeneration and bladder activity after bilateral lumbosacral dorsal rhizotomy in the adult rat. J. Urol. 167(3):1522–1526; 2002. [PubMed] [Google Scholar]
- 290. Patel A. N.; Park E.; Kuzman M.; Benetti F.; Silva F. J.; Allickson J. G. Multipotent menstrual blood stromal stem cells: Isolation, characterization, and differentiation. Cell Transplant. 17(3):303–311; 2008. [DOI] [PubMed] [Google Scholar]
- 291. Patel U.; Bernstein J. J. Growth, differentiation, and viability of fetal rat cortical and spinal cord implants into adult rat spinal cord. J. Neurosci. Res. 9(3):303–310; 1983. [DOI] [PubMed] [Google Scholar]
- 292. Pellitteri R.; Spatuzza M.; Russo A.; Stanzani S. Olfactory ensheathing cells exert a trophic effect on the hypothalamic neurons in vitro. Neurosci. Lett. 417(1):24–29; 2007. [DOI] [PubMed] [Google Scholar]
- 293. Pellitteri R.; Spatuzza M.; Russo A.; Zaccheo D.; Stanzani S. Olfactory ensheathing cells represent an optimal substrate for hippocampal neurons: An in vitro study. Int. J. Dev. Neurosci. 27(5):453–458; 2009. [DOI] [PubMed] [Google Scholar]
- 294. Perlow M. J.; Freed W. J.; Hoffer B. J.; Seiger A.; Olson L.; Wyatt R. J. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 204(4393):643–647; 1979. [DOI] [PubMed] [Google Scholar]
- 295. Peterson D. A. Umbilical cord blood cells and brain stroke injury: Bringing in fresh blood to address an old problem. J. Clin. Invest. 114(3):312–314; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Pizzorusso T.; Fagiolini M.; Fabris M.; Ferrari G.; Maffei L. Schwann cells transplanted in the lateral ventricles prevent the functional and anatomical effects of monocular deprivation in the rat. Proc. Natl. Acad. Sci. USA 91(7):2572–2576; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Plant G. W.; Christensen C. L.; Oudega M.; Bunge M. B. Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the contused adult rat spinal cord. J. Neurotrauma 20(1):1–16; 2003. [DOI] [PubMed] [Google Scholar]
- 298. Pluchino S.; Gritti A.; Blezer E.; Amadio S.; Brambilla E.; Borsellino G.; Cossetti C.; Del, Carro U.; Comi G.; ’t Hart B.; Vescovi A.; Martino G. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann. Neurol. 66(3):343–354; 2009. [DOI] [PubMed] [Google Scholar]
- 299. Pluchino S.; Quattrini A.; Brambilla E.; Gritti A.; Salani G.; Dina G.; Galli R.; Del Carro U.; Amadio S.; Bergami A.; Furlan R.; Comi G.; Vescovi A. L.; Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 422(6933):688–694; 2003. [DOI] [PubMed] [Google Scholar]
- 300. Pluchino S.; Zanotti L.; Brambilla E.; Rovere-Querini P.; Capobianco A.; Alfaro-Cervello C.; Salani G.; Cossetti C.; Borsellino G.; Battistini L.; Ponzoni M.; Doglioni C.; Garcia-Verdugo J. M.; Comi G.; Manfredi A. A.; Martino G. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PLoS One 4(6):e5959; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Polentes J.; Stamegna J. C.; Nieto-Sampedro M.; Gauthier P. Phrenic rehabilitation and diaphragm recovery after cervical injury and transplantation of olfactory ensheathing cells. Neurobiol. Dis. 16(3):638–653; 2004. [DOI] [PubMed] [Google Scholar]
- 302. Polezhaev L. V.; Alexandrova M. A. Transplantation of embryonic brain tissue into the brain of adult rats after hypoxic hypoxia. J. Hirnforsch. 25(1):99–106; 1984. [PubMed] [Google Scholar]
- 303. Pollock K.; Stroemer P.; Patel S.; Stevanato L.; Hope A.; Miljan E.; Dong Z.; Hodges H.; Price J.; Sinden J. D. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol. 199(1):143–155; 2006. [DOI] [PubMed] [Google Scholar]
- 304. Portaccio E.; Amato M. P.; Siracusa G.; Pagliai F.; Sorbi S.; Guidi S.; Bosi A.; Saccardi R. Autologous hematopoietic stem cell transplantation for very active relapsing-remitting multiple sclerosis: Report of two cases. Mult. Scler. 13(5):676–678; 2007. [DOI] [PubMed] [Google Scholar]
- 305. Prewitt C. M.; Niesman I. R.; Kane C. J.; Houlé J. D. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp. Neurol. 148(2):433–443; 1997. [DOI] [PubMed] [Google Scholar]
- 306. Prieto M.; Chauvet N.; Alonso G. Tanycytes transplanted into the adult rat spinal cord support the regeneration of lesioned axons. Exp. Neurol. 161(1):27–37; 2000. [DOI] [PubMed] [Google Scholar]
