Abstract
Although the oral route remains the most favored route of drug administration, major scientific obstacles prevent the effective and efficient delivery of low-molecular-mass drugs, peptides and proteins that exhibit poor solubility and permeability. Mucoadhesive dosage forms and the associated drug carriers have the ability to interact at a molecular level with the mucus gel layer that lines the epithelial surfaces of the major absorptive regions of the body. This interaction provides an increased residence time of the therapeutic formulation while localizing the drug at the site of administration. Such local, non-specific targeting leads to an increase in both oral absorption and bioavailability. Fundamental understanding of the biological processes encountered along the gastrointestinal tract can provide a sufficient engineer of carriers that are capable to provide this increase in residence time. Here we discuss the theoretical framework for achieving mucoadhesive systems as related to biomaterials science and the structure of the biomaterials used.
Keywords: Hydrogels, mucoadhesives, bioadhesives, diffusion, interpenetration
Introduction
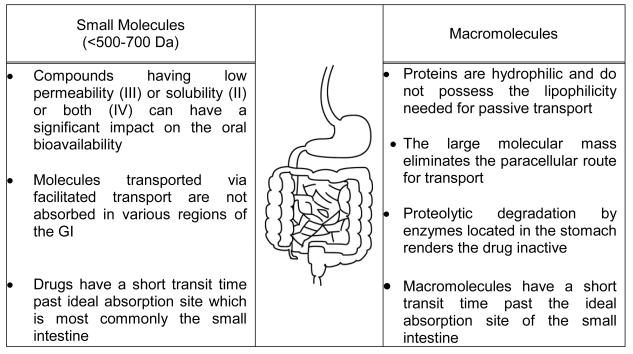
The oral delivery of low molecular weight drugs, or macromolecular drugs such as peptides and proteins and vaccines, remains the most favored route of administration. However, barriers exist in successfully administering drugs via the gastrointestinal tract and achieving a predictable and increased absorption and oral bioavailability. These barriers include but are not limited to a mucous gel layer lining the gastrointestinal epithelia, a degradative pH that varies from patient to patient, a relatively short transit time past the ideal absorption sites, and a system of degradative enzymes (Figure 1).
Figure 1.
Physiological challenges encountered in both the development of modified or controlled release oral dosage forms for low molecular weight compounds and the effective delivery of macromolecules.
For low molecular mass drugs (500-700 daltons), the narrow absorption window of the proximal intestine poses the greatest challenge in the development of a modified or controlled release product (1). The successful oral delivery of macromolecules faces major challenges which are a result of their much larger size (> 500-700 Da), hydrophilicity, vulnerability to proteolytic degradation, and narrow window of effective absorption, all of which have limited their commercial and clinical development (2). Drug carriers have shown great promise in enabling the successful delivery of proteins and peptides by overcoming the aforementioned challenges without modifying the structure of the macromolecular therapeutic, damaging epithelial membranes, or creating new toxicities (3-7). However, rapid transit of the drug carrier past the ideal site of absorption still poses a challenge in developing an effective and efficient oral macromolecular drug delivery system (8).
Two physical processes or phenomena have been used to solve the problem of rapid transit of a therapeutic past the location of maximal absorption: gastroretention and bioadhesion. Both address the need for controlling the spatial location of an oral drug delivery system to achieve a maximal bioavailability. Gastroretentive dosage forms provide a system that maintains the drug above the compound's window of absorption, releasing the therapeutic slowly so that the time available for the body to effectively absorb the pharmaceutical ingredient is increased (9). Since the early 1980s, mucoadhesion has offered promise in enabling the development of drug delivery carriers and systems that are capable of an increased residence time in the narrow absorption window of the drug. Mucoadhesion is the attachment of a biological or synthetic moiety to a biological substrate such as the mucous gel layer lining the oral cavities of the body.
Mucoadhesive oral drug delivery systems allow for intimate contact to develop between the system and the ideal absorption site (most cases, the small intestine), which aids in maximizing the rate and extent to which a drug absorbs. Major adversities encountered in designing superior mucoadhesive systems include the motility of the digestive tract which is designed to move contents, the rate of mucus turnover and the amount of sloughed or free mucous gel that resides in the lumenal environment.
A mucoadhesive is the synthetic or semi-natural material, most commonly a polymer, which is responsible for the interaction between the drug carrier and the mucous gel layer. Recent advances in mucoadhesives include polymer networks that contain thiolated moieties available for disulfide bond formation with mucin molecules (10), polymer tethers capable of interpenetrating the mucous gel layer to create a bridge between the system and the mucous gel layer (11, 12), and mucin recognitive molecules (or their carbohydrate side-chains) such as lectins (13, 14), fimbrial proteins (15), and chitosan (16-18). These advances offer promise in that the technology is advancing and systems are evolving to become more effective. However, there is much knowledge to be gained in order for biomimetic systems to be developed that offer characteristics representative of biological mucoadhesive organisms, namely the bacteria comprising the intestinal microflora (19-21). The surface of the polymeric drug delivery carrier remains the integral part in obtaining a mucoadhesive interaction with the mucous gel layer, as it is this surface that is at the interface with the mucosal lining. A surface layer of polymer has been shown to have the capacity to act as an adhesion promoter in developing intimate contact between the carrier and the mucosal lining (22).
Mucoadhesion
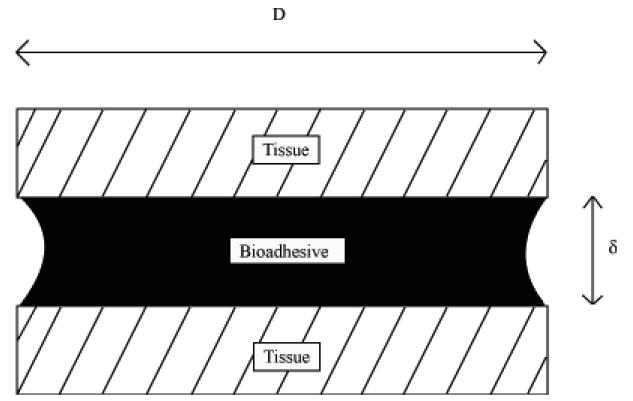
Adhesion refers to the molecular contact (adherence) of two substances, which typically involves the application of an adhesive between these two entities (Figure 2). Historically, adhesives (23) and bioadhesives have been used as terms in two distinctly different areas of thought with very little overlap and continuity. The term biological adhesive has been used to describe a particular biomaterial or system that is responsible for adherence. Bioadhesion has largely remained well entrained in the field of biology referring to cellular interactions (cytoadhesion) or to mucosal adhesion (mucoadhesion).
Figure 2.
Bioadhesion refers to the molecular contact (adherence) of two tissues through the application of a bioadhesive between these two surfaces. Adapted from Ref (23).
Whether it is cell-to-cell, soft or hard tissue, or mucosal adhesion, bioadhesion is the phenomenon of long term contact of a synthetic or biological macromolecule or hydrocolloid with a biological substrate (13, 24-28). Therefore, bioadhesion can be extended to include any type of adhesion process in contact with or within the biological milieu. The term bioadhesive refers to the particular substance, natural or synthetic, responsible for this adhesion. Surfaces usually employed in bioadhesive applications involve synthetic, natural, or hybrid macromolecules, which can be found on biological components such as cells composing tissue and blood platelets. Mucoadhesion is often used to describe bioadhesion where mucus is the substrate, whereas cytoadhesion refers to cell-to-cell bioadhesion.
The substrate to which bioadhesion occurs is quite different in its physical properties than those employed in conventional adhesion. Soft tissue or a synthetic material is often used to adhere another soft tissue. Bioadhesives have found success in hard tissue applications such as orthopedics and dentistry due to the relatively more favorable biological environment presented in these areas (29). Recently there has been significant interest in the development of more effective bioadhesives for tissue engineering (30-32) and carriers for drug delivery applications (13, 24-28) , both areas in which clinical success has not been as successful. Also, the understanding of bacterial adhesion has received considerable attention to further the knowledge behind biofouling (biofilm formation) and the role bacterial bioadhesives play.
Theoretical and Dynamic Aspects of Bioadhesion
The mechanisms that describe the phenomena of bioadhesion and the structural characteristics of bioadhesives were originally proposed by Peppas and Buri (26). The components of importance for obtaining adhesion are the surface containing the bioadhesive, the surface of the biological substrate, and the interface between these two. The bioadhesive must come into intimate contact with the substrate (either with an external applied force, through an attractive force, or by collision). For liquid bioadhesive formulations, the bioadhesive fills the inclusions provided by the surface roughness and develops a physical or mechanical bond, which is quite common in orthopedic or dental applications. Once contact has been achieved, the macromolecules responsible for the adhesive character are then able to interact with specific moieties present on the substrates' surface. In some instances, due to the high mobility of the surface macromolecules, interdiffusion occurs and the interdigitated macromolecules are capable of interacting with other moieties present subsurface or entangling with other macromolecules composing the surface of the substrate to develop a bioadhesive bond. The molecule to molecule bioadhesion is attributed to bonding derived from either covalent or noncovalent interactions.
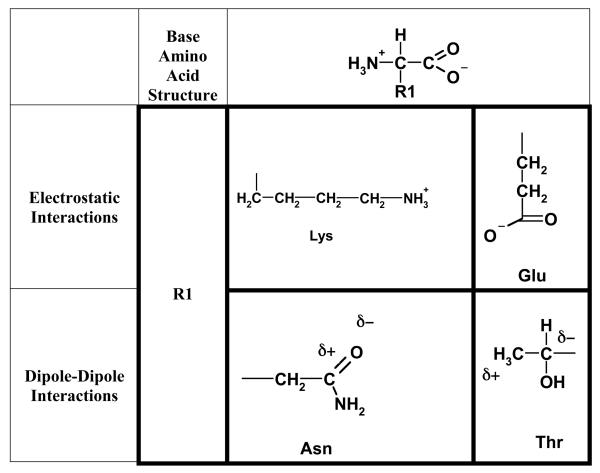
Primary chemical interactions occur through ionic bonding, disulfide bonds, or a chemical reaction between the functional groups of the adhesive and the substrate. Secondary chemical interactions are attributed to electrostatic forces (ionic and dipole-dipole, Figure 3), hydrogen bonding, or hydrophobic forces. The theories that describe the chemical mechanisms include the electronic theory and the adsorption theory, and those that describe the physical mechanisms include the wetting theory, the interpenetration or diffusion theory, and the fracture theory (13, 24, 26).
Figure 3.
Examples of electrostatic forces occurring between amino acids. These forces are due to (a) the association of two ionic protein groups such as Lysine (Lys) and Glutamic acid (GLU) or (b) the dipole-dipole interactions such as the dipole-induced dipole interaction between Asparagine (Asn) and Threonine (Thr).
The electronic theory describes adhesion in terms of the differences in the electronic structures of the materials involved. After the materials come into contact, a double layer of charge forms at the interface, and adhesion occurs due to these attractive forces (33). The adsorption theory describes materials that adhere to mucus due to secondary forces such as van der Waals, hydrogen bonding, or hydrophobic interactions (34, 35). Due to the presence of a large number of these types of forces, the net force is greater than that which is created by electrostatic forces. The wetting theory describes the ability of a mucoadhesive to spread over the mucus gel layer and develop an intimate contact (36-38). The parameters used to correlate a materials mucoadhesive capacity based upon this theory are the individual materials' spreading coefficients which aid in calculating the interfacial energy. The diffusion theory describes mucoadhesion as a process where the chains of the materials interpenetrate to a sufficient depth to create a semipermanent bond (12, 39).
Each individual theory fails to sufficiently describe the phenomenon of bioadhesion; therefore, combining various aspects of the theories enables a more appropriate description (40). As a bioadhesive approaches the biological substrate, it reaches equilibrium with the biological environment, which can include wetting and swelling. As the material comes into contact with the biological substrate, non-covalent secondary bonds are formed at the bioadhesive-substrate interface through either non-specific or specific interactions. As the bioadhesive continues to reside in contact with the substrate, macromolecules of the respective materials interdiffuse into one another while forming more and more noncovalent bonds.
The field of bioadhesion is receiving a great deal of attention in a wide variety of fields related to biomedical engineering. These main areas of thrust are discussed along with the current research efforts.
Drug Delivery
Drug-delivery applications utilize bioadhesive macromolecules to localize treatment to a specific area of the body thereby increasing the residence time of the therapeutic and improving the oral bioavailability (1, 41, 42). For intravascular applications, a targeting agent such as a ligand is incorporated into the drug delivery system, creating adhesion through the ligand-receptor interaction present at the endothelium surface. Bioadhesion in this instance is governed by (i) the shear stress caused by the hemodynamic force exerted over the cell/particle, the loading rate which is affected by the viscosity of the biological environment, and the ligand/receptor density ratio which can be controlled during the fabrication of the system (43). For oral drug delivery systems, mucoadhesion is the specific type of bioadhesion responsible for localizing the system at the mucous gel layer, which lines the absorptive regions of the alimentary canal.
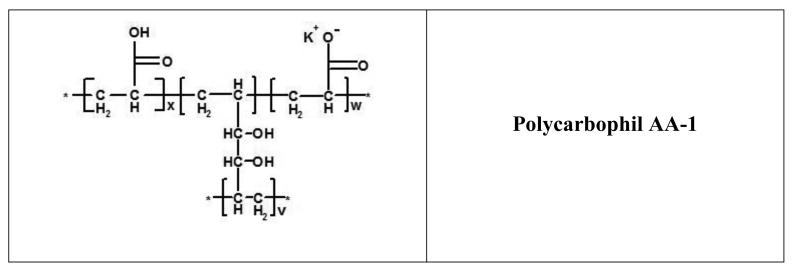
Mucoadhesive polymer-based drug delivery systems were first utilized by Nagai and collaborators as carriers for local treatment to the buccal cavity (44, 45). Mucus is also present in the nasal and gastrointestinal cavity, the vagina, and other hollow organs, providing a diverse arena for the application of mucoadhesive drug delivery systems. Polymers that have typically been utilized in the development of mucoadhesive controlled release formulations include hydrophilic macromolecules containing numerous hydrogen bonding groups such as poly((meth)acrylic acid) which include the carbomer and polycarbophil families (Figure 4), cellulosics, and (semi)natural ones such as chitosan and alginate (46). Like so many other materials that were first utilized in biomedical applications (cellulous acetate dialysis tubing, Dacron® vascular grafts, and polyurethane heart molds), these materials were available off-the-shelf. Because their use were originally intended for other applications and were only transitioned into oral drug delivery systems, the drug delivery systems comprised of these materials have been classified as the first generation bioadhesive-based dosage forms (13).
Figure 4.
Chemical structure of commonly employed poly(acrylic acid)-based materials in mucoadhesive drug delivery systems. 934P is crosslinked with allyl sucrose, 974P with allyl pentaerythritol, and AA-1 with divinyl glycol. A small amount of the acrylic acids are neutralized to potassium acrylate prior to polymerization.
Dosage forms have been engineered so that they take advantage of the abilities to molecularly design polymer networks to impart specific structural characteristics so that polymers comprising the dosage form are multifunctional. This new generation of bioadhesives has employed hybrid materials such as lectins, fimbrial proteins, or ligands which have specific interaction sites within the body. Biomimetic design of devices can take advantage of the natural processes of the body to be highly selective and effective, enabling the ability to localize and maintain treatment at a specific area within the body. However, to be both effective and selective, a thorough knowledge and understanding of the biological environment is a necessity.
Gastrointestinal Motility
The alimentary canal's physiology and motility play a key role in determining the fate of a drug delivery system. The specific state of the digestive system poses particular obstacles in allowing the therapeutic to be delivered as engineered. In developing site-specific delivery systems, one must take into consideration both the physiology and motility to better design the dosage form. After passing through the mouth and esophagus, the delivery system enters the stomach. The stomach is composed of three distinct muscular components, the fundus, the antrum, and the pylorus, and its primary function is to mix and grind food up into small enough particles to pass through the pylorus (24).
The fundus is composed of three layers of muscle—the inner circular layer, outer circular layer, and outermost oblique layer—which relax upon the entry of food and contract to force the food towards the antrum region. Through phases and contractile activity, the antrum functions both as a pump and a grinding mill. The pylorus possesses the ability to restrict the exit of liquids, prevent the passage of large particles, and close completely during the antral stroke. Once passing through the pylorus sphincter into the duodenum, the contents of the GI tract can be subjected to propagated bursts of contractions that often occur in association with propagated antropyloric pressure waves as the antroduodenal emptying stroke, isolated contractions, or retroperistaltic contractions that return the contents back into the stomach. The major motility function of the duodenum area is to further move the contents and the emptied gastric chyme downstream and is the site where the major digestive processes occur. The stomach and the duodenum work in a closely integrated fashion to control gastric emptying that is suitable for the person's physiological and emotional state.
Intestinal motility (small and large) is a broad generalization that refers to intraluminal flow, the motions of the wall that cause this flow, and the control systems that regulate these wall motions (47). The two muscle layers responsible for most of the motions associated with movement are the outer longitudinal and the inner circular muscle layers. The epithelium layers consists of the various types of cells lining the intestinal lumen including enterocytes and goblet cells, the cells responsible for absorption and mucus secretion, respectively. The small intestine moves its contents caudally with accompanying stirring and mixing. The two wall contractions responsible for the motility are ring contractions and sleeve contractions. Ring contractions are the type of muscle movement most often associated with intestinal transit. The rings begin as circumferential indentations and then sweep across the small intestine. The point of inception and distance of movement is controlled by both the nervous system and hormones, reflecting the digestive needs of the luminal contents. These contractions are associated with a periodicity at a specific level of the small intestine with a drop in frequency caudally along the intestine. This particular type of contraction is what is often referred to as peristalsis.
Sleeve contractions are responsible for the mixing of the lumenal contents by shifting fluid between the inside and outside of the lumen. Both types of major contractions work in a concerted effort to spread and mix the lumenal contents across the mucosal surface to achieve maximum absorption. The viscosity of a suspension in the lumen has an effect on its GI transit due to its greater inertial ability to resist propulsive contractions (24). Therefore, a dosage form with a high viscosity has the capability of increasing the residence time of the therapeutic. To further enhance the intimate contact of the dosage form with the absorption site, the delivery system can be engineered with molecular characteristics capable of achieving greater bioadhesion, specifically mucoadhesion.
Mucous Gel Layer
The mucous gel layer, the target site for mucoadhesion, serves to protect and lubricate epithelial surfaces such as those of the gastrointestinal, respiratory, and urogenital tracts while still allowing specific interactions to occur by means of their complex structure (48). Mucins are filamentous glycoproteins that are the major component of the mucus that lines most hollow organs of the body and those organs that come into contact with exogenous environments. The family of mucin biopolymers is characterized by a protein backbone that consists of tandem repeat units of peptide sequences, typically serine- and threonine-rich, which contain O-linked glycans (49). There are currently 17 different mucins (Table 1) all of which contain extended domains of the variable number of tandem repeats (VNTR) which are serine-, threonine-, and/or proline-rich (STP-rich regions). There are two structurally and functionally distinct classes of mucins which can either be classified as secreted gel-forming mucins or transmembrane mucins (50).
Table 1.
Mucins found in the human body. Adapted from Ref. (49).
| Mucin species | Secretory/membraneous (S/M) |
Chromosonal localization |
|---|---|---|
| MUC2 | S | 11p15.5 |
| MUC5AC | S | 11p15.5 |
| MUC5B | S | 11p15.5 |
| MUC6 | S | 11p15.5 |
| MUC7 | S | 4q13-21 |
| MUC8 | S | 12q24.3 |
| MUC1 | M,S | 1q21 |
| MUC3 | M,S | 7q22 |
| MUC4 | M,S | 3q29 |
| MUC11 | M | 7q22 |
| MUC12 | M | 7q22 |
| MUC13 | M | 3q13.3 |
| MUC15 | M | 11p14.3 |
| MUC16 | M | 19p13.2 |
| MUC17 | M | 7q22 |
| MUC20 | M | 3q29 |
| MUC9 | ? | 1p13 |
Secretory mucins such as MUC2, MUC5AC, MUC5B, MUC6, MUC7, and MUC8 are secreted by the goblet cells and receive extensive O-glycosylation in the cis to trans Golgi of the cell. These mucins have the capability to polymerize through disulfide bond linkages formed between the cysteine residues found in the von Willebrand factor (VWF-)-like D domains (51). O-glycosylation begins in the cis Golgi and is completed after passing through the trans Golgi after which a significant increase occurs in the biopolymers' apparent molecular weight (49). The non-tandem repeating regions of MUC2 have over an 8% cysteine content which are positioned at the ends of the proteins and form dimers in the endoplasmic reticulum of the goblet cells. Along with glycosylation, polymerization occurs in the cis to trans compartments of the Golgi. Polymerization of the biopolymers begins with tail-to-tail dimerization at the C-terminal followed by head-to-head multimerization at the N-terminals (52).
The gel layer can be divided into one that is loosely adhered and one that is firmly adhered. The firmly adhered is composed of the membrane mucins and is approximately 20 μm in the upper small intestine for rats. The loosely adhered mucus is composed of the secreted mucins such as MUC2 and MUC3. This gel layer is approximately 150 μm in the upper small intestine for rats (53). The mean turnover time of the mucous gel layer has been shown to vary between 47 and 270 min, which would indicate a significant factor in designing mucoadhesive drug delivery systems (54). The mucous gel layer itself represents an unstirred water layer which impedes drug diffusion and adsorption across the epithelium.
MUC2 and MUC3 mucin mRNAs are prominently expressed in the small intestine, the most favorable site for orally delivered therapeutic absorption. The goblet cells are responsible for the MUC2 synthesis, whereas the enterocytes produce MUC3 (55). MUC2 is also the prominent mucin in the large intestine; therefore, its structure and functionality plays a key role in the successful development of mucoadhesive drug delivery systems. The biopolymer MUC2 has a molecular weight of approximately 3,000 kDa (56) and is responsible for the formation of the viscoelastic gel (57) that covers the lumen of the small and large intestine. The protein backbone consists of over 4,500 residues which can be divided into three major structural domains: tandem repeat array, carboxyl-terminal domain, and amino-terminal domain.
Taking into consideration the steric constraints imposed by a relatively heavily O-glycosylated tandem repeat array domain, the mucin molecules are rigid and extended, taking on a filamentous structure (58). The other two domains are non-glycosylated and cysteine-rich which provide the groups necessary for disulfide bond formation and mucin polymerization. The mucus that is formed by multimers of MUC2 protects the underlying epithelial cells along the digestive tract from the acidic and proteolytic environment presented by the lumen, the shear forces naturally exerted during the digestive process, and the microorganisms and bacteria responsible for infections and disease. The gel-forming capabilities are directly affected by proteolytic degradation or mercaptoethanol reduction, signifying the importance of the presence of disulfide bonds and biopolymerization on the formation and stability of the gel layer (59). The mucous gel layer can be considered a loosely, physically entangled, biopolymeric network which is weakly viscoelastic in nature.
Analysis of Recent Mechanistic Studies
Mucoadhesive Surfaces Decorated With Tethered Stuctures
The mucous gel network serves as a target for mucoadhesive biomaterials composed of polymer chains with the ability to interpenetrate and bridge the interface between the drug carrier and the mucus (39, 60). Polymer chains available at the surface of the biomaterial are often employed to serve as adhesion promoters (22, 61-63). With the polymer chains at the periphery of the delivery system, they are able to develop an intimate contact with the mucous gel layer, diffuse across the interface, and form entanglements with the mucous gel layer. Once across the polymer/gel boundary, further non-covalent mechanisms can occur to strengthen the bond. The enhanced bridging is a result of further chain entanglement, secondary bonding, or electrostatic interactions. For further enhancement of the adhesive bond formed between a delivery system and the mucous gel layer, tethered polymers, both nonionic and cationic, can be used to create a synergistic effect in developing a more effective mucoadhesive delivery system (64-67).
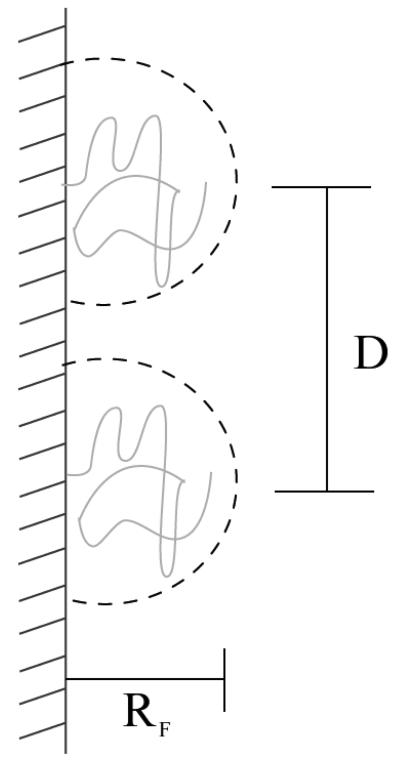
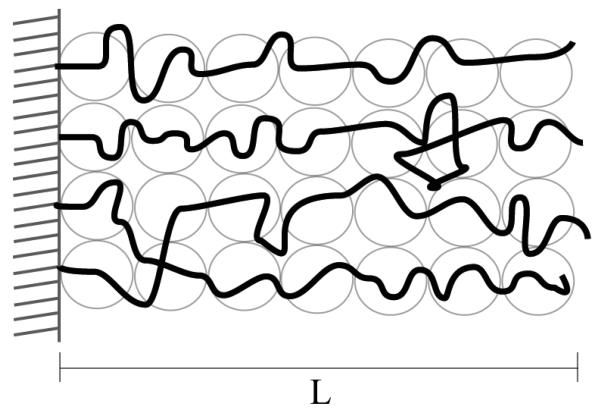
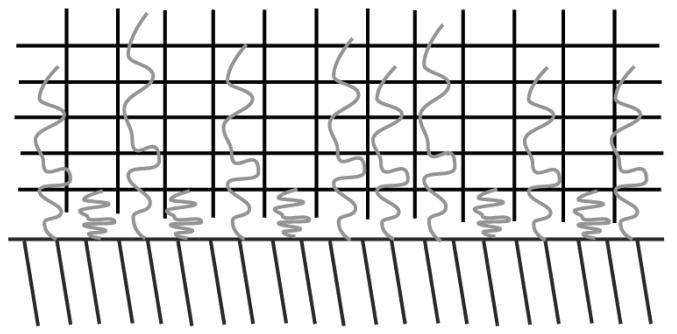
Polymer chains with one of their ends tethered onto a surface have found uses in a wide variety of applications such as surface energy enhancement (wetting), colloidal stability, biocompatibility, and adhesion (68-70). The pioneering work on the conformation of tethered polymers (Figure 5) was developed by de Gennes (69). The thickness of the polymer layer was shown to be dependent on the surface coverage, σ, of the chains, and either a mushroom or a stretched configuration is developed when in the low or high surface coverage regime, respectively. The thickness of the layer is directly proportional to the degree of polymerization and the width of the monomer (Figure 6).
Figure 5.
Polymer tethers grafted at a low surface coverage (σ → 0) and in a pure solvent. The dashed circle has a diameter which is 2 × RF (Flory radius). The grafted chains are separated by a distance, D, that is directly proportional to the monomer size (a) and inversely proportional to the square root of the surface coverage (σ). Adapted from Ref. (69).
Figure 6.
Tethered polymers in a good solvent take a strongly stretched conformation, and the thickness of this stretched polymer layer, L, is directly proportional to the degree of polymerization (N), the monomer size (a), and the surface coverage (σ) to the 1/3 power. Adapted from Ref. (69).
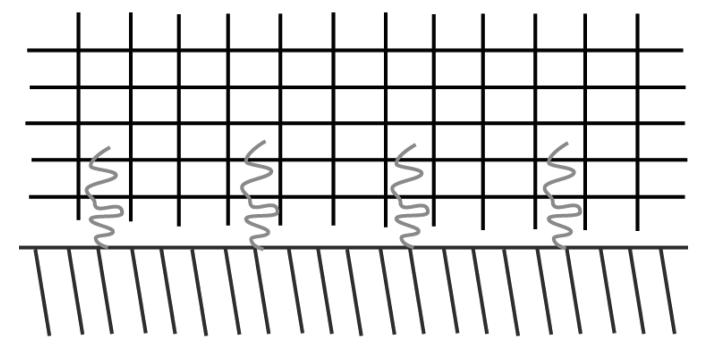
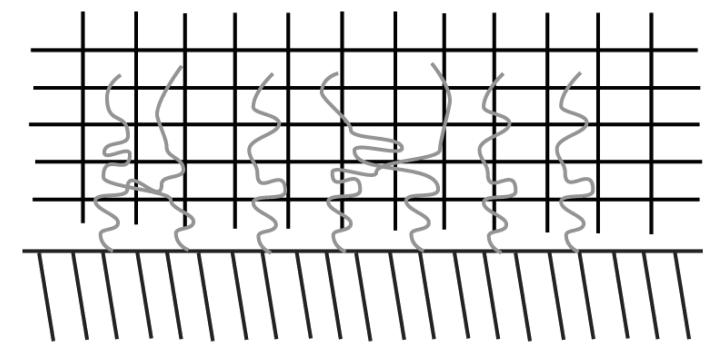
Brochard-Wyart et al. (68) further elucidated on the role of grafted chains as adhesion promoters and provided evidence of maximum adhesion enhancement at an intermediate surface coverage (Figures 7, 8, and 9). These results provided evidence that polymers attached to a surface take on three distinct regimes: separate mushrooms, overlapping mushrooms, and partial interdigitation. The intermediate regime provides the greatest adhesion energy due to a higher percentage of chains participating in interdigitation.
Figure 7.
Tethered polymers take on distinct conformations when exposed to a crosslinked network. Polymers take on a separate mushroom regime at low surface coverages.
Figure 8.
At intermediate surface coverages, the tethered polymer layers interpenetrate into the crosslinked polymer with some of the polymers overlapping.
Figure 9.
At high surface coverages, some polymers interpenetrate into the crosslinked network while others collapse onto the tethered surface resulting in partial interdigitation.
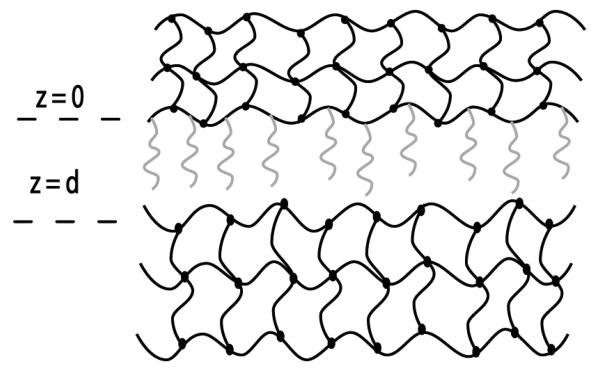
The theory surrounding polymers attached to hydrogels was further developed in our laboratory by Huang et al. (66, 67) and included systems composed of two hydrogels such as those involved with the process of mucoadhesion (Fig. 10). This theoretical framework provided evidence of the probability of finding both the chains and the free end segment outside the base gel available for interpenetration. Both were dependent on the surface coverage of the polymer tethers, with a large number of the chains and free ends present outside the base gel at low and intermediate coverage. However, at high coverages the chains penetrated back into the base gel due to the intermolecular interactions between neighboring chains. Adhesion enhancement associated with the base and target gel (mucus layer) was also shown to be maximized at an intermediate coverage. Therefore, in designing biomaterials for mucoadhesive applications, the surface coverage of grafted polymer chains can be controlled to achieve adhesion enhancement through polymer-gel interpenetration (71).
Figure 10.
The theoretical framework developed in our laboratory by Huang et al. (66) includes the tethered polymers, the base gel to which these polymers are tethered, and the target gel representative of the tissue to be adhered. The position at z = 0 represents the base gel surface plane and d is the distance between the two hydrogel networks.
Thiomers
Thiolated polymers, or thiomers, have received considerable attention as potential mucoadhesive polymers (10). The list of thiol-bearing polymers includes chito-sancysteine, chitosan-thiobutylamidine, chitosan-thioglycolic acid, poly(acrylic acid)-cysteine, poly(acrylic acid)-cysteamine, carboxy-methylcellulose-cysteine, and alginate cysteine. The nature of their action differs as compared to PEG adhesion promoters in that a covalent disulfide bond is formed between the cysteine residues on the mucin backbone and the free thiols along the mucoadhesive polymer backbone. This provides a stronger bond as compared to the formation of weak non-covalent bonds such as hydrogen bonds, van der Waals forces, and electrostatic interactions. These thiomers have the ability to mimic what naturally occurs in the secretion of the mucous gel layer where oligomers of mucins are joined by disulfide bonds.
Cationic thiomers have largely been thiolated chitosan whereas anionic thiomers exhibit carboxylic groups as the anionic moieties. The reactions typically employ carbodiimide chemistry to form an amide bond between the primary amino group of the chitosan and the carboxylic acid group of the attached ligand, or the reverse for anionic polymers. Stability of the free thiol is maintained through an inert environment or a lowered pH. At a pH < 5, the number of thiolate anions is significantly reduced, thereby decreasing the oxidation of the thiol groups. Ellman's agent can be used to determine the amount of thiol groups present on the polymers' backbone.
The interaction between the thiomers and mucin molecules can be attributed to the formation of a disulfide bond which occurs through either a thiol/disulfide exchange reaction or through the oxidation process of free thiols. Mucins covering the surfaces of the body typically all possess cysteine-rich subdomains along the mucin backbone which are free of O-glycosylation, allowing for interaction between other mucins and thiomers to occur. Disulfide bond formation is largely dependent on the thiolate anion concentration. The anion concentration is further dependant on the pKa of the thiol group, the pH of the thiomer (microclimate), and the pH of the surrounding medium.
Biopolymers
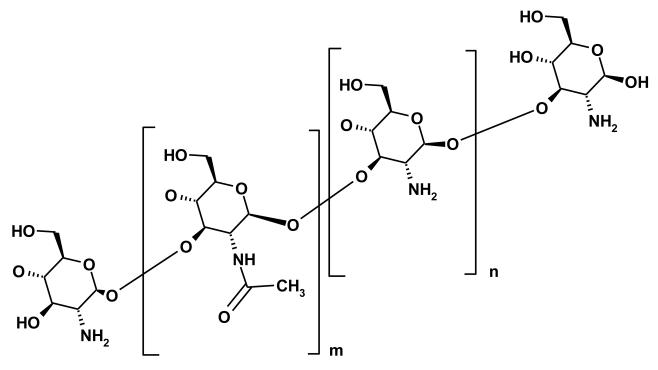
Biopolymers include polymers that are found in nature such as chitosan, alginates, and lectins, to name a few. Chitosan (Figure 11) has been used as a bioadhesive due to its ability to bind mucin through both i) electrostatic forces between the positively charged amine and the charged acidic groups of sialic acid and sulphonated residues and ii) hydrophobic forces between the acetyl groups and fucose residues (17). Chitosan coated microparticles have been shown to adhere well to the intestine of animals when administered intraduodenally to rats, and the system maintained the plasma concentration for an extended period of time (72). Other polysaccharides can be found widely in the literature for their use as mucoadhesives, but their interactions are based on hydrogen bonding with their sedimentation ratios being closed to unity. This sedimentation ratio indicates that there is little to no interaction on the molecular level between these other polysaccharides and mucin (17).
Figure 11.
Structure of chitosan, which is derived by partially deacetylating chitin. The number of acetyl groups (m) and primary amine groups (n) is controlled through the extent of deacetylation.
Lectins, plant proteins that bind specifically to carbohydrate groups found on mucins and cell membranes, have been introduced as a way to circumvent the problems associated with non-specific binding of the first generation bioadhesives to sloughed mucus or other components present in the gastrointestinal tract (14, 73, 74). Binding affinity relies heavily upon the conformation the lectin takes which can be significantly affected by the immobilization procedure and the application environment. Biomimetic mucoadhesive drug delivery systems have further evolved to employ fimbriae, long filamentous protein projections found on the surface of bacteria, as specific mucin binding moieties (15).
Bioadhesives in Tissue Engineering
Biomaterials (polymers, ceramics or metals) have led to significant advances in the treatment of damaged tissue and organs and organ dysfunction. They have the capacity to restore biological function while maintaining compatibility with the surrounding biological environment (biocompatibility). The complexity and number of variables involved in achieving success with the usage of biomaterials has limited the understanding to a generalized scale without focusing on the fundamental mechanisms. An objective of recent biomaterial development has been to interact with the biological environment more specifically as opposed to non-specific interactions based on the generalized properties such as cell's negative surface charge and common characteristics of the extracellular matrix.
The use of biomimetic materials, materials that mimic a biological environment to elicit a desired cellular response, provides a means to obtain specific interactions (75). One of the critical steps in achieving success with the application of a biomaterial is cytoadhesion. The critical processes involved with cell adhesion include cell attachment, cell spreading, organization of an actin cytoskeleton, and formation of focal adhesions. Cellular adhesion, more specifically cell attachment, can be made more effective through the deployment of ligands that interact specifically with integrins (receptors present at the surface of a cell) present in the environment where the biomaterial is applied (76-78). One of the more commonly used approaches is the attachment of an RGD (Arg-Gly-Asp, Arginine-Glycine-Aspartic acid) peptide to the biomaterial surface to enhance cell adhesion by targeting the integrin receptors on a cell's surface (78).
Biomaterial surface modifications are not limited to ligand-integrin receptor mediated adhesion. Heparin-binding peptides and lectins have also been used to increase cell attachment. Proteoglycans are the targets for the heparin-binding peptides whereas the carbohydrate-rich glycocalyx serves as the binding target for lectins. As the knowledge of the processes involved with cellular adhesion is increased, more effective biomaterials can be developed which provide the necessary surface characteristics to promote adhesion. More effective cellular adhesion will aid in a biomaterials' success which will result in better treatments for diseases and increased quality of life for these patients.
Adhesion of Microbes and Pathogens
The fundamental understanding of how microorganisms are able to adhere to materials has a broad impact that ranges from having the ability to effectively clean contaminated soil, developing materials resistant to biofouling, and preventing inflammation and rejection due to biofilms formed on a biomedical implant's surface. Just as in the case of a bioadhesive polymer, the surface characteristics of the bacterium, the bacterium/material interface, and the surface characteristics play a pivotal role in determining whether or not a bacterium is capable of initial attachment.
Forces responsible for this initial attachment can be attributed to electrostatic forces, van der Waals, hydrophobic, water movement, or other specific interactions. Factors such as the residence time, loading force, pH, and ionic strength have been shown to be important in the adhesion of microbes to surfaces (79). Atomic force microscopy (AFM) has provided valuable information regarding molecular-level interactions between microbial and material surfaces through the use of novel biopolymer immobilization techniques such as biopolymer-coupled-carboxylated dextrans (80). Entire bacteria have been immobilized to glass to study the effect of pH on the surface polymers' conformation which drives the ability of the microbe to adhere to a surface (81).
Developing a fundamental understanding of the receptor/binding molecule interaction has implications in understanding how diseases are spread. Human-to-human virus transmission of certain strains of influenza, or the lack thereof, can be linked to the binding preference of the virus to certain carbohydrates present on epithelium surfaces. Human and avian influenza viruses bind specific sialic acids linked to galactose through an α-2,6 or an α-2,3 linkage, respectively (82). The reason why widespread transmission of this virus from infected avian populations to human ones has not occurred can be attributed to this subtle binding preference.
Future Directions and Conclusions
The field of bioadhesion is one that is rapidly evolving to aid in the development of materials that are capable of more effective drug delivery, enhanced disease treatment, and the prevention and understanding of disease transmission. Biomimetic materials are quickly replacing those first-generation materials that relied heavily on the non-specific interactions they had with the natural tissue. Drug delivery systems are employing nature's method of covalent bond formation (thiomers) and specific interactions (lectins) through carefully designed surfaces (tethered surfaces). Tissue engineering matrices are incorporating macromolecules that the body is capable of recognizing and utilizing in the rehabilitation of organ dysfunction and failure. Some disease transmissions can be attributed to the ability of a microorganism or pathogen to adhere to an epithelial surface, and the fundamental understanding of this binding event could lead to more effective ways of prevention and treatment.
Successfully engineered materials for bioadhesive applications, or the prevention of bioadhesion in some instances, will continue to incorporate biologically relevant moieties to aid in their effective use by the biological host. Carbohydrates and their role in cell-cell and cell-matrix interactions are becoming increasingly important, and both the incorporation of carbohydrate structures and their binding molecules will be crucial for the success of biomedical devices (83).
References
- 1.Davis SS. Formulation strategies for absorption windows. Drug Discov. Today. 2005;10:249–257. doi: 10.1016/S1359-6446(04)03351-3. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discov. 2003;2:289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RA, Kapoor SC, Agarwal RK, Kisicki J, Catella-Lawson F, FitzGerald GA. Oral delivery of anticoagulant doses of heparin - A randomized, double-blind, controlled study in humans. Circulation. 1998;98:1610–1615. doi: 10.1161/01.cir.98.16.1610. [DOI] [PubMed] [Google Scholar]
- 4.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J. Pharm. Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 5.Morishita M, Goto T, Nakamura K, Lowman AM, Takayama K, Peppas NA. Novel oral insulin delivery systems based on complexation polymer hydrogels: Single and multiple administration studies in type 1 and 2 diabetic rats. J. Control. Release. 2006;110:587–594. doi: 10.1016/j.jconrel.2005.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morishita M, Goto T, Peppas NA, Joseph JI, Torjman MC, Munsick C, Nakamura K, Yamagata T, Takayama K, Lowman AM. Mucosal insulin delivery systems based on complexation polymer hydrogels: effect of particle size on insulin enteral absorption. J. Control. Release. 2004;97:115–124. doi: 10.1016/j.jconrel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Torres-Lugo M, Peppas NA. Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules. 1999;32:6646–6651. [Google Scholar]
- 8.Lehr CM. Bioadhesion Technologies For The Delivery Of Peptide And Protein Drugs To The Gastrointestinal-Tract. Crit. Rev. Ther. Drug Carr. Syst. 1994;11:119–160. [PubMed] [Google Scholar]
- 9.Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J. Control. Release. 2000;63:235–259. doi: 10.1016/s0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 10.Bernkop-Schnürch A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005;57:1569–1582. doi: 10.1016/j.addr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Peppas NA, Huang YB. Nanoscale technology of mucoadhesive interactions. Adv. Drug Deliv. Rev. 2004;56:1675–1687. doi: 10.1016/j.addr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials. 1996;17:1553–1561. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 13.Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Lehr CM, Bouwstra JA, Kok W, Noach ABJ, Deboer AG, Junginger HE. Bioadhesion By Means of Specific Binding of Tomato Lectin. Pharm. Res. 1992;9:547–553. doi: 10.1023/a:1015804816582. [DOI] [PubMed] [Google Scholar]
- 15.Caston A, Davis S, Williams P. The potential of fimbrial proteins for delaying intestinal transit of oral drug delivery system. Proceed. Intern. Symp. Controlled Release Bio. Mat. 1990;17:313–314. [Google Scholar]
- 16.Felt O, Buri P, Gurny R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998;24:979–993. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 17.Harding SE. Analysis of polysaccharides by ultracentrifugation. Size, conformation and interactions in solution. Polysaccharides 1: Structure, Characterization And Use. 2005;186:211–254. Advances In Polymer Science. [Google Scholar]
- 18.Hejazi R, Amiji M. Chitosan-based gastrointestinal delivery systems. J. Control. Release. 2003;89:151–165. doi: 10.1016/s0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 19.Boekhorst J, Helmer Q, Kleerebezem M, Siezen RJ. Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology-(UK) 2006;152:273–280. doi: 10.1099/mic.0.28415-0. [DOI] [PubMed] [Google Scholar]
- 20.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 22.De Ascentiis A, Degrazia JL, Bowman CN, Colombo P, Peppas NA. Mucoadhesion Of Poly(2-Hydroxyethyl Methacrylate) Is Improved When Linear Poly(Ethylene Oxide) Chains Are Added To The Polymer Network. J. Control. Release. 1995;33:197–201. [Google Scholar]
- 23.Zisman WA. Influence of Constitution on Adhesion. In: Skeist I, editor. Handbook of Adhesives. Van Nostrand Reinhold Company; New York, NY: 1977. pp. 33–66. I. Skeist, ed. [Google Scholar]
- 24.Chickering DE, Mathiowitz E. Definitions, Mechanisms, and Theories of Bioadhesion. In: Mathiowitz E, Chickering DE, Lehr CM, editors. Bioadhesive Drug Delivery Systems: Fundamentals, Novel Approaches, and Development. Marcel Dekker, Inc.; New York, NY: 1999. pp. 1–10. E. Mathiowitz, D. E. Chickering, and C. M. Lehr, eds. [Google Scholar]
- 25.Junginger HE, Thanou M, Verhoef JC. Mucoadhesive Hydrogels in Drug Delivery. In: Swarbrickand J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology. Marcel Dekker, Inc.; New York, NY: 2002. pp. 1848–1863. J. Swarbrickand J. C. Boylan, eds. [Google Scholar]
- 26.Peppas NA, Buri PA. Surface, Interfacial and Molecular Aspects of Polymer Bioadhesion on Soft Tissues. J. Control. Release. 1985;2:257–275. [Google Scholar]
- 27.Peppas NA, Little MD, Huang Y. Bioadhesive Controlled Release Systems. In: Wise DL, editor. Handbook of Pharmaceutical Controlled Release Technology. Marcel Dekker, Inc.; New York, NY: 2000. pp. 255–269. D. L. Wise, ed. [Google Scholar]
- 28.Smart JD. Recent developments in the use of bioadhesive systems for delivery of drugs to the oral cavity. Crit. Rev. Ther. Drug Carr. Syst. 2004;21:319–344. doi: 10.1615/critrevtherdrugcarriersyst.v21.i4.20. [DOI] [PubMed] [Google Scholar]
- 29.Smith DC. Adhesives and Sealants. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science: An Introduction to Materials in Medicine. Academic Press; San Diego, CA: 2004. pp. 572–583. B. D. Ratner, A. S. Hoffman, F. J. Schoen, and J. E. Lemons, eds. [Google Scholar]
- 30.Hubbell JA. Biomaterials In Tissue Engineering. Bio-Technology. 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 31.Kast CE, Frick W, Losert U, Bernkop-Schnurch A. Chitosan-thioglycolic acid conjugate: a new scaffold material for tissue engineering? Int. J. Pharm. 2003;256:183–189. doi: 10.1016/s0378-5173(03)00076-0. [DOI] [PubMed] [Google Scholar]
- 32.Reyes CD, Garcia AJ. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J. Biomed. Mater. Res. Part A. 2003;65A:511–523. doi: 10.1002/jbm.a.10550. [DOI] [PubMed] [Google Scholar]
- 33.Derjaguin BV, Aleinikova IN, Toporov YP. On The Role Of Electrostatic Forces In The Adhesion Of Polymer Particles To Solid-Surfaces. Prog. Surf. Sci. 1994;45:119–123. [Google Scholar]
- 34.Gu JM, Robinson JR, Leung SHS. Binding Of Acrylic Polymers To Mucin Epithelial Surfaces - Structure-Property Relationships. Crit. Rev. Ther. Drug Carr. Syst. 1988;5:21–67. [PubMed] [Google Scholar]
- 35.Mikos AG, Peppas NA. Measurement Of The Surface-Tension Of Mucin Solutions. Int. J. Pharm. 1989;53:1–5. [Google Scholar]
- 36.Kaelble DH, Moacanin J. Surface-Energy Analysis Of Bioadhesion. Polymer. 1977;18:475–482. [Google Scholar]
- 37.Lehr CM, Bodde HE, Bouwstra JA, Junginger HE. A Surface-Energy Analysis Of Mucoadhesion.2. Prediction Of Mucoadhesive Performance By Spreading Coefficients. Eur. J. Pharm. Sci. 1993;1:19–30. [Google Scholar]
- 38.Lehr CM, Bouwstra JA, Bodde HE, Junginger HE. A Surface-Energy Analysis Of Mucoadhesion - Contact-Angle Measurements On Polycarbophil And Pig Intestinal-Mucosa In Physiologically Relevant Fluids. Pharm. Res. 1992;9:70–75. doi: 10.1023/a:1018931811189. [DOI] [PubMed] [Google Scholar]
- 39.Ponchel G, Touchard F, Wouessidjewe D, Duchene D, Peppas NA. Bioadhesive Analysis Of Controlled-Release Systems.3. Bioadhesive And Release Behavior Of Metronidazole-Containing Poly(Acrylic Acid)-Hydroxypropyl Methylcellulose Systems. Int. J. Pharm. 1987;38:65–70. [Google Scholar]
- 40.Dodou D, Breedveld P, Wieringa PA. Mucoadhesives in the gastrointestinal tract: revisiting the literature for novel applications. Eur. J. Pharm. Biopharm. 2005;60:1–16. doi: 10.1016/j.ejpb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Hou SYE, Cowles VE, Berner B. Gastric retentive dosage forms: A review. Crit. Rev. Ther. Drug Carr. Syst. 2003;20:461–497. doi: 10.1615/critrevtherdrugcarriersyst.v20.i6.30. [DOI] [PubMed] [Google Scholar]
- 42.Talukder R, Fassihi R. Gastroretentive delivery systems: A mini review. Drug Dev. Ind. Pharm. 2004;30:1019–1028. doi: 10.1081/ddc-200040239. [DOI] [PubMed] [Google Scholar]
- 43.Decuzzi P, Lee S, Decuzzi M, Ferrari M. Adhesion of microfabricated particles on vascular endothelium: A parametric analysis. Ann. Biomed. Eng. 2004;32:793–802. doi: 10.1023/b:abme.0000030255.36748.d3. [DOI] [PubMed] [Google Scholar]
- 44.Ishida M, Machida Y, Nambu N, Nagai T. Pharmaceutical Interactions In Dosage Forms And Processing.21. New Mucosal Dosage Form Of Insulin. Chem. Pharm. Bull. 1981;29:810–816. doi: 10.1248/cpb.29.810. [DOI] [PubMed] [Google Scholar]
- 45.Nagai T, Machida Y. Advances In Drug Delivery - Mucosal Adhesive Dosage Forms. Pharm. Int. 1985;6:196–200. [Google Scholar]
- 46.Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Christensen J. Motility of the intestine. In: Sleisengerand MH, Fordtran JS, editors. Gastrointestinal Disease: Pathophysiology/Diagnosis/Management. W.B. Saunders Co.; Philadelphia, PA: 1993. pp. 822–837. M. H. Sleisengerand J. S. Fordtran, eds. [Google Scholar]
