Abstract
Regardless of the level of experience, surgeons need convenient times and locations to practice laparoscopic skills. Although attendance at formal courses is essential for continuing surgical education, reinforcement is still needed by other means to allow surgeons to learn new operations as they advance. A method is presented here that allows surgeons to practice laparoscopic skills while following their own time schedules. Novice surgeons can also benefit from this method by practicing basic laparoscopic skills in a cost-effective simulated operative environment.
The Borinquen Ring pelvic trainer was originally described in 1993. It is now reintroduced along with an additional device called a Tissue Suspender to create a new laparoscopic simulation training system. Instruction is provided for the use of these 2 devices in practicing basic surgical skills. Different simulation models are used to substitute for human organs and vessels. These consist of both artificial and animal tissue specimens.
Keywords: Laparoscopy, Simulator, Trainer, Laparoscopic education
INTRODUCTION
To provide satisfactory simulation of the laparoscopic environment, one must establish what the minimum criteria of that environment are. The first requirement is the presence of set entry ports by which laparoscopic instruments are directed into the operative practice field. The second is a system by which to project the work area onto a video monitor. The third is a method that allows use of as many different tissue substitutes as possible, thereby broadening the practice experience. Presumably, meeting the above criteria in a simulation model should allow practice in such a model to transfer to the real environment.
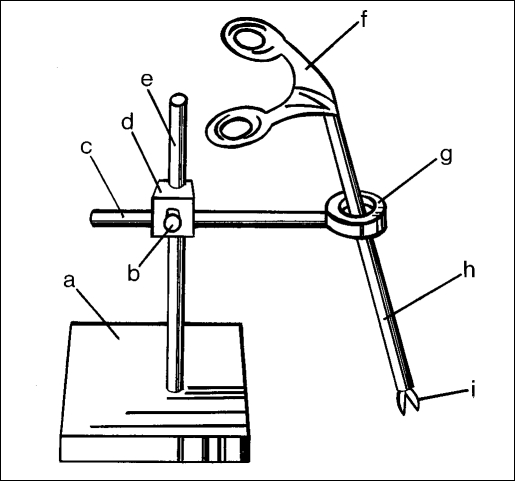
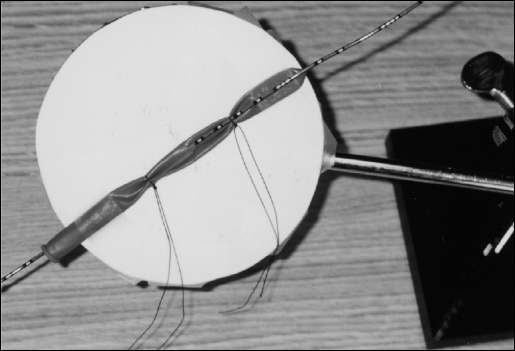
An abstract drawing of the Borinquen ring (BR)1 shows that it consists of a base holding up a vertical pole that is attached to a ring with a horizontal stem (Figure 1). A coupling device and screw are used to hold both the vertical pole and the ring stem together. Through the center of this small ring, laparoscopic instruments can be placed during training sessions. The BR limits hand movements intentionally, as is the case in laparoscopic operations. The coupler provides a means to change the angle of the ring and allow it to simulate the outer convex abdominal wall surface. A flexible membrane can also be attached to the ring to simulate the local elasticity of the abdominal wall and allow trocar placement.
Figure 1.
The Borinquen Ring. (a) stand, (b) turning screw, (c) ring stem, (d) coupler, (e) vertical pole, (f) instrument handle, (g) entry port ring, (h) instrument shaft, (i) instrument blades.
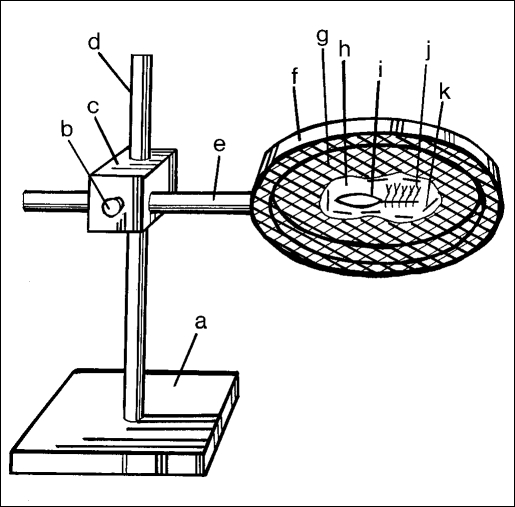
The Tissue Suspender (TS) device has a similar abstract design as the BR, but the ring in this case is much larger and has a mesh surface attached to it (Figure 2). The coupling device allows variation in placement of the mesh surface. This simulates the different tissue plane angles present along the inner concave surface of the different cavities encountered while performing video surgery. These can be the peritoneal, retroperitoneal, thoracic, or other formed working cavities. Multiple working angles are involved in laparoscopic surgery, and training sessions should be used to maximize practice in as many of these as possible.2 The Laparoscopic-Ring (Lap-Ring) Simulation Trainer comprises the TS, two BR, a cam-corder, and a television monitor.
Figure 2.
(a) stand, (b) turning screw, (c) coupler, (d) vertical pole, (e) ring stem, (f) large ring, (g) support mesh, (h) tissue specimen, (i) incision site, (j) practice sutures, (k) restraining wire.
The above 2 USA patented devices and the work that follows represent the author's attempt to create a learning environment that is unique to the needs of each individual surgeon.
MATERIALS AND METHODS
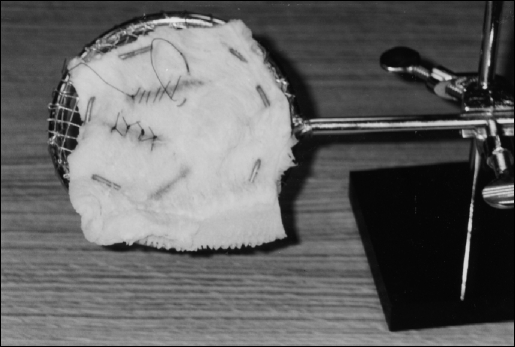
Figure 3 shows a TS with a piece of bovine stomach, which has been placed on the mesh surface, with U-shaped nails passed through it. Wire or hocks can also be used for restraint purposes. This material can be used to practice suturing and stapling. To remove some of the strong odor of bovine stomach when preparing it for use, it is best to soak it in sodium bicarbonate solution. During suturing practice, it is helpful to stain the “incision” sites with color markers to provide a contrast to the white tissue. This maneuver enhances the camcorder's automatic focusing mechanism. The Lap-Ring Simulation Trainer is demonstrated in Figure 4 with the video monitor visible in the background. Suturing practice being done at the TS is displayed on the monitor. In this exercise, the TS is placed at approximately 110° to simulate the location of a ventral hernia.
Figure 3.
Tissue Suspender (TS) with beef stomach (tripe) as practice tissue. Incision sites stained pink to facilitate camcorder focusing.
Figure 4.
Lap-Ring Simulation Trainer with TS at 110° and television monitor in background.
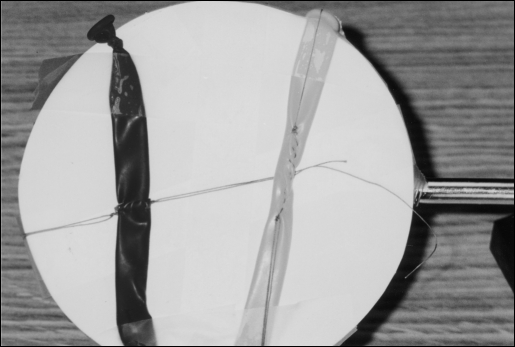
The TS wire mesh can be covered with a white circular plastic cover, on top of which artificial models can be placed. These can be used for additional practice sessions. To simulate vascular and other tubular structures, narrow balloons can be placed on the TS and used for practicing interrupted and continuous anastomoses. They are taped onto a white circular plastic cover. Figure 5 shows the general appearance of this model. In Figure 6, a close-up of a completed practice session, demonstrates an end-to-end and a running anastomosis.
Figure 5.
Lap-Ring Simulation Trainer using thin rubber balloons to simulate vessels. These have been placed on a white plastic disk that is covering the TS mesh.
Figure 6.
Anastomosis practice on TS. Red balloon (left): running end-to-end. Yellow balloon (right): running straight.
Narrow tubular balloons can also be used to simulate ductal structures, such as the common bile ducts, ureters, the urethra, and fallopian tubes. Catheterization practice can also be performed using these simple models, as demonstrated in Figure 7. When practicing anastomosis with narrow balloons, it is best to place small cylindrical plugs just inside the cut ends. This maneuver helps maintain the 2 ends open, while the surgeon is trying to anastomose them together.
Figure 7.
Catheterization practice using a cone tip ureteral stent placed into a pink balloon and tied in 2 places.
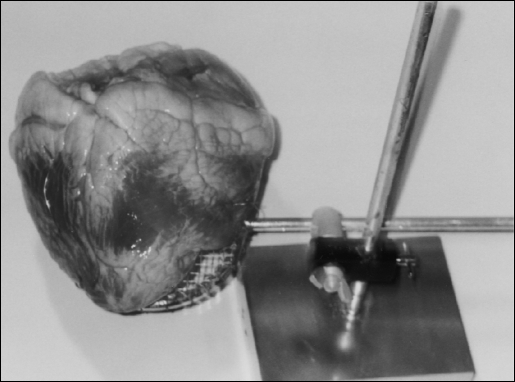
When practice on more complex natural tissues is desired, the TS can be used to suspend pieces of small bowel, or an entire animal heart, as demonstrated in Figure 8. Bovine hearts, or bowel, can be ordered from local meat markets and used repeatedly. All that is required is to occasionally sprinkle the specimens with dilute salt water solution during practice sessions. For short-term storage, the specimens can then be placed in a refrigerator. For long-term storage, a freezer is recommended. The tissue can be frozen and defrosted repeatedly for training purposes. The heart model allows practice dissecting the cardiac arteries and practice tying off these vessels. These exercises can be done either manually or with mechanical devices.
Figure 8.
Bovine heart placed on TS to use the cardiac vessels to practice dissection techniques and vessel tying or stapling.
For more advanced dissections, one can dispense with the TS and use larger natural tissue specimens placed on a flat surface below the Lap-Ring Simulation Trainer. Different animals like rabbits, pigs, and dogs can be used for this purpose. The animals are first sacrificed in a humane fashion, and then an immediate laparotomy is performed to remove the large and small intestines. Once these stool- and bacteria-containing organs are removed, the bladder is emptied by exerting pressure on the dome. The stomach can be emptied through the opening in the pylorus and irrigated with saline. The entire specimen is then washed down with saline to enhance preservation.
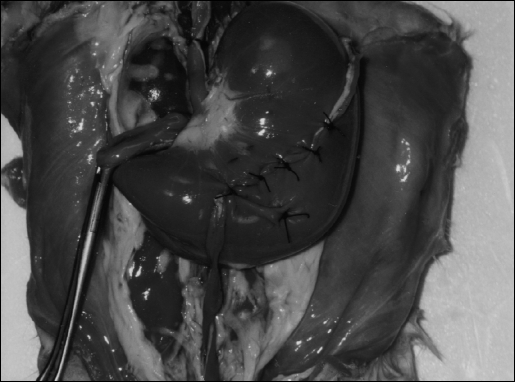
A Rabbit Tissue Model (RTM) is demonstrated in Figure 9. Here the rabbit stomach has been filled with salt water that was introduced through a catheter in the esophagus. This was done as part of a catheterization simulation exercise. An atraumatic clamp is placed across the pylorus to prevent saline loss. Once full, the rabbit stomach can be used to perform different exercises. In this figure, the reimplantation of a ureter is simulated by using a segment of the rabbit rectosigmoid. A “submucosal” tunnel was developed by dividing and dissecting free the stomach muscle for approximately 6 cm and then approximating the muscle over the “ureter” with interrupted sutures. Due to rabbit pellet stools, this portion of the bowel can be retained during the initial preparation of the RTM by simply removing the pellets and irrigating the lumen.
Figure 9.
Rabbit Tissue Model (RTM). Stomach filled with saline and used for practicing suturing and dissecting techniques.
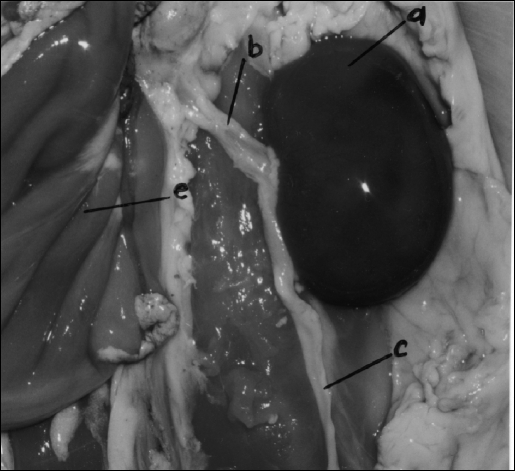
Fine dissection technique can also be practiced using the RTM as seen in Figure 10. Here the left kidney, renal vessels, and ureter down to the bladder were dissected free of their adhering fibrous tissue. The bladder can be used for placing incisions and then practicing their closure. Female rabbits are preferable because end-to-end anastomosis can be performed on the bicornate uterus. Practicing on these structures should allow transfer of skills later to human ureters or fallopian tubes (M.M., unpublished data, 2000).3
Figure 10.
RTM following dissection. (a) left kidney, (b) renal vessels, (c) left ureter, (d) bladder, (e) empty stomach, (f) sigmoid remnant.
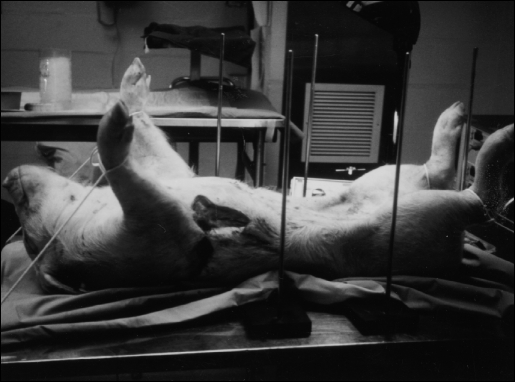
Even larger animal carcasses can be placed under the Lap-Ring Simulation Trainer. In Figure 11, a small pig is used as a pig tissue model (PTM). The pig was sacrificed, after its parts were no longer needed in an independent laboratory research protocol. The animal carcass was then recycled for laparoscopic training purposes. A similar preservation technique as that described for the RTM was used. Five stands and poles are then placed around the PTM, 4 to hold BRs and 1 between the legs to suspend a laparoscopic camera.
Figure 11.
Pig Tissue Model (PTM) with surrounding stands and vertical poles, prior to setup of Lap-Ring Simulation Trainer.
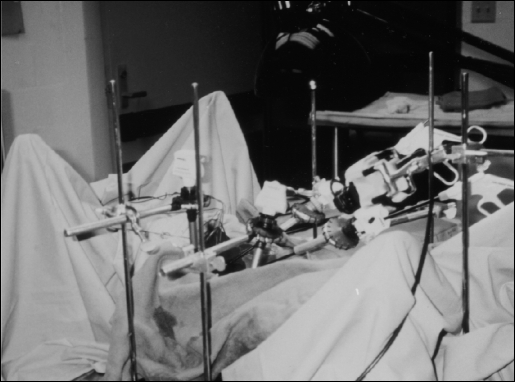
Figure 12 demonstrates the Lap-Ring Simulation Trainer using the PTM. Drapes were placed around the carcass to simulate an operating room environment. The exercise taking place at this time is a cholecystectomy. Using this arrangement, the maneuvers practiced to remove the gall-bladder are almost exactly the same as if performed on a live animal or human. The major difference is the minimal cost of this method of training, vis-à-vis maintaining a live animal operating room.
Figure 12.
Covered PTM with equipment set up and simulation cholecystectomy performed. Instead of a camcorder, a laparoscopic camera without the telescope is used to provide monitor display.
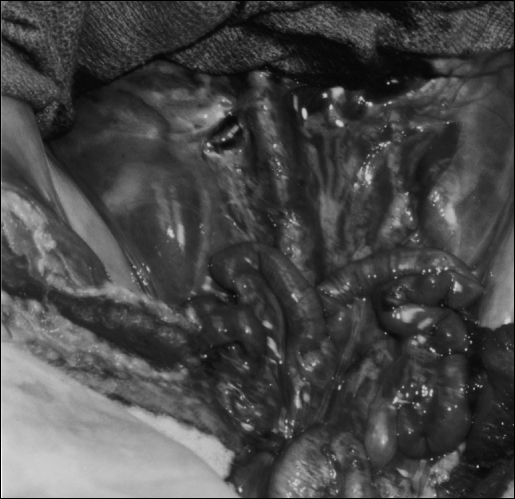
Some of the organs of a PTM are illustrated in Figure 13. The kidneys, great vessels, genitalia, and bladder are clearly delineated prior to their dissection. Because the bowels have to be removed as part of preparing the animal carcasses for practice sessions, female pigs are the prefered choice for the PTM. In this case, the bicornate uterus and fallopian tubes can be used as bowel substitutes. On the pig uterus, surgeons can practice laparoscopic manual and mechanical anastomoses, mastering techniques that can then be transferred to bowel surgery.
Figure 13.
Close-up of PTM after removal of entire bowel. Large kidneys are evident as are vena cavae, bicornate uterus, and bladder.
DISCUSSION
The ability of laparoscopic surgery to shorten hospital stays and lessen patient morbidity has been well established over the past decade. Some specialty areas, such as general surgery and gynecology, embraced the new technology with a passion and created multiple new laparoscopic operations. Success in this area is attributed to the existence of initial “easy” operations, such as laparoscopy, fallopian tubal ligation, and laparoscopic cholecystectomies. By means of these frequently performed operations, gynecological and general surgeons were able to advance their skills and confidence. Once enough experience was achieved by performing “easy” operations, these specialty surgeons went on to undertake more technically difficult and challenging operations.
General urologists, on the other hand, have been less successful in incorporating the advantages of the laparoscopic surgery into their practices. Although brilliant advances to replace traditional retroperitoneal surgery have been achieved by a few shining stars in university urology departments, these have not filtered down to the general nonacademic urologist. The major difficulty urologists have encountered in taking an aggressive leap into laparoscopic surgery has been the lack of an “easy” laparoscopic urologic operation, one that can be performed frequently enough to develop and maintain basic laparoscopic skills.
Urologists who initially started by performing laparoscopic pelvic lymph node dissections soon realized they needed more practice to perform these difficult operations. Due to lack of adequate training programs, many urologists became discouraged and gave up on laparoscopic surgery. Unless performed frequently, laparoscopic skills are difficult to maintain.
The initial learning curve for major laparoscopic operations can be steep. It first requires training under the auspices of a mentor or university directed course. It then secondly requires periodic practice of basic skills, such as instrument handling, suturing, knot tying, and dissecting. All this is required to keep the learning curve down and minimize complications. Although best ac-complished by frequent operating room experience, it can also be achieved by working in a simulated laparoscopic environment.
CONCLUSION
To encourage the return of the general urologist to the laparoscopic arena, more “user friendly” operations and training techniques must be made available. The recent description of laparoscopic hands-assisted nephrectomy4–6 may be a step in that direction. The Lap-Ring Simulation Trainer introduced here may be another one. It offers a system that allows easy reference back to the open field during training exercises. This ability enhances skills by allowing surgeons who are experiencing difficulty to look down away from the monitor to study instrument and tissue angle relationships. They are then able to compare the monitor display field with what is actually happening at the specimen site in 3 dimensions. This facilitates making timely corrections.
When using the Lap-Ring Simulation Trainer, the natural tendency is always to look at the monitor because of its magnification, not the open practice field as one might suspect seeing the trainer initially. It also allows use of diversified tissue specimens and permits variations in angles between the laparoscopic instruments and target tissues. This training system is in its infancy and is presented here to encourage its use by surgeons interested in continued laparoscopic training. Its final success will be determined after training protocols are set up to analyze how easily surgeons using it are able to acquire new skills and refine old ones.
Acknowledgments and Disclosures:
The animal carcasses used in this presentation were sacrificed following approved protocols at the TUFTS-New England Medical Center in Boston between 1991 and 1992 while the author was working as a fellow in the surgery department research laboratories.
The Lap-Ring Simulation Trainer is currently being developed by the author with the intention to make it available to surgeons' interested in its purchase in the future.
References:
- 1. Medina M. The Borinquen ring: introduction of a new laparoscopic simulation surgery training instrument. J Laparoendosc Surg. 1993;3(6):593–597 [DOI] [PubMed] [Google Scholar]
- 2. Medina M. Formidable challenges to teaching advanced laparoscopic skills. JSLS. 2001;5:153–158 [PMC free article] [PubMed] [Google Scholar]
- 3. Medina M. Rabbit tissue model (RTM) harvesting technique. JSLS. In press [PMC free article] [PubMed] [Google Scholar]
- 4. Stifelman M, Sosa ER, Shichman S. Hand-assisted laparoscopy (HAL) Curr Surg Tech Urol. 1999;12(2):2–8 [Google Scholar]
- 5. Bishoff JT. Basic techniques in laparoscopic surgery. In: Bishoff JT, Kavoussi LR. eds. Atlas of laparoscopic retroperitoneal surgery. Philadelphia, PA: WB Saunders; 2000:25 [Google Scholar]
- 6. McDougall EM. [unpublished course training manuscript] Laparoscopic nephroureterectomy: hand-assisted approach. 2000 [Google Scholar]