- 307. Qu R.; Li Y.; Gao Q.; Shen L.; Zhang J.; Liu Z.; Chen X.; Chopp M. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology 27(4):355–363; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Rabinovich S. S.; Seledtsov V. I.; Banul N. V.; Poveshchenko O. V.; Senyukov V. V.; Astrakov S. V.; Samarin D. M.; Taraban V. Y. Cell therapy of brain stroke. Bull. Exp. Biol. Med. 139(1):126–128; 2005. [DOI] [PubMed] [Google Scholar]
- 309. Rabinovich S. S.; Seledtsov V. I.; Poveschenko O. V.; Senuykov V. V.; Taraban V. Y.; Yarochno V. I.; Kolosov N. G.; Savchenko S. A.; Kozlov V. A. Transplantation treatment of spinal cord injury patients. Biomed. Pharmacother. 57(9):428–433; 2003. [DOI] [PubMed] [Google Scholar]
- 310. Radtke C.; Akiyama Y.; Brokaw J.; Lankford K. L.; Wewetzer K.; Fodor W. L.; Kocsis J. D. Remyelination of the nonhuman primate spinal cord by transplantation of H-transferase transgenic adult pig olfactory ensheathing cells. FASEB J. 18(2):335–357; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Ramer L. M.; Au E.; Richter M. W.; Liu J.; Tetzlaff W.; Roskams A. J. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J. Comp. Neurol. 473(1):1–15; 2004. [DOI] [PubMed] [Google Scholar]
- 312. Ramón-Cueto A.; Cordero M. I.; Santos-Benito F. F.; Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25(2):425–435; 2000. [DOI] [PubMed] [Google Scholar]
- 313. Ramón-Cueto A.; Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp. Neurol. 127(2):232–244; 1994. [DOI] [PubMed] [Google Scholar]
- 314. Ramón-Cueto A.; Plant G. W.; Avila J.; Bunge M. B. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J. Neurosci. 18(10):3803–3815; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Redmond D. E. Jr.; Elsworth J. D.; Roth R. H.; Leranth C.; Collier T. J.; Blanchard B.; Bjugstad K. B.; Samulski R. J.; Aebischer P.; Sladek J. R. Jr. Embryonic substantia nigra grafts in the mesencephalon send neurites to the host striatum in non-human primate after overexpression of GDNF. J. Comp. Neurol. 515(1):31–40; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Rehni A. K.; Singh N.; Jaggi A. S.; Singh M. Amniotic fluid derived stem cells ameliorate focal cerebral ischaemiareperfusion injury induced behavioural deficits in mice. Behav. Brain Res. 183(1):95–100; 2007. [DOI] [PubMed] [Google Scholar]
- 317. Reier P. J.; Bregman B. S.; Wujek J. R. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J. Comp. Neurol. 247(3):275–296; 1986. [DOI] [PubMed] [Google Scholar]
- 318. Reier P. J.; Perlow M. J.; Guth L. Development of embryonic spinal cord transplants in the rat. Brain Res. 312(2):201–219; 1983. [DOI] [PubMed] [Google Scholar]
- 319. Ren Z.; Zhang Y. Cells therapy for Parkinson’s disease—so close and so far away. Sci. China C. Life Sci. 52(7):610–614; 2009. [DOI] [PubMed] [Google Scholar]
- 320. Rice H. E.; Hsu E. W.; Sheng H.; Evenson D. A.; Freemerman A. J.; Safford K. M.; Provenzale J. M.; Warner D. S.; Johnson G. A. Superparamagnetic iron oxide labeling and transplantation of adipose-derived stem cells in middle cerebral artery occlusion-injured mice. AJR Am. J. Roentgenol. 188(4):1101–1108; 2007. [DOI] [PubMed] [Google Scholar]
- 321. Richardson R. M.; Varenika V.; Forsayeth J. R.; Bankiewicz K. S. Future applications: Gene therapy. Neurosurg. Clin. N. Am. 20(2):205–210; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Roberts T. J.; Price J.; Williams S. C.; Modo M. Preservation of striatal tissue and behavioral function after neural stem cell transplantation in a rat model of Huntington’s disease. Neuroscience 139(4):1187–1199; 2006. [DOI] [PubMed] [Google Scholar]
- 323. Ronaghi M.; Erceg S.; Moreno-Manzano V.; Stojkovic M. Challenges of stem cell therapy for spinal cord injury: Human embryonic stem cells, endogenous neural stem cells or induced pluripotent stem Cells? Stem Cells 28(1):93–99; 2010. [DOI] [PubMed] [Google Scholar]
- 324. Rosenbluth J.; Hasegawa M.; Shirasaki N.; Rosen C. L.; Liu Z. Myelin formation following transplantation of normal fetal glia into myelin-deficient rat spinal cord. J. Neurocytol. 19(5):718–730; 1990. [DOI] [PubMed] [Google Scholar]
- 325. Roszek K.; Komoszyński M. Regulation and direction of umbilical cord blood stem cells differentiation and their therapeutic application. Postepy. Hig. Med. Dosw (Online). 62:660-667; 2008. [PubMed] [Google Scholar]
- 326. Ryu H. H.; Lim J. H.; Byeon Y. E.; Park J. R.; Seo M. S.; Lee Y. W.; Kim W. H.; Kang K. S.; Kweon O. K. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J. Vet. Sci. 10(4):273–284; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Saberi H.; Moshayedi P.; Aghayan H. R.; Arjmand B.; Hosseini S. K.; Emami-Razavi S. H.; Rahimi-Movaghar V.; Raza M.; Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci. Lett. 443(1):46–50; 2008. [DOI] [PubMed] [Google Scholar]
- 328. Saccardi R.; Kozak T.; Bocelli-Tyndall C.; Fassas A.; Kazis A.; Havrdova E.; Carreras E.; Saiz A.; Löwenberg B.; te Boekhorst P. A.; Gualandio F.; Openshaw H.; Longo G.; Pagliai F.; Massacesi L.; Deconink E.; Ouyang J.; Nagore F. J.; Besalduch J.; Lisukov I. A.; Bonini A.; Merelli E.; Slavino S.; Gratwohl A.; Passweg J.; Tyndall A.; Steck A. J.; Andolina M.; Capobianco M.; Martin J. L.; Lugaresi A.; Meucci G.; Sáez R. A.; Clark R. E.; Fernandez M. N.; Fouillard L.; Herstenstein B.; Koza V.; Cocco E.; Baurmann H.; Mancardi G. L.; Autoimmune Diseases Working Party of EBMT. Autologous stem cell transplantation for progressive multiple sclerosis: Update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult. Scler. 12(6):814–823; 2006. [DOI] [PubMed] [Google Scholar]
- 329. Safford K. M.; Rice H. E. Stem cell therapy for neurologic disorders: Therapeutic potential of adipose-derived stem cells. Curr. Drug Targets. 6(1):57–62; 2005. [DOI] [PubMed] [Google Scholar]
- 330. Saiz A.; Blanco Y.; Berenguer J.; Gómez-Choco M.; Carreras E.; Arbizu T.; Graus F. Clinical outcome 6 years after autologous hematopoietic stem cell transplantation in multiple sclerosis. Neurologia 23(7):405–407; 2008. [PubMed] [Google Scholar]
- 331. Sanberg P. R.; Borlongan C. V.; Othberg A. I.; Saporta S.; Freeman T. B.; Cameron D. F. Testis-derived Sertoli cells have a trophic effect on dopamine neurons and alleviate hemiparkinsonism in rats. Nat. Med. 3(10):1129–1132; 1997. [DOI] [PubMed] [Google Scholar]
- 332. Sanberg P. R.; Borlongan C. V.; Saporta S.; Cameron D. F. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat. Biotechnol. 14(13):1692–1695; 1996. [DOI] [PubMed] [Google Scholar]
- 333. Sanberg P. R.; Park D. H.; Kuzmin-Nichols N.; Cruz E.; Hossne N. A. Jr.; Buffolo E.; Willing A. E. Monocyte transplantation for neural and cardiovascular ischemia repair. J. Cell. Mol. Med. 14(3):553–563; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Sanberg P. R.; Willing A. E.; Garbuzova-Davis S.; Saporta S.; Liu G.; Sanberg C. D.; Bickford P. C.; Klasko S. K.; El-Badri N. S. Umbilical cord blood-derived stem cells and brain repair. Ann. NY Acad. Sci. 1049:67–83; 2005. [DOI] [PubMed] [Google Scholar]
- 335. Sankar V.; Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience 118(1):11–17; 2003. [DOI] [PubMed] [Google Scholar]
- 336. Santiago L. Y.; Clavijo-Alvarez J.; Brayfield C.; Rubin J. P.; Marra K. G. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 18(2):145–158; 2009. [DOI] [PubMed] [Google Scholar]
- 337. Saporta S.; Kim J. J.; Willing A. E.; Fu E. S.; Davis C. D.; Sanberg P. R. Human umbilical cord blood stem cells infusion in spinal cord injury: Engraftment and beneficial influence on behavior. J. Hematother. Stem Cell Res. 12(3):271–278; 2003. [DOI] [PubMed] [Google Scholar]
- 338. Sasaki M.; Black J. A.; Lankford K. L.; Tokuno H. A.; Waxman S. G.; Kocsis J. D. Molecular reconstruction of nodes of Ranvier after remyelination by transplanted olfactory ensheathing cells in the demyelinated spinal cord. J. Neurosci. 26(6):1803–1812; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Sasaki M.; Hains B. C.; Lankford K. L.; Waxman S. G.; Kocsis J. D. Protection of corticospinal tract neurons after dorsal spinal cord transection and engraftment of olfactory ensheathing cells. Glia 53(4):352–359; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Sasaki M.; Honmou O.; Akiyama Y.; Uede T.; Hashi K.; Kocsis J. D. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia 35(1):26–34; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Sasaki M.; Li B.; Lankford K. L.; Radtke C.; Kocsis J. D. Remyelination of the injured spinal cord. Prog. Brain Res. 161:419–433; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Satake K.; Lou J.; Lenke L. G. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine 29(18):1971–1979; 2004; [DOI] [PubMed] [Google Scholar]
- 343. Schmidt R. H.; Björklund A.; Stenevi U. Intracerebral grafting of dissociated CNS tissue suspensions: A new approach for neuronal transplantation to deep brain sites. Brain Res. 218(1–2):347–356; 1981. [DOI] [PubMed] [Google Scholar]
- 344. Schmidtmayerová B.; Cendelín J.; Korelusová I.; Vozeh F. Various methods of Purkinje cells transplantation and their functional response in Lurcher mutant mice. Prague Med. Rep. 106(1):79–84; 2005. [PubMed] [Google Scholar]
- 345. Seledtsov V. I.; Kafanova M. Y.; Rabinovich S. S.; Poveshchenko O. V.; Kashchenko E. A.; Fel’de M. A.; Samarin D. M.; Seledtsova G. V.; Kozlov V. A. Cell therapy of cerebral palsy. Bull. Exp. Biol. Med. 139(4):499–503; 2005. [DOI] [PubMed] [Google Scholar]
- 346. Seledtsov V. I.; Rabinovich S. S.; Parlyuk O. V.; Kafanova M. Y.; Astrakov S. V.; Seledtsova G. V.; Samarin D. M.; Poveschenko O. V. Cell transplantation therapy in re-animating severely head-injured patients. Biomed. Pharmacother. 59(7):415–420; 2005. [DOI] [PubMed] [Google Scholar]
- 347. Seledtsov V. I.; Rabinovich S. S.; Parlyuk O. V.; Poveshchenko O. V.; Astrakov S. V.; Samarin D. M.; Seledtsova G. V.; Senyukov V. V.; Taraban V. Y.; Kozlov V. A. Cell therapy of comatose states. Bull. Exp. Biol. Med. 142(1):129–132; 2006. [DOI] [PubMed] [Google Scholar]
- 348. Shechter R.; London A.; Varol C.; Raposo C.; Cusimano M.; Yovel G.; Rolls A.; Mack M.; Pluchino S.; Martino G.; Jung S.; Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 6(7):e1000113; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Shen L. H.; Li Y.; Chen J.; Zacharek A.; Gao Q.; Kapke A.; Lu M.; Raginski K.; Vanguri P.; Smith A.; Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J. Cereb. Blood Flow Metab. 27:6–13; 2007. [DOI] [PubMed] [Google Scholar]
- 350. Shi X.; Kang Y.; Hu Q.; Chen C.; Yang L.; Chen L.; Huang H.; Zhou C. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain Res. 1317:257–267; 2010. [DOI] [PubMed] [Google Scholar]
- 351. Shimazaki T. Biology and clinical application of neural stem cells. Horm. Res. 60(Suppl. 3):1–9; 2003. [DOI] [PubMed] [Google Scholar]
- 352. Shyu W. C.; Lin S. Z.; Chiang M. F.; Su C. Y.; Li H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J. Neurosci. 26(13):3444–3453; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Sieradzan K.; Vrbová G. Replacement of missing motoneurons by embryonic grafts in the rat spinal cord. Neuroscience 31(1):115–130; 1989. [DOI] [PubMed] [Google Scholar]
- 354. Sievers J.; Bamberger C.; Debus O. M.; Lucius R. Regeneration in the optic nerve of adult rats: influences of cultured astrocytes and optic nerve grafts of different ontogenetic stages. J. Neurocytol. 24(10):783–793; 1995. [DOI] [PubMed] [Google Scholar]
- 355. Sladek J. R.; Bjugstad K. B.; Collier T. J.; Bundock E. A.; Blanchard B. C.; Elsworth J. D.; Roth R. H.; Redmond D. E. Jr. Embryonic substantia nigra grafts show directional outgrowth to cografted striatal grafts and potential for pathway reconstruction in nonhuman primate. Cell Transplant. 17(4):427–444; 2008. [PubMed] [Google Scholar]
- 356. Song M.; Kim Y.; Kim Y.; Ryu S.; Song I.; Kim S. U.; Yoon B. W. MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci. Res. 64(2):235–239; 2009. [DOI] [PubMed] [Google Scholar]
- 357. Sotelo C. Neuronal transplantation: Purkinje cell replacement and reconstruction of a defective cerebellar circuitry in mice with heredo-degenerative ataxia. Boll. Soc. Ital. Biol. Sper. 62(12):1479–1485; 1986. [PubMed] [Google Scholar]
- 358. Sotelo C.; Alvarado-Mallart R. M. Reconstruction of the defective cerebellar circuitry in adult Purkinje cell degeneration mutant mice by Purkinje cell replacement through transplantation of solid embryonic implants. Neuroscience 20(1):1–22; 1987. [DOI] [PubMed] [Google Scholar]
- 359. Srivastava N.; Seth K.; Khanna V. K.; Ansari R. W.; Agrawal A. K. Long-term functional restoration by neural progenitor cell transplantation in rat model of cognitive dysfunction: Co-transplantation with olfactory ensheathing cells for neurotrophic factor support. Int. J. Dev. Neurosci. 27(1):103–110; 2009. [DOI] [PubMed] [Google Scholar]
- 360. Stein D. G. Fetal brain tissue grafting as therapy for brain dysfunctions: Unanswered questions, unknown factors, and practical concerns. J. Neurosurg. Anesthesiol. 3(3):170–189; 1991. [DOI] [PubMed] [Google Scholar]
- 361. Stein D. G.; Labbe R.; Attella M. J.; Rakowsky H. A. Fetal brain tissue transplants reduce visual deficits in adult rats with bilateral lesions of the occipital cortex. Behav. Neural Biol. 44(2):266–277; 1985. [DOI] [PubMed] [Google Scholar]
- 362. Stichel C. C.; Lips K.; Wunderlich G.; Muller H. W. Reconstruction of transected postcommissural fornix in adult rat by Schwann cell suspension grafts. Exp. Neurol. 140(1):21–36; 1996. [DOI] [PubMed] [Google Scholar]
- 363. Stroemer P.; Patel S.; Hope A.; Oliveira C.; Pollock K.; Sinden J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil. Neural Repair 23(9):895–909; 2009. [DOI] [PubMed] [Google Scholar]
- 364. Suárez-Monteagudo C.; Hernández-Ramírez P.; Alvarez-González L.; García-Maeso I.; de la Cuétara-Bernal K.; Castillo-Díaz L.; Bringas-Vega M. L.; Martínez-Aching G.; Morales-Chacón L. M.; Báez-Martín M. M.; Sánchez-Catasús C.; Carballo-Barreda M.; Rodríguez-Rojas R.; Gómez-Fernández L.; Alberti-Amador E.; Macías-Abraham C.; Balea E. D.; Rosales L. C.; Del Valle Pérez L.; Ferrer B. B.; González R. M.; Bergado J. A. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor. Neurol. Neurosci. 27(3):151–161; 2009. [DOI] [PubMed] [Google Scholar]
- 365. Subramanian T. Cell transplantation for the treatment of Parkinson’s disease. Semin. Neurol. 21(1):103–115; 2001. [DOI] [PubMed] [Google Scholar]
- 366. Subramanian T.; Marchionini D.; Potter E. M.; Cornfeldt M. L. Striatal xenotransplantation of human retinal pigment epithelial cells attached to microcarriers in hemiparkinsonian rats ameliorates behavioral deficits without provoking a host immune response. Cell Transplant. 11(3):207–214; 2002. [PubMed] [Google Scholar]
- 367. Sugaya K.; Merchant S. How to approach Alzheimer’s disease therapy using stem cell technologies. J. Alzheimers Dis. 15(2):241–254; 2008. [DOI] [PubMed] [Google Scholar]
- 368. Syková E.; Homola A.; Mazanec R.; Lachmann H.; Konrádová S. L.; Kobylka P.; Pádr R.; Neuwirth J.; Komrska V.; Vávra V.; Stulík J.; Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 15(8–9):675–687; 2006. [DOI] [PubMed] [Google Scholar]
- 369. Taira T.; Hori T. Clinical application of drug pump for spasticity, pain, and restorative neurosurgery: Other clinical applications of intrathecal baclofen. Acta Neurochir. Suppl. 87:37–38; 2003. [DOI] [PubMed] [Google Scholar]
- 370. Takahashi K.; Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676; 2006. [DOI] [PubMed] [Google Scholar]
- 371. Takizawa S. Differentiation of adult bone marrow cells into neurons and endothelial cells in rat brain after stroke in the presence of cytokines. Rinsho Shinkeigaku 43(11):830–831; 2003. [PubMed] [Google Scholar]
- 372. Tamariz E.; Díaz-Martínez N. E.; Díaz N. F.; García-Peña C. M.; Velasco I.; Varela-Echavarría A. Axon responses of embryonic stem cell-derived dopaminergic neurons to semaphorins 3A and 3C. J. Neurosci. Res. 88(5):971–980; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Taylor S. M. Electroconvulsive therapy, brain-derived neurotrophic factor, and possible neurorestorative benefit of the clinical application of electroconvulsive therapy. J. ECT 24(2):160–165; 2008. [DOI] [PubMed] [Google Scholar]
- 374. Tello F. La influencia del neurotropismo en la regeneration de los centros nerviosos. Trab. Lab. Invest. Biol. 9:123–159; 1911. [Google Scholar]
- 375. Tessler A. Intraspinal transplants. Ann. Neurol. 29(2):115–123; 1991. [DOI] [PubMed] [Google Scholar]
- 376. The International Association of Neurorestoratology. Beijing Declaration of International Association of Neurorestoratology (IANR). Cell Transplant. 18(4):487; 2009. [DOI] [PubMed] [Google Scholar]
- 377. Thompson T. P.; Lunsford L. D.; Kondziolka D. Restorative neurosurgery: Opportunities for restoration of function in acquired, degenerative, and idiopathic neurological diseases. Neurosurgery 45(4):741–752; 1999. [DOI] [PubMed] [Google Scholar]
- 378. Toft A.; Scott D. T.; Barnett S. C.; Riddell J. S. Electrophysiological evidence that olfactory cell transplants improve function after spinal cord injury. Brain 130(Pt. 4):970–984; 2007. [DOI] [PubMed] [Google Scholar]
- 379. Torrente Y.; Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. 17(10–11):1103–1113; 2008. [DOI] [PubMed] [Google Scholar]
- 380. Toyoda M.; Cui Ch.; Umezawa A. Myogenic transdifferentiation of menstrual blood-derived cells. Acta Myol. 26(3):176–178; 2007. [PMC free article] [PubMed] [Google Scholar]
- 381. Trujillo C. A.; Schwindt T. T.; Martins A. H.; Alves J. M.; Mello L. E.; Ulrich H. Novel perspectives of neural stem cell differentiation: From neurotransmitters to therapeutics. Cytometry A 75(1):38–53; 2009. [DOI] [PubMed] [Google Scholar]
- 382. Uchida K.; Momiyama T.; Okano H.; Yuzaki M.; Koizumi A.; Mine Y.; Kawase T. Potential functional neural repair with grafted neural stem cells of early embryonic neuroepithelial origin. Neurosci. Res. 52(3):276–286; 2005. [DOI] [PubMed] [Google Scholar]
- 383. van de Ven C.; Collins D.; Bradley M. B.; Morris E.; Cairo M. S. The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp. Hematol. 35(12):1753–1765; 2007. [DOI] [PubMed] [Google Scholar]
- 384. Verdu E.; Garcia-Alias G.; Fores J.; Gudino-Cabrera G.; Muneton V. C.; Nieto-Sampedro M.; Navarro X. Effects of ensheathing cells transplanted into photochemically damaged spinal cord. Neuroreport 12(11):2303–2309; 2001. [DOI] [PubMed] [Google Scholar]
- 385. Verdu E.; Navarro X.; Gudino-Cabrera G.; Rodriguez F. J.; Ceballos D.; Valero A.; Nieto-Sampedro M. Olfactory bulb ensheathing cells enhance peripheral nerve regeneration. Neuroreport 10(5):1097–1101; 1999. [DOI] [PubMed] [Google Scholar]
- 386. Vick R. S.; Neuberger T. J.; DeVries G. H. Role of adult oligodendrocytes in remyelination after neural injury. J. Neurotrauma 9(Suppl. 1):S93–S103; 1992. [PubMed] [Google Scholar]
- 387. Vignais L.; Nait Oumesmar B.; Mellouk F.; Gout O.; Labourdette G.; Baron-Van Evercooren A.; Gumpel M. Transplantation of oligodendrocyte precursors in the adult demyelinated spinal cord: Migration and remyelination. Int. J. Dev. Neurosci. 11(5):603–612; 1993. [DOI] [PubMed] [Google Scholar]
- 388. Vincent A. J.; Lau P. W.; Roskams A. J. SPARC is expressed by macroglia and microglia in the developing and mature nervous system. Dev. Dyn. 237(5):1449–1462; 2008. [DOI] [PubMed] [Google Scholar]
- 389. Wallace M. C.; Tator C. H.; Lewis A. J. Chronic regenerative changes in the spinal cord after cord compression injury in rats. Surg. Neurol. 27(3):209–219; 1987. [DOI] [PubMed] [Google Scholar]
- 390. Wan H.; An Y. H.; Sun M. Z.; Zhang Y. Z.; Wang Z. C. Schwann cells transplantation promoted and the repair of brain stem injury in rats. Biomed. Environ. Sci. 16(3):212–218; 2003. [PubMed] [Google Scholar]
- 391. Wang C.; Shi Z.; Wang K. Effect of olfactory ensheathing cells transplantation on protecting spinal cord and neurons after peripheral nerve injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 19(11):875–878; 2005. [PubMed] [Google Scholar]
- 392. Wang J. J.; Chuah M. I.; Yew D. T.; Leung P. C.; Tsang D. S. Effects of astrocyte implantation into the hemisected adult rat spinal cord. Neuroscience 65(4):973–981; 1995. [DOI] [PubMed] [Google Scholar]
- 393. Wei X.; Zhao L.; Zhong J.; Gu H.; Feng D.; Johnstone B. H.; March K. L.; Farlow M. R.; Du Y. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neurosci. Lett. 462(1):76–79; 2009. [DOI] [PubMed] [Google Scholar]
- 394. Weiss M. L.; Medicetty S.; Bledsoe A. R.; Rachakatla R. S.; Choi M.; Merchav S.; Luo Y.; Rao M. S.; Velagaleti G.; Troyer D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 24(3):781–792; 2006. [DOI] [PubMed] [Google Scholar]
- 395. Wenning G. K.; Odin P.; Morrish P.; Rehncrona S.; Widner H.; Brundin P.; Rothwell J. C.; Brown R.; Gustavii B.; Hagell P.; Jahanshahi M.; Sawle G.; Björklund A.; Brooks D. J.; Marsden C. D.; Quinn N. P.; Lindvall O. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Ann. Neurol. 42(1):95–107; 1997. [DOI] [PubMed] [Google Scholar]
- 396. Wernig M.; Benninger F.; Schmandt T.; Rade M.; Tucker K. L.; Büssow H.; Beck H.; Brüstle O. Functional integration of embryonic stem cell-derived neurons in vivo. J. Neurosci. 24(22):5258–5268; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Wewetzer K.; Brandes G. Axonal signalling and the making of olfactory ensheathing cells: A hypothesis. Neuron Glia Biol. 2(3):217–224; 2006. [DOI] [PubMed] [Google Scholar]
- 398. Wewetzer K.; Kern N.; Ebel C.; Radtke C.; Brandes G. Phagocytosis of O4+ axonal fragments in vitro by p75– neonatal rat olfactory ensheathing cells. Glia 49(4):577–587; 2005. [DOI] [PubMed] [Google Scholar]
- 399. Willing A. E.; Cameron D. F.; Sanberg P. R. Sertoli cell transplants: Their use in the treatment of neurodegenerative disease. Mol. Med. Today 4(11):471–477; 1998. [DOI] [PubMed] [Google Scholar]
- 400. Willing A. E.; Lixian J.; Milliken M.; Poulos S.; Zigova T.; Song S.; Hart C.; Sanchez-Ramos J.; Sanberg P. R. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 73(3):296–307; 2003. [DOI] [PubMed] [Google Scholar]
- 401. Wirth E. D. 3rd; Theele D. P.; Mareci T. H.; Anderson D. K.; Brown S. A.; Reier P. J. In vivo magnetic resonance imaging of fetal cat neural tissue transplants in the adult cat spinal cord. J. Neurosurg. 76(2):261–274; 1992. [DOI] [PubMed] [Google Scholar]
- 402. Woerly S. Restorative surgery of the central nervous system by means of tissue engineering using NeuroGel implants. Neurosurg. Rev. 23(2):59–77; 2000. [DOI] [PubMed] [Google Scholar]
- 403. Wrathall J. R.; Rigamonti D. D.; Braford M. R.; Kao C. C. Reconstruction of the contused cat spinal cord by the delayed nerve graft technique and cultured peripheral non-neuronal cells. Acta Neuropathol. (Berl.) 57(1):59–69; 1982. [DOI] [PubMed] [Google Scholar]
- 404. Wu S.; Suzuki Y.; Noda T.; Bai H.; Kitada M.; Kataoka K.; Nishimura Y.; Ide C. Immunohistochemical and electron microscopic study of invasion and differentiation in spinal cord lesion of neural stem cells grafted through cerebrospinal fluid in rat. J. Neurosci. Res. 69(6):940–945; 2002. [DOI] [PubMed] [Google Scholar]
- 405. Wu Z. Y.; Hui G. Z.; Lu Y.; Wu X.; Guo L. H. Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury. Chin. Med. J. (Engl.) 119(24):2101–2107; 2006. [PubMed] [Google Scholar]
- 406. Wunderlich G.; Stichel C. C.; Schroeder W. O.; Müller H. W. Transplants of immature astrocytes promote axonal regeneration in the adult rat brain. Glia 10(1):49–58; 1994. [DOI] [PubMed] [Google Scholar]
- 407. Xu B.; Jiang C. C.; Zhang L.; Li B. M.; Chen Z. P.; Guan Y. H.; Liu X. D.; Zhang F. L. Therapeutic study of autologous Schwann cells’ bridge graft into the brain of hemiparkinsonian monkey. Zhonghua Yi Xue Za Zhi 84(4):318–322; 2004. [PubMed] [Google Scholar]
- 408. Xu X. M.; Guenard V.; Kleitman N.; Bunge M. B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 351(1):145–160; 1995. [DOI] [PubMed] [Google Scholar]
- 409. Yamamoto M.; Raisman G.; Li D.; Li Y. Transplanted olfactory mucosal cells restore paw reaching function without regeneration of severed corticospinal tract fibres across the lesion. Brain Res. 1303:26–31; 2009. [DOI] [PubMed] [Google Scholar]
- 410. Yan J.; Xu L.; Welsh A. M.; Chen D.; Hazel T.; Johe K.; Koliatsos V. E. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells 24(8):1976–1985; 2006. [DOI] [PubMed] [Google Scholar]
- 411. Yang C. C.; Shih Y. H.; Ko M. H.; Hsu S. Y.; Cheng H.; Fu Y. S. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS ONE 3(10):e3336; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Yasuhara T.; Hara K.; Maki M.; Mays R. W.; Deans R. J.; Hess D. C.; Carroll J. E.; Borlongan C. V. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J. Cereb. Blood Flow Metab. 28(11):1804–1810; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Yasuhara T.; Hara K.; Maki M.; Xu L.; Yu G.; Ali M. M.; Masuda T.; Yu S. J.; Bae E. K.; Hayashi T.; Matsukawa N.; Kaneko Y.; Kuzmin-Nichols N.; Ellovitch S.; Cruz E. L.; Klasko S. K.; Sanberg C. D.; Sanberg P. R.; Borlongan C. V. Mannitol facilitates neurotrophic factor upregulation and behavioral recovery in neonatal hypoxic-ischemic rats with human umbilical cord blood grafts. J. Cell. Mol. Med. 14(4):914–921; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Yasuhara T.; Matsukawa N.; Hara K.; Maki M.; Ali M. M.; Yu S. J.; Bae E.; Yu G.; Xu L.; McGrogan M.; Bankiewicz K.; Case C.; Borlongan C. V. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev. 18(10):1501–1514; 2009. [DOI] [PubMed] [Google Scholar]
- 415. Yasuhara T.; Matsukawa N.; Yu G.; Xu L.; Mays R. W.; Kovach J.; Deans R. J.; Hess D. C.; Carroll J. E.; Borlongan C. V. Behavioral and histological characterization of intrahippocampal grafts of human bone marrow-derived multipotent progenitor cells in neonatal rats with hypoxic-ischemic injury. Cell Transplant. 15(3):231–238; 2006. [DOI] [PubMed] [Google Scholar]
- 416. Yoon S. H.; Shim Y. S.; Park Y. H.; Chung J. K.; Nam J. H.; Kim M. O.; Park H. C.; Park S. R.; Min B. H.; Kim E. Y.; Choi B. H.; Park H.; Ha Y. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells 25(8):2066–2073; 2007. [DOI] [PubMed] [Google Scholar]
- 417. Yu S. J.; Soncini M.; Kaneko Y.; Hess D. C.; Parolini O.; Borlongan C. V. Amnion: A potent graft source for cell therapy in stroke. Cell Transplant. 18(2):111–118; 2009. [DOI] [PubMed] [Google Scholar]
- 418. Yu T. B.; Cheng Y. S.; Zhao P.; Kou D. W.; Sun K.; Chen B. H.; Wang A. M. Immune therapy with cultured microglia grafting into the injured spinal cord promoting the recovery of rat’s hind limb motor function. Chin. J. Traumatol. 12(5):291–295; 2009. [PubMed] [Google Scholar]
- 419. Yu X. D.; Luo Z. J.; Zhang L.; Gong K. Effects of olfactory ensheathing cells on hydrogen peroxide-induced apoptosis in cultured dorsal root ganglion neurons. Chin. Med. J. (Engl.) 120(16):1438–1443; 2007. [PubMed] [Google Scholar]
- 420. Zhang J.; Li Y.; Chen J.; Cui Y.; Lu M.; Elias S. B.; Mitchell J. B.; Hammill L.; Vanguri P.; Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 195(1):16–26; 2005. [DOI] [PubMed] [Google Scholar]
- 421. Zhang L.; Zhang H. T.; Hong S. Q.; Ma X.; Jiang X. D.; Xu R. X. Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem. Res. 34(11):2030–2039; 2009. [DOI] [PubMed] [Google Scholar]
- 422. Zhang Y. N.; Lie P. C.; Wei X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy 11(5):548–558; 2009. [DOI] [PubMed] [Google Scholar]
- 423. Zhang Z. G.; Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 8(5):491–500; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Zhang Z. X.; Guan L. X.; Zhang K.; Zhang Q.; Dai L. J. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy 10(2):134–139; 2008. [DOI] [PubMed] [Google Scholar]
- 425. Zhao L. R.; Duan W. M.; Reyes M.; Keene C. D.; Verfaillie C. M.; Low W. C. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 174(1):11–20; 2002. [DOI] [PubMed] [Google Scholar]
- 426. Zhao Z. M.; Li H. J.; Liu H. Y.; Lu S. H.; Yang R. C.; Zhang Q. J.; Han Z. C. Intraspinal transplantation of CD34+ human umbilical cord blood cells after spinal cord hemisection injury improves functional recovery in adult rats. Cell Transplant. 13(2):113–122; 2004. [DOI] [PubMed] [Google Scholar]
- 427. Zhou C.; Wen Z. X.; Wang Z. P.; Guo X.; Shi D. M.; Zuo H. C.; Xie Z. P. Green fluorescent protein-labeled mapping of neural stem cells migrating towards damaged areas in the adult central nervous system. Cell Biol. Int. 27(11):943–945; 2003. [DOI] [PubMed] [Google Scholar]
- 428. Zhu J.; Zhou L.; XingWu F. Tracking neural stem cells in patients with brain trauma. N. Engl. J. Med. 355(22):2376–2378; 2006. [DOI] [PubMed] [Google Scholar]
- 429. Zwart I.; Hill A. J.; Al-Allaf F.; Shah M.; Girdlestone J.; Sanusi A. B.; Mehmet H.; Navarrete R.; Navarrete C.; Jen L. S. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp. Neurol. 216(2):439–448; 2009. [DOI] [PubMed] [Google Scholar]