Abstract
Background and Objectives:
Gallbladder carcinoma is found in 0.2 % to 5% of patients undergoing cholecystectomy, and gallstones are found in 70% to 98% of patients with gallbladder carcinoma. Early diagnosis of carcinoma is difficult because of the absence of specific symptoms and the frequent association with chronic cholecystitis and gallstones. At present, laparoscopic cholecystectomy is the gold standard for the surgical treatment of symptomatic cholelithiasis and other benign gallbladder diseases. The aims of this study were to evaluate retrospectively the incidence of occasional and occult gallbladder carcinomas to ascertain the effect of laparoscopy on diagnosis and treatment of unexpected extrahepatic biliary tree carcinomas and to assess possible guidelines that can be taken into consideration when the problem is encountered.
Methods:
Clinical records of 3900 patients undergoing laparoscopic cholecystectomy were reviewed. Patients with occasional (intraoperative = Group A) or occult (postoperative = Group B) diagnosis of gallbladder or common bile duct carcinoma entered the study group. Follow-up data were obtained in June 2000.
Results:
A total of 14 patients (0.35%), 3 men and 11 women, mean age 60.8 years (range 37 to 73) with extra-hepatic biliary tree carcinoma were found. Occasional carcinomas occurred in 8 patients, occult carcinomas in 6. No deaths occurred in either group. The overall survival at mean follow-up of 30.5 months is 50%. Five patients are disease free, and 2 are alive with evidence of recurrence.
Discussion:
In 2 large series of unselected consecