Abstract
Objective:
The development of a thoracoscopically assisted technique to be performed with the patient under local anesthesia for both diagnostic and therapeutic purposes when treating pleural effusions and empyemas in high-risk surgical patients.
Methods:
Twenty patients with pleural effusion or empyema who were also determined to be at high risk for complications following a thoracotomy, pleural biopsy, general anesthesia, or all of these, underwent placement of a thoracoscope while under local anesthesia followed by thoracic fluid drainage, pleural biopsy, and pleurodesis as required. Patients were retrospectively evaluated for a variety of factors including personal history, pre-existing medical conditions, and pre- and postoperative course.
Results:
The average age of the patients was 59 years (18 to 89) with a 55% male/45% female sex distribution. Patients had this procedure as a consequence of malignancy (50%), empyema (30%), spontaneous pneumothorax (10%), bronchiectasis (5%), or heart failure (5%). The average duration of the procedure was 62 minutes (20 to 190), with an average of 861 mL of fluid drainage, and 114 mL of estimated blood loss. The tube thoracostomy was usually removed on the sixth (0 to 13) postprocedure day. This procedure was well tolerated by the patients with the majority of pain management being achieved with patient controlled analgesia (58%). The direct complication rate was 10%, with 2 patients requiring endotracheal intubation.
Conclusion:
This novel thoracoscopic procedure represents an acceptable alternative to the traditional treatment of pleural effusions and empyema with comparable outcome parameters and morbidity. This technique may eventually become the standard of care for the treatment of pleural effusions.
Keywords: Thoracoscopy, Pleural effusion, Pleural biopsy, Pleurodesis.
INTRODUCTION
Thoracoscopy, in an attempt to decrease the morbidity associated with a thoracotomy, was first introduced into clinical medicine by Jacobaeus in 1912.1 During the ensuing decades, it was utilized extensively for the diagnosis of pleural disease, particularly in patients with suspected tuberculosis.2
Recently, with the advent of equipment allowing better visualization and instrumentation, thoracoscopy is currently being utilized not only for diagnostic purposes but also for therapeutic purposes. The benefits of this less invasive technique are numerous, namely: (1) an increase in both immediate and late postoperative patient comfort, (2) less impairment of pulmonary function, (3) ability to obtain tissue for histologic and microbiologic analysis, (4) avoidance of large thoracotomy incisions that impede adjuvant radiotherapy in the early postoperative period, (5) a reduction in length of hospital stay as related to the procedure.3
Approximately 40% of all pleural effusions are secondary to malignancy, which accounts for nearly 100,000 cases annually in the United States alone.4,5 Nearly half of the patients with disseminated cancers eventually develop pleural effusion.4,6 Malignant pleural effusions are most commonly associated with lung carcinoma (17-56%); breast carcinoma (15-38%); lymphoma, leukemia (6-17%); ovarian carcinoma (7-16%); and gastrointestinal malignancies usually in the stomach (3-6%).4,7–11
Furthermore, diagnostic and therapeutic interventions related to pleural effusions represent a tremendous source of morbidity and mortality in this setting. This represents a diagnostic and therapeutic dilemma in these high-risk surgical patients. Perhaps by the introduction of thoracoscopically assisted pleural drainage, acquisition of tissue specimens and pleurodesis with local anesthesia, a tremendous source of morbidity and mortality can be reduced without affecting patient care. We compared in our study the traditional technique of evaluating pleural effusion (puncture thoracentesis, repeated chest tube insertion, and bedside or operating room pleurodesis blindly), with our direct visualization technique and direct biopsy, but we are not comparing this technique in our study with double lumen intubation with thoracotomy for evaluating the pleural cavity.
METHODS
Patient Selection
Twenty patients admitted to or consulted at the thoracic surgical service of a large, urban, teaching hospital from 1996 to 1998, with the diagnosis of symptomatic pleural effusions were included in the study. All had cardiopulmonary complaints as a consequence of the pleural effusions and required evaluation or diagnosis of the underlying medical problem that caused the pleural effusion. This then necessitated the pleural fluid aspiration, pleural biopsy, and pleurodesis.
A retrospective analysis was performed via a chart review with respect to sex, race, underlying medical condition, preoperative diagnosis, duration of procedure, amount of pleural fluid drained, the time tube thoracostomy remained in place, postoperative histologic diagnosis, postoperative pain management, and complications.
Equipment
The procedures were performed in a fully equipped operating room. A rigid thoracoscope with cold light source was used.12,13 The equipment required for rigid thoracoscopy are trocars, telescopes, and forceps (Figure 1). Eleven-mm trocars, which are smaller than the commonly used trocars, with obturators and cannulas were used because they were easier to introduce and less painful to the patient. Operating telescopes with 30∞ side view capability were coupled with television monitoring and inserted through endoscopy ports for use in this study. In this manner, we were able to view the entire pleural surface of the thorax as well as a majority of the thoracic contents after gentle retraction of the lung.
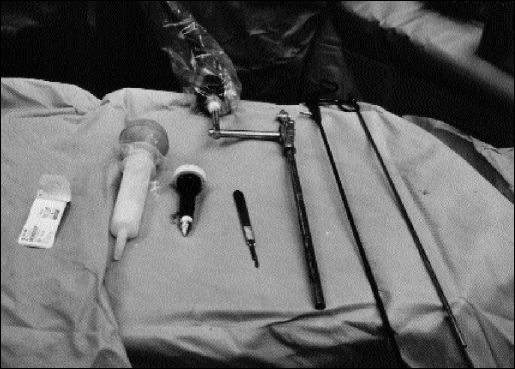
Figure 1.
Equipment: (1) suture material, (2) talcum powder, (3) 10-mm trocar with obturator, (4) scalpel, (5) right-angle telescope (0 degree), (6) dissector with diathermy, (7) dissector with diathermy.
Illuminated forceps are ideal for biopsies of the parietal pleura, diaphragmatic pleura, and fibrous pleural lesions under direct vision. This is made possible through the use of 1 port with the aid of 5-mm coagulating forceps, again introduced through the same port (Figure 2).
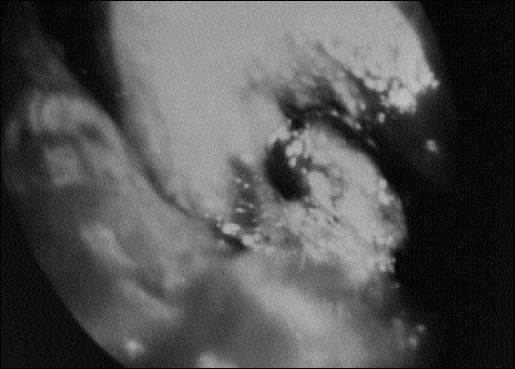
Figure 2.
Thoracoscopy with video recorder: Complete evaluation of the pleural cavity is possible. The lung is retracted for parietal and visceral pleural biopsies. Lysis of adhesions and lung biopsies could be easily illustrated on the video screen.
Technique
Previous experience with thoracoscopic procedures and techniques are required in performing this procedure. The patients were placed in the supine position, with the head of the bed elevated as needed. The surgeon and the assistant should be on the side of the effusion while performing the procedure.
Anesthesia with Lidocaine 1% (2 mg/kg) about 30 to 40 mL was given along with a single agent for sedation, analgesia, and amnesia [Droperidol (5 mg), Pethidine (5 to 10 mg), Propofol (400 mg), and Fentanyl (5 mcg)]. The patient was masked with 40% oxygen, and a simple operating room protocol was followed including pulse, blood pressure, and blood-gas saturation monitoring (Figure 3).
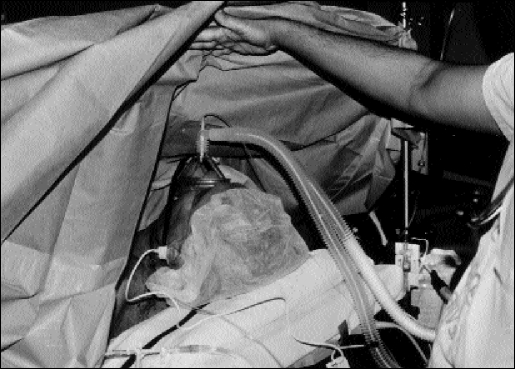
Figure 3.
The patient is in the supine position, and the procedure performed with the patient under local anesthesia without intubation.
A needle thoracentesis with an 18-gauge needle was performed to confirm the presence of the fluid before the start of the procedure. The incision was usually along the anterior axillary line between the fifth and sixth inter-costal spaces. However, the choice of entry can vary depending on the indication of thoracoscopy and the location of the fluid. A small skin incision is made along the axillary line followed by introduction of a 10-mm trocar with a cannula into the thoracic cavity. The pleural fluid was aspirated and sent for cytologic, microbiologic, and biochemical analysis.
Next, the 30∞ side arm operating telescope was introduced through the same port after the trocar was removed. Almost complete visualization of parietal and visceral pleura is possible after gentle retraction of the lung. Blunt and sharp dissection can be easily performed through the same port with a blunt probe, forceps, or electrocautery. Biopsies can be easily performed with illuminated forceps introduced through the same port. Bleeding after entry into the thorax or from the biopsy site is possible but is easily controlled with diathermy forceps. After completion of all biopsies, pleurodesis was performed by introduction of 16g of sterilized asbestos free, talcum powder. At the end of the procedure, a 36 Fr. right-angle tube thoracostomy was inserted and secured in place. This was left in place until the fluid drainage was less than 100 mL/day, the lung completely expanded, and no evidence existed of an air leak. Chest radiographs were obtained on a daily basis to assess the position on the tube thoracostomy and progress of the patient. The same standard of care was applied to all patients; however, the variation in particular parts was modified according to the underlying diagnosis and pathology.
RESULTS
The average age of the patients was 59 years (18 to 89) with a 55% male/45% female sex distribution. Sixty-five percent were white and 30% were Hispanic.
The underlying pathologic diagnoses were malignancy (50%), empyema (30%), spontaneous pneumothorax (10%), bronchiectasis (10%) or heart failure (5%) (Table 1). The patients had a variety of underlying medical problems including malignancies, whether primary or metastatic, cardiac problems, infectious diseases, such as tuberculosis, peripheral vascular diseases, and finally diabetes.
Table 1.
Underlying Pathology of Pleural Biopsies
| Diagnosis | Percentage |
|---|---|
| Malignancy (primary and secondary) | 50% (10/20) |
| Empyema | 30% (6/20) |
| Hemothorax and Pneumothorax | 10% (2/20) |
| Bronchiectasis | 5% (1/20) |
| Other causes | 5% (1/20) |
The average duration of the procedure was 62 minutes (20 to 190) with an average of 861 mL of fluid drainage from the pleural cavity and 114 mL of estimated blood loss during the procedure.
No patients required conversion to an open thoracotomy or evaluation of the pleural space under general anesthesia. Two patients (10%) required endotracheal intubation in the early postoperative period due to underlying pulmonary insufficiency.
The chest tube was usually removed on the sixth (0 to 13) postprocedure day. The procedure was generally well tolerated by the patients with the majority of pain management being achieved by patient-controlled analgesia (58%) (Table 2). The histological diagnosis confirmed that malignancy was the main etiological factor in 60% of the cases (Table 3). No postoperative mortality occurred, and the morbidity rate was 10% due to 2 patients requiring short-term postoperative intubation.
Table 2.
Pain Control in the Postoperative Period
| Postoperative Pain Control | Percentage |
|---|---|
| Patient control analgesia | 55.0% (11/20) |
| Duragesic patches and local anesthesia | 25.0% (5/20) |
| Opioid and Nonsteroidal anti- inflammatory drugs (NSIAD) | 10.0% (2/20) |
| Epidural anesthesia | 10.0% (2/20) |
Table 3.
Postoperative Histopathological Diagnosis
| Histopathological Diagnosis | Percentage |
|---|---|
| Metastatic carcinoma | 20.0% (4/20) |
| Primary lung squamous cell carcinoma | 15.0% (3/20) |
| Primary lung adenocarcinoma | 15.0% (3/20) |
| Lung oat cell carcinoma | 5.0% (1/20) |
| Mesothelioma | 5.0% (1/20) |
| Lung tuberculosis | 5.0% (1/20) |
| Infectious origin | 15.0% (3/20) |
| Nonspecific | 20.0% (4/20) |
The longest follow-up was 12 months (2 to 26 months). No long-term sequelae of the procedure occurred, and no patient developed a recurrent pleural effusion.
DISCUSSION
Minimal access surgery has become an accepted approach for the diagnosis and treatment of many surgical diseases. Several distinct advantages of minimal access surgery have been described in the literature in the past few years.3 It is well known that thoracoscopy is a procedure in the pleural cavity with 92% to 97% sensitivity and 99% specificity.8 Traditionally, patients with pleural effusion require multiple diagnostic and therapeutic procedures including needle thoracentesis with cytological and bacteriological evaluation, multiple blind pleural biopsies, tube thoracostomy, and finally pleurodesis using talcum powder or chemical substances. These procedures require multiple hospital admissions and their associated costs.
Evaluation of the pleural space under general anesthesia with the double lumen endotracheal tube and possible mini-thoracotomy especially in high-risk patients increases the hospital mortality to approximately 10%.14 Repeated pleural fluid drainage and tube thoracostomy are very painful procedures to the patient and require a tremendous expenditure of resources with an unacceptably high recurrence rate of 97% within 30 days.9 Tube thoracostomy drainage alone is effective in only 22%.14
Our procedure is of short duration with an average time of 62 minutes and can be easily performed by a trained surgeon. Adequate visualization of the parietal and visceral pleura can be obtained by manipulating the thoracoscope, and even lung biopsy with limited lung resection is possible. In 18 out of 20 patients, the procedure was very successful and full visualization of the pleural space was complete.
We emphasize the advantage of this procedure over the traditional methods and over thoracotomy or thoracoscopy with the patient under general anesthesia in all areas of the thoracic cavity.15 It was found that a combination of pleural fluid cytological examination and pleural biopsy histological examination can provide a definitive diagnosis in 73% to 90% of the cases; these results are comparable to those of our study.14 It should be noted that when a biopsy cannot be obtained, as with tube thoracostomy drainage and pleurodesis, the cytological diagnosis rate is 67%, and the blind pleural biopsy accuracy rate is 58% to 71%.10,16,17 This procedure was well tolerated by the patients, and postoperative pain was relatively easily controlled when compared with pain control in more traditional procedures. Our complication rate was acceptable (10%), and we had no case of mortality related to this procedure; however, the pain was easily controlled postoperatively in spite of the fact that this procedure was performed with the patient under local anesthesia. Further analgesia might be required depending on the patient's pain threshold.
This technique may have some utility. However, if a patient is deemed too frail for general anesthesia and has a suspected malignancy, one could argue that perhaps the procedure be performed with our technique or that bedside pleurodesis be performed without the benefit of tissue diagnosis.
In conclusion, this procedure can be easily performed with the patient under local anesthesia, general or loculated pleural effusions can be easily evaluated, pleural biopsies can be obtained, and pleurodesis can be performed all in a single procedure with low morbidity. These characteristics make it useful in the evaluation of high-risk patients forsaking the more traditional procedures.
Currently, a prospective study evaluating this procedure and the traditional multi-step technique with respect to the criteria of patient participation, cost, hospital stay, pain control, recurrence rate, and patient satisfaction, is under development.
Footnotes
This paper was presented at the conference of the New York Society of Thoracic Surgery, New York, NY, November 19, 1998; the International College of Surgeons meeting, July 24-26, 1999, Cancun, Mexico; and as a poster presentation at the first International Chicago Symposium on Malignancies of the Chest, Head, and Neck, October 1-2, 1999, Chicago, IL.
References:
- 1. Jacobaeus HC. Uber lapro-und thorakoskopie. Beitr Klin Tuberk. 1912;25:334–341 [Google Scholar]
- 2. Hau T, Forster E, Gandawidjaja L, Heemken R. Thoracoscopic pulmonary surgery: indications and results. Eur J Surg. 1996;162:23–28 [PubMed] [Google Scholar]
- 3. Saenz NC, Conlon KCP, Aronson DA, LaQuaglia MP. The application of malignancies. J Laparosc Adv Surg Tech. 1997;7(5):110–117 [DOI] [PubMed] [Google Scholar]
- 4. Matthay RA, Coppage L, Shaw C, Fielderman AE. Malignancies metastatic to the pleura. Inves Radiol. 1990;25:601–619 [DOI] [PubMed] [Google Scholar]
- 5. Lynch TE. Management of malignant pleural effusion. Chest. 1993;103:385S–389S [DOI] [PubMed] [Google Scholar]
- 6. Rusch VW, Harper GR. Pleural effusion in patients with malignancy. In: Roth JA, Ruckdeschel JC, Weisenburger TH. eds. Thorac Oncol. Philadelphia: W.B. Saunders; 1989:594–605 [Google Scholar]
- 7. Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1997;63:695–702 [DOI] [PubMed] [Google Scholar]
- 8. Sahn SA. Pleural effusion in lung cancer. Clin Chest Med. 1993;14:189–200 [PubMed] [Google Scholar]
- 9. Monte SA, Ehya H, Lang WR. Positive effusion cytology as the initial presentation of malignancy. Acta Cytol. 1987;31:448–452 [PubMed] [Google Scholar]
- 10. Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van de, Molengraft FJM, Vooijs GP. Survival of patients with malignancy associated effusion. Acta Cytol. 1989;33:911–916 [PubMed] [Google Scholar]
- 12. Brandt HJ, Loddenkemper R, Mai J. Atlas of Diagnostic Thoracoscopy. Stuttgart: George Thieme Verlag; 1985 [Google Scholar]
- 13. Boutin C, Viallat JR, Aelony Y. Practical Thoracoscopy. New York: Springer Verlag; 1991:85–97 [Google Scholar]
- 14. Fenton KN, Richardson DJ. Diagnosis and management of malignant pleural effusions. Am J Surg. 1995;170:69–74 [DOI] [PubMed] [Google Scholar]
- 15. Boutin C, Loddenkemper R, Astoul P. Diagnostic and therapeutic thoracoscopy: Techniques and indications in pulmonary medicine. Tuber Lung Dis. 1993;74:225–239 [DOI] [PubMed] [Google Scholar]
- 16. Emad A, Razaian GR. Closed percutaneous pleural brushing: A new method for diagnosis of malignant pleural effusion. Respir Med. 1998;92(4):659–63 [DOI] [PubMed] [Google Scholar]
- 17. Christopher DJ, Peter JV, Cherian AM. Blind pleural biopsy using Tru-Cut needle in moderate to large pleural effusion–an experience. Singapore Med J. 1998;39(50):196–199 [PubMed] [Google Scholar]