Abstract
Objectives:
In the early 1990s, minimally invasive videoscopy was applied to numerous operations. After undertaking more than 50 “open” Heller myotomies, our experience with videoscopic Heller myotomy began in 1992. We sought to determine whether the outcome following videoscopic Heller myotomy is influenced by surgeon experience.
Methods:
Seventy-eight patients with severe dysphagia secondary to achalasia underwent videoscopic Heller myotomy between 1992 and 1998. Intraoperative endoscopy was utilized to ensure adequate myotomy in all patients. Patients were stratified into 3 groups: the first 25 patients (group I), the second 25 patients (group II), and the last 28 patients (group III). Clinical outcome was based on length of stay, incidence of intraoperative complications, conversion to an ‘open’ procedure, and postoperative symptoms.
Results:
Perioperative complications occurred in 20% of patients in group I compared with 8% and 12% in groups II and III, respectively (P = NS). Only 3 patients required conversion to an ‘open’ procedure, all in group I (P < 0.05). Symptomatic improvement was achieved in 80% of patients in group I, 100% in group II, and 96% in group III (P < 0.05). Significant reductions in conversions to ‘open,’ length of stay, and postoperative symptoms were seen after 20 myotomies were undertaken.
Conclusion:
Outcome following videoscopic Heller myotomy, like other videoscopic operations, improves as surgeons progress along the videoscopic “learning curve.” After approximately 20 videoscopic Heller myotomies, surgeons can expect fewer conversions to open procedures, shorter hospital stays, and better symptomatic relief.
Keywords: Achalasia, Heller, Videoscopy, Learning curve
INTRODUCTION
In the late 1980s, videoscopy began to be widely applied to the surgical management of diseases of the gallbladder. Since its inception, established surgeons and residents alike have embarked upon the “learning curve” of laparoscopy and thoracoscopy. Many studies have suggested that the true efficacy of laparoscopic cholecystectomy is related to the surgeon's experience with laparoscopy.1–3 Similar conclusions have been drawn in laparoscopic gynecologic procedures,4 colectomy,5 inguinal hernia repair,6 Nissen fundoplication,7 and nephrectomy/adrenalectomy.8 The utility of videoscopy ultimately requires acceptance and teachability to surgeons, including those in training. This subject has been addressed in several studies showing that less experienced surgeons can benefit from their senior counterparts when properly supervised during videoscopy.9–12
As videoscopic techniques were rapidly applied to a variety of operations, surgeons had to modify their approaches to diseases. As such, the “learning curve” involved more than just learning how to use new instruments. It also involved modification of surgical procedures as surgeons learned by trial and error and added new experiences to their databases.13–14 Experiences gleamed from 1 procedure, in turn, could be applied to other videoscopic operations.15
Videoscopy was quickly embraced at our institution and applied to a variety of procedures. Our experience with advanced videoscopy began early and much of our interest has focused on diseases involving the upper gastrointestinal tract (ie, gastroesophageal reflux, achalasia, esophageal cancer). Although the literature is replete with reports on laparoscopic antireflux procedures, few studies have addressed the utility of videoscopy in the management of achalasia. We discuss our experience with videoscopic Heller myotomy and how we progressed along the “learning curve.” Our purpose in undertaking this study was to review our experience with videoscopic Heller myotomy and to determine the ‘break point’ at which improved outcomes can be expected.
METHODS
Between June of 1992 and November of 1998, patients with dysphagia, often with the diagnosis of achalasia, were referred to our institution by their primary care physicians and gastroenterologists. The diagnosis of achalasia was secured by barium esophagram and esophageal manometry in all patients. Videoscopic Heller myotomy was undertaken in these patients via either a thoracoscopic or laparoscopic approach. Our techniques for laparoscopic and thoracoscopic Heller myotomy have been described previously16 and will be reviewed briefly.
Thoracoscopic Heller myotomy was undertaken utilizing a 5-port approach with the patient in the right lateral decubitus position. Concomitant endoscopy was undertaken per os and used to assist in the localization of the gastroesophageal junction. Myotomy was completed along the posterolateral wall of the lower esophageal sphincter using the 90°-angle hook cautery and extended down onto the gastric cardia until the endoscope was easily passed into the stomach. Myotomy was considered complete once the endoscopist could easily distend the lower esophageal sphincter with gentle insufflation using the endoscope. The integrity of the myotomized segment was verified by instilling saline around the esophageal hiatus and insufflating the esophagus using the endoscope. The presence of extraluminal bubbles suggested esophageal perforation. An angled thoracostomy tube was left in place through the most inferior and posterior port at the conclusion of the operation.
Laparoscopic Heller myotomy also utilized 5 ports with the patient in the supine position. Again, the gastroesophageal junction was localized with the aid of the endoscopist. The myotomy was completed along the anterior wall of the esophagus and extended caudally onto the cardia. Once the endoscope could be easily passed into the stomach and the lower esophageal sphincter easily distended with gentle insufflation, the myotomy was considered complete. The esophagus was examined for leaks by instilling saline around the esophageal hiatus and looking for bubbles as the endoscopist insufflated the esophagus.
Fundoplication was undertaken selectively in patients with a large hiatus hernia, a patulous esophageal hiatus, or as part of a repair of an intraoperative esophageal perforation. A floppy 270° posterior (Toupet) fundoplication was undertaken after division of the short gastric vessels and closure of the esophageal hiatus and secured to the right crus of the diaphragm. Tension-free Dor fundoplication was completed prior to hiatus closure by securing the anterior fundus along the perimeter of the myotomized segment of esophagus.
All patients were studied in the early postoperative period with an esophagram to evaluate for esophageal perforation and to assess esophageal motility. If no leak was detected and esophageal emptying was swift, patients were started on a soft mechanical diet and discharged. Patients with slow postoperative esophageal emptying secondary to edema were maintained on liquid diets for 1 to 3 days until swallowing improved. They were then advanced to soft mechanical diets and discharged.
Patients were followed in the clinic and by telephone postoperatively. They were queried about symptoms and asked to grade their symptoms relative to their preoperative status. Outcome was considered excellent if their symptoms, by their estimation, had completely resolved, good if they were much improved, fair if they were slightly improved, and poor if they had no improvement or had worsening of symptoms. Patients were also asked to grade the severity of their reflux symptoms on a scale of 0 to 5 (0-absent/rare, 1-occasional, 2-monthly, 3-bimonthly, 4-weekly, 5-daily).
Patients were arbitrarily divided into 3 groups: the first 25 patients (group I), the second 25 patients (group II), and the last 28 patients (group III). Data were maintained in a Microsoft® Excel spreadsheet. Data were compared utilizing Student t tests, Fisher's exact test, and chi-square analysis when appropriate. Statistical comparisons were completed utilizing True Epistat (Epistat Services, Richardson, TX).
RESULTS
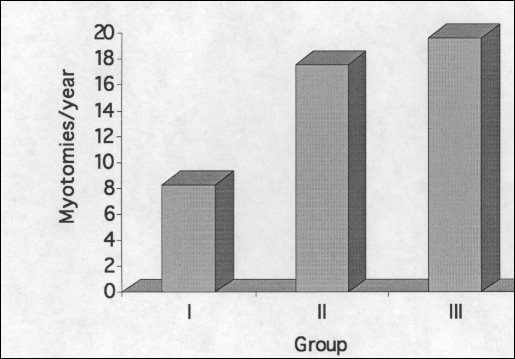
Between June 1992 and November 1998, 78 consecutive patients underwent videoscopic Heller myotomy. Videoscopic Heller myotomy was undertaken in group I between June 1992 and June 1995, in group II between September 1995 and May 1997, and in group III between June 1997 and November 1998 (Figure 1). Each group of patients had similar sex and age distributions (Table 1). All patients in each group complained of dysphagia. Postprandial chest pain and regurgitation were also common. Heartburn was rarely experienced in group I prior to myotomy although it was fairly common in the remaining patients. Most patients in each group had undergone previous esophageal dilations or Botox injections with many patients undergoing both of these nonoperative treatment modalities. Few patients in any group were taking calcium channel blockers or long-acting nitrates at the time of operation although most patients had tried these medications at some point in the past.
Figure 1.
Frequency of Heller Myotomy by group.
Table 1.
Patient Demographics.
| Group | I | II | III | |
|---|---|---|---|---|
| Male/Female | 14/11 | 13/12 | 16/12 | |
| Age ± SD (range) | 56.6 ± 20.2 (18-91) | 48.2 ± 18.1 (20-80) | 47.9 ± 18.2 (14-89) | |
| Postprandial Symptoms | Dysphagia | 25 (100%) | 25 (100%) | 28 (100%) |
| Regurgitation* | 11 (44%) | 2 (8%) | 19 (68%) | |
| CP | 11 (44%) | 11 (44%) | 11 (39%) | |
| Heartburn* | 4 (16%) | 14 (56%) | 13 (46%) | |
| Preoperative Interventions | Dilation | 16 (64%) | 13 (52%) | 11 (39%) |
| Botox | 8 (32%) | 15 (60%) | 14 (36%) | |
| Both | 7 (25%) | 8 (32%) | 14 (36%) | |
| Neither | 8 (32%) | 5 (20%) | 3 (11%) | |
| Nifedipine | 7 (25%) | 6 (24%) | 6 (21%) | |
| Isordil | 3 (12%) | 2 (8%) | 1 (4%) | |
CP = Chest pain;
= P < 0.05, Chi-square analysis.
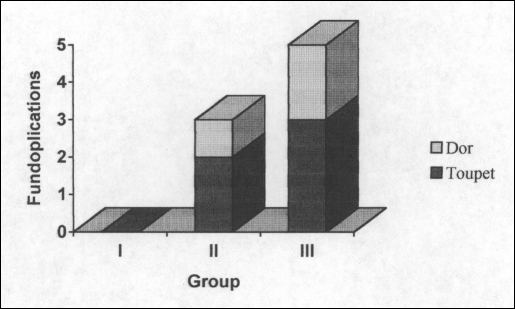
Thoracoscopic Heller myotomy was undertaken in 11 of the first 14 patients (Table 2). All remaining patients underwent laparoscopic Heller myotomy. Epiphrenic diverticulectomy and cholecystectomy were rarely undertaken. An antireflux procedure was not routinely applied, with only 8 patients undergoing fundoplication at the time of their myotomy (Figure 2). Toupet fundoplication was undertaken in 5 patients (2 in group II and 3 in group III), and 3 patients underwent Dor fundoplication (1 in group II and 2 in group III). No patients in group I underwent fundoplication. More complications occurred in the first 25 patients compared with the other 2 groups, and no procedures were converted to “open” after the fourteenth operation (Table 2).
Table 2.
Videoscopic Heller Myotomy.
| Group | I | II | III | |
|---|---|---|---|---|
| Thoracoscopic/Laparoscopic* | 11/14 | 0/25 | 0/28 | |
| Concomitant Procedures | Fundoplication | 0 | 3 (12%) | 5 (18%) |
| Diverticulectomy | 2 (8%) | 0 | 1 (4%) | |
| Cholecystectomy | 0 | 1 (4%) | 0 | |
| Complications | 5 (20%) | 2 (8%) | 3 (12%) | |
| Conversions to ‘open’† | 3 (12%) | 0 | 0 | |
= P < 0.05, Chi-square analysis;
= P < 0.05, Log-likelihood ratio.
Figure 2.
Frequency of fundoplication by group.
Complications occurred in 10 patients (Table 3). Three esophageal perforations occurred in the first 25 patients. These all occurred during thoracoscopy and all required “mini-thoracotomy” for repair. Two of the 3 perforations were recognized and addressed at the time of the index operation. The third perforation was recognized on a routine postoperative esophagram and was repaired in the operating room via a “mini-thoracotomy.” One enterotomy occurred during laparoscopy in the first group of patients. This patient had undergone previous thoracic, abdominal, and thoracoabdominal operations. The patient developed intraabdominal sepsis requiring celiotomy and enterorrhaphy. After a prolonged hospital course, the patient was discharged in good condition. One patient in the first group developed transient renal insufficiency, which was self-limiting. One esophageal perforation occurred in the second group of 25 patients. This perforation was recognized at the time of operation and repaired laparoscopically. An anterior fundoplication was constructed in this patient to secure the esophageal repair. The pleural space was violated in 1 patient in group II. The resulting pneumothorax was managed expectantly and resolved without the need for tube thoracostomy. One patient in group III suffered an esophageal perforation. This was also repaired laparoscopically and buttressed with an anterior fundoplication. One patient in the last group of patients was readmitted for nausea and vomiting that resolved spontaneously, and urinary retention developed in 1 elderly male with prostatism. No deaths occurred in any group.
Table 3.
Complications Following Videoscopic Heller Myotomy.
| Total | 10 |
| Group I | 3 esophageal perforations |
| 1 enterotomy | |
| 1 renal insufficiency | |
| Group II | 1 esophageal perforation |
| 1 pleura violation | |
| Group III | 1 esophageal perforation |
| 1 nausea/vomiting | |
| 1 urinary retention | |
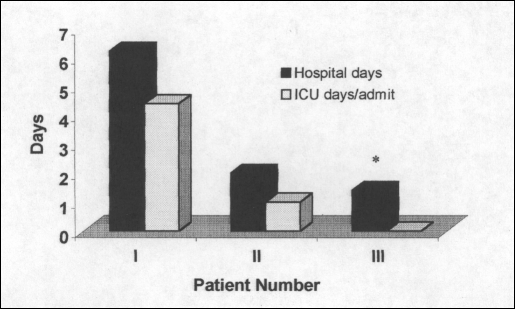
The average length of hospitalization and need for ICU monitoring decreased steadily throughout the study (Figure 3). The mean length of stay for the first 25 patients was 6.2 days ± 11.5. This compares with 2.0 days ± 1.6 for the second group of 25 patients (P = 0.08 vs group I) and 1.4 days ± 1.1 for the last 28 patients (P =0.05 vs group I). Seven patients were admitted to the ICU for an average of 4.4 days each in group I with 2 patients spending 1 day each in the ICU in group II. No patients in group III required ICU monitoring.
Figure 3.
Length of stay and ICU admissions.
All patients were available for follow-up in groups I and II, and 93% were available from group III (Table 4). As expected, the mean follow-up for group I was significantly longer than that for either group II or III. At least mild postoperative dysphagia occurred more frequently in the first 25 patients when compared with that in the remaining 2 groups, although this did not reach statistical significance (P = 0.07) (Table 4). Postoperative reflux scores were similar for all groups (Table 4). Based on recommendations from their gastroenterologists, 4 patients in group I underwent postoperative balloon dilation, as did 1 patient in group III. Similarly, 2 additional patients in group I underwent further Botox injections. One patient in each group underwent esophagectomy for severe symptoms due to proximal esophageal dysfunction, 2 at another institution. One patient in group I underwent Toupet fundoplication to treat symptomatic acid reflux 4 years after myotomy. In total, 8 patients in group I underwent further intervention after myotomy compared with 3 patients out of the remaining 53 patients (P = 0.004, Fisher's exact test). Improvement in symptoms was seen in 80% of patients in group I after videoscopic Heller myotomy. This compares to 100% and 96% in groups II and III, respectively (P = 0.02, chi-square analysis) (Table 4).
Table 4.
Follow-up.
| Group | I | II | III | |
|---|---|---|---|---|
| Patients available for F/U | 25 (100%) | 25 (100%) | 26 (93%) | |
| Mean F/U (Months ± SD) | 36.5 ± 19.7 | 30.9 ± 17.6 | 18.8 ± 13.4*† | |
| Postop Symptoms | Dysphagia§ | 56% | 24% | 44% |
| Regurgitation | 12% | 4% | 19% | |
| Chest pain | 16% | 8% | 19% | |
| Reflux Score (Mean ± SD) | 1.2 ± 1.7 | 1.2 ± 1.2 | 1.7 ± 2.1 | |
| Follow-up Procedures | Dilation† | 4 (16%) | 0% | 1 (4%) |
| Botox | 2 (8%) | 0% | 0% | |
| Other | 2 (8%) | 1 (4%) | 1 (4%) | |
| Outcome | Excellent | 56% | 76% | 73% |
| Good | 16% | 12% | 15% | |
| Fair | 8% | 12% | 8% | |
| Poor‡ | 20% | 0% | 4% | |
= P < 0.05 vs group I, Student t test;
= P < 0.01 vs group II, Student t test;
= P < 0.05, chi-square analysis;
= P = 0.07, Chi-square analysis.
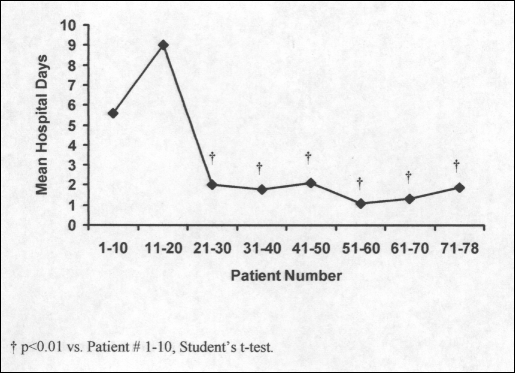
The mean length of hospitalization decreased significantly after 20 consecutive videoscopic Heller myotomies (Figure 4). The average length of stay for the first 20 patients was 7.9 days and fell to 1.7 days for the remaining 58 patients. As such, the overall rate of complication, esophageal perforation, and conversion to “open” fell dramatically after 20 myotomies were completed (Table 5). Similarly, improvements in patients' symptoms were much more common after completion of 20 or more myotomies.
Figure 4.
Length of stay per 10 patients.
Table 5.
The “Magic Number.”
| Patient #1-20 | Patient #21-78 | |
|---|---|---|
| Complications | 5 (25%) | 5 (9%) |
| Perforations | 3 (15%) | 2 (3%) |
| Conversions | 3 (15%)* | 0 |
| Months F/U | ||
| (Mean ± SD) | 37.4 ± 20.4† | 25.0 ± 16.6 |
| Postoperative dysphagia | 50% | 39% |
| % improvement | 80%* | 96% |
| Postoperative reflux score | 0.8 ± 1.6 | 1.5 ± 1.7 |
= P < 0.05, Fisher's exact test;
= P < 0.05, Student t test.
DISCUSSION
Since the advent of videoscopy, many authors have written about the “learning curve” in terms of operative time, complications, length of stay, procedure-related costs, and outcomes following videoscopic surgery. Although the literature is replete with studies assessing the “learning curve” in a variety of videoscopic procedures, little has been written about videoscopic Heller myotomy. This study includes a relatively large number of patients with relatively longer follow-up after undergoing videoscopic Heller myotomy for definitive management of achalasia. We have shown that after we undertook 20 videoscopic Heller myotomies, we noted improved outcomes with fewer intraoperative and postoperative complications.
The patients in this clinical series were generally older, though both young and old patients were operated upon. Generally, symptoms had been noted for years and reflected the sensation of a delayed passage of a food bolus, usually solids. Notably, chest pain and reflux were common complaints as well. Generally, myotomy was undertaken as a salvage procedure, as most patients had undergone either esophageal dilation or Botox injection, and sometimes both, prior to referral for myotomy. In summary, these were not well-selected groups of patients, but rather “all comers.”
Early in our experience, we undertook relatively fewer myotomies per year and, initially, utilized a thoracoscopic approach. This approach was abandoned for a laparoscopic (or celioscopic) approach because of the anticipated ease of the procedure. The higher complication rate seen by our first patients undoubtedly reflects several factors: the thoracoscopic approach, the advent of videoscopic application to achalasia, and the advent of videoscopy in general. In addition, it can be argued that the first patients referred for videoscopic myotomy had the most severe and complicated achalasia, as if they had been waiting for the advent of what they hoped would be a less invasive operative therapy. Though no data exist to strongly support this premise, isolated patients in our first 25 videoscopic myotomies had exceptionally difficult operations (ie, concomitant diverticulectomies, adhesiolysis, and others.). Nonetheless, along broad schemes, the patient groups are comparable and meaningful conclusions can be drawn.
Notable complications, thankfully infrequent, occurred in our first group of patients. Esophageal perforations in the later groups of patients did not add to postoperative morbidity, only to the length of the operative procedure. A decrease in postoperative symptoms and the need for remedial postoperative interventions in the later groups reflected improved technical success with myotomy. In other words, with experience, we got better at doing enough without doing too much. Possibly, this was assisted by our growing experience with intraoperative endoscopy and the guidance it provides. For all these reasons, our best results occurred in our last group of patients.
We suggest that after 20 myotomies, the operating surgeon and team have accumulated sufficient experience and confidence such that care is more appropriately safe and effective. We suggest that these first 20 operations are the “learning curve” and that 20 operations is the “magic number” after which myotomy can be undertaken with less trepidation, insecurity, and uncertainty. But, this series includes not only our first experiences with videoscopic Heller myotomy, but also our early experience with videoscopy in general. As such, we were progressing along the learning curve for a variety of advanced videoscopic procedures and, therefore, several factors were involved in improving outcomes after our first 20 videoscopic Heller myotomies. First, and most obvious, was simply becoming more familiar with the videoscopic instruments and becoming adept at using them. Because we were beginning our experience with a variety of videoscopic procedures in the early 1990s, including cholecystectomy, fundoplication, adrenalectomy, and esophagectomy, lessons learned from other procedures were applied to videoscopic Heller myotomy. Additionally, videoscopic equipment improved at all levels. Cameras, monitors, insufflators, graspers, retractors, and others all improved. Assistance to the operating surgeon also improved, whether we consider nursing help, resident surgeon skills, or attending surgeon skills. As such, the “magic number” may actually be less than 20 now, because most surgeons are more familiar with videoscopy in general. Even as surgeons become adept at other advanced videoscopic techniques, the necessary coordination with the gastroenterologist undertaking concomitant intraoperative endoscopy and the radiolo-gist completing the proper postoperative contrast study in addition to the surgeon achieving an appropriate comfort level in postoperative management limit how short the learning curve can be.
Videoscopy, as we start the new millennium, is certainly better, more efficacious, and safer than it was from 1992 to 1998. Improvements continue, though any curve drawn to reflect these measures of our care and expertise are flatter than they were. We continue to experience perforations and postoperative complaints and not all patients today have perfect outcomes, but such outcomes are more likely than when we first embarked upon the “learning curve.”
Footnotes
Presented at the 9th International Meeting of Laparoendoscopic Surgeons, SLS Annual Meeting, Endo Expo 2000, December 6-9, 2000 in Orlando, Florida.
Contributor Information
Mark Bloomston, Department of Surgery..
Francesco Serafini, Department of Surgery..
H. Worth Boyce, Center for Swallowing Disorders..
Alexander S. Rosemurgy, Department of Surgery..
References:
- 1. Sariego J, Spitzer L, Matsumoto T. The ‘learning curve’ in the performance of laparoscopic cholecystectomy. Int Surg. 1993; 78(1):1–3 [PubMed] [Google Scholar]
- 2. Lekawa M, Shapiro SJ, Gordon LA, et al. The laparoscopic learning curve. Surg Laparosc Endosc. 1995;5(6):455–458 [PubMed] [Google Scholar]
- 3. Moore MJ, Bennett CL. The learning curve for laparoscopic cholecystectomy. The Southern Surgeons Club. Am J Surg. 1995; 170(1):55–59 [DOI] [PubMed] [Google Scholar]
- 4. Lee YS. Early experience with laparoscopic pelvic lymphadenectomy in women with gynecologic malignancy. J Am Assoc Gynecol Laparosc. 1999;6(1):59–63 [DOI] [PubMed] [Google Scholar]
- 5. Simons AJ, Anthone GJ, Ortega AE, et al. Laparoscopic-assisted colectomy learning curve. Dis Colon Rectum. 1995; 38(6):600–603 [DOI] [PubMed] [Google Scholar]
- 6. Voitk AJ. The learning curve in laparoscopic inguinal hernia repair for the community general surgeon. Can J Surg. 1998;41(6):446–450 [PMC free article] [PubMed] [Google Scholar]
- 7. Deschamps C, Allen MS, Trastek VF, et al. Early experience and learning curve with laparoscopic Nissen fundoplication. J Thorac Cardiovasc Surg. 1998;115(2):281–285 [DOI] [PubMed] [Google Scholar]
- 8. Higashihara E, Baba S, Nakagawa K, et al. Learning curve and conversion to open surgery in cases of laparoscopic adrenalectomy and nephrectomy. J Urol. 1998;159(3):650–653 [PubMed] [Google Scholar]
- 9. Soot SJ, Eshraghi N, Farahmand M. Transition from open to laparoscopic fundoplication: the learning curve. Arch Surg. 1999; 134(3):278–282 [DOI] [PubMed] [Google Scholar]
- 10. Schol FP, Go PM, Gouma DJ, et al. Laparoscopic cholecystectomy in a surgical training programme. Eur J Surg. 1996;162(3):193–197 [PubMed] [Google Scholar]
- 11. Watson DI, Baigrie RJ, Jamieson GG. A learning curve for laparoscopic fundoplication. Definable, avoidable, or a waste of time? Ann Surg. 1996;224(2):198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rege RV, Joehl RJ. A learning curve for laparoscopic splenectomy at an academic institution. J Surg Res. 1999;81(1):27–32 [DOI] [PubMed] [Google Scholar]
- 13. Poulin EC, Mamazza J. Laparoscopic splenectomy: lessons from the learning curve. Can J Surg. 1998;41(1):28–36 [PMC free article] [PubMed] [Google Scholar]
- 14. Shwayder JM. The learning curve for laparoscopically assisted vaginal hysterectomy/laparoscopic hysterectomy. J Am Assoc Gynecol Laparosc. 1994;1(4, Part 2):S33. [DOI] [PubMed] [Google Scholar]
- 15. Agachan F, Joo JS, Sher M, et al. Laparoscopic colorectal surgery. Do we get faster? Surg Endosc. 1997;11(4):331–335 [DOI] [PubMed] [Google Scholar]
- 16. Bloomston M, Boyce W, Mamel J, et al. Videoscopic Heller myotomy for achalasia- results beyond short-term follow-up. J Surg Res. 2000;92(2):150–155 [DOI] [PubMed] [Google Scholar]