- 48.Dekker J, Rossen JWA, Buller HA, Einerhand AWC. The MUC family: an obituary. Trends Biochem.Sci. 2002;27:126–131. doi: 10.1016/s0968-0004(01)02052-7. [DOI] [PubMed] [Google Scholar]
- 49.Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: A family of human mucins with impact in cancer biology. Crit. Rev. Clin. Lab. Sci. 2004;41:189–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- 50.Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 51.Moncada DM, Kammanadiminti SJ, Chadee K. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 2003;19:305–311. doi: 10.1016/s1471-4922(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 52.Gum JR. Human mucin glycoproteins: Varied structures predict diverse properties and specific functions. Biochem. Soc. Trans. 1995;23:795–799. doi: 10.1042/bst0230795. [DOI] [PubMed] [Google Scholar]
- 53.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol.-Gastroint. Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 54.Lehr CM, Poelma FGJ, Junginger HE, Tukker JJ. An Estimate Of Turnover Time Of Intestinal Mucus Gel Layer In The Rat Insitu Loop. Int. J. Pharm. 1991;70:235–240. [Google Scholar]
- 55.Van Klinken BJW, Dekker J, Buller HA, DeBolos C, Einerhand AWC. Biosynthesis of mucins (MUC2-6) along the longitudinal axis of the human gastrointestinal tract. Am. J. Physiol.-Gastroint. Liver Physiol. 1997;36:G296–G302. doi: 10.1152/ajpgi.1997.273.2.G296. [DOI] [PubMed] [Google Scholar]
- 56.Van Klinken BJW, Dekker J, Buller HA, Einerhand AWC. Mucin Gene Structure And Expression - Protection Vs Adhesion. Am. J. Physiol.-Gastroint. Liver Physiol. 1995;32:G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- 57.Sellers LA, Allen A, Morris ER, Rossmurphy SB. Mucus Glycoprotein Gels - Role Of Glycoprotein Polymeric Structure And Carbohydrate Side-Chains In Gel-Formation. Carbohydr. Res. 1988;178:93–110. doi: 10.1016/0008-6215(88)80104-6. [DOI] [PubMed] [Google Scholar]
- 58.Gum JR, Hicks JW, Toribara NW, Rothe EM, Lagace RE, Kim YS. The Human Muc2 Intestinal Mucin Has Cysteine-Rich Subdomains Located Both Upstream And Downstream Of Its Central Repetitive Region. J. Biol. Chem. 1992;267:21375–21383. [PubMed] [Google Scholar]
- 59.Allen A, Hutton DA, Pearson JP, Sellers LA. Mucus Glycoprotein Structure, Gel Formation And Gastrointestinal Mucus Function. Ciba Foundation Symp. 1984;109:137–156. doi: 10.1002/9780470720905.ch10. [DOI] [PubMed] [Google Scholar]
- 60.Jabbari E, Wisniewski N, Peppas NA. Evidence Of Mucoadhesion By Chain Interpenetration At A Poly(Acrylic Acid) Mucin Interface Using Atr-Ftir Spectroscopy. J. Control. Release. 1993;26:99–108. [Google Scholar]
- 61.Peppas NA. Molecular calculations of poly(ethyleneglycol) transport across a swollen poly(acrylic acid) mucin interface. J. Biomater. Sci.-Polym. Ed. 1998;9:535–542. doi: 10.1163/156856298x00028. [DOI] [PubMed] [Google Scholar]
- 62.Peppas NA, Mongia NK. Ultrapure poly(vinyl alcohol) hydrogels with mucoadhesive drug delivery characteristics. Eur. J. Pharm. Biopharm. 1997;43:51–58. [Google Scholar]
- 63.Sahlin JJ, Peppas NA. Enhanced hydrogel adhesion by polymer interdiffusion: Use of linear poly(ethylene glycol) as an adhesion promoter. J. Biomater. Sci.-Polym. Ed. 1997;8:421–436. doi: 10.1163/156856297x00362. [DOI] [PubMed] [Google Scholar]
- 64.Efremova NV, Huang Y, Peppas NA, Leckband DE. Direct measurement of interactions between tethered poly(ethylene glycol) chains and adsorbed mucin layers. Langmuir. 2002;18:836–845. [Google Scholar]
- 65.Huang YB, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: Tethered structures and site-specific surfaces. J. Control. Release. 2000;65:63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 66.Huang YB, Szleifer I, Peppas NA. Gel-gel adhesion by tethered polymers. J. Chem. Phys. 2001;114:3809–3816. [Google Scholar]
- 67.Huang YB, Szleifer I, Peppas NA. A molecular theory of polymer gels. Macromolecules. 2002;35:1373–1380. [Google Scholar]
- 68.Brochard-Wyart F, Degennes PG, Leger L, Marciano Y, Raphael E. Adhesion Promoters. J. Phys. Chem. 1994;98:9405–9410. [Google Scholar]
- 69.Degennes PG. Conformations Of Polymers Attached To An Interface. Macromolecules. 1980;13:1069–1075. [Google Scholar]
- 70.Leger L, Raphael E, Hervet H. Surface-anchored polymer chains: Their role in adhesion and friction. Polymers In Confined Environments. 1999;138:185–225. Advances In Polymer Science. [Google Scholar]
- 71.Serra L, Doménech J, Peppas NA. Design of poly(ethylene glycol)-tethered copolymers as novel mucoadhesive drug delivery systems. Eur. J. Pharm. Biopharm. 2006;63:11–18. doi: 10.1016/j.ejpb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Takishima J, Onishi H, Machida Y. Prolonged intestinal absorption of cephradine with chitosan-coated ethylcellulose microparticles in rats. Biol. Pharmacol. Bull. 2002;25:1498–1502. doi: 10.1248/bpb.25.1498. [DOI] [PubMed] [Google Scholar]
- 73.Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv. Drug Deliv. Rev. 2004;56:425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 74.Lehr CM. Lectin-mediated drug delivery: The second generation of bioadhesives. J. Control. Release. 2000;65:19–29. doi: 10.1016/s0168-3659(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 75.Drotleff S, Lungwitz U, Breunig M, Dennis A, Blunk T, Tessmar J, Gopferich A. Biomimetic polymers in pharmaceutical and biomedical sciences. Eur. J. Pharm. Biopharm. 2004;58:385–407. doi: 10.1016/j.ejpb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 76.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 77.Mann BK, Tsai AT, Scott-Burden T, West JL. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20:2281–2286. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 78.Ruoslahti E, Pierschbacher MD. New Perspectives In Cell-Adhesion - Rgd And Integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 79.Xu LC, Vadillo-Rodriguez V, Logan BE. Residence time, loading force, pH, and ionic strength affect adhesion forces between colloids and biopolymer-coated surfaces. Langmuir. 2005;21:7491–7500. doi: 10.1021/la0509091. [DOI] [PubMed] [Google Scholar]
- 80.Stevens MM, Allen S, Davies MC, Roberts CJ, Schacht E, Tendler SJB, VanSteenkiste S, Williams PM. The development, characterization, and demonstration of a versatile immobilization strategy for biomolecular force measurements. Langmuir. 2002;18:6659–6665. [Google Scholar]
- 81.Camesano TA, Logan BE. Probing bacterial electrosteric interactions using atomic force microscopy. Environ. Sci. Technol. 2000;34:3354–3362. [Google Scholar]
- 82.Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 83.Kelm S, Schauer R. International Review Of Cytology - A Survey Of Cell Biology, Vol 175. Vol. 175. Academic Press Inc; San Diego: 1997. Sialic acids in molecular and cellular interactions; pp. 137–240. International Review Of Cytology-A Survey Of Cell Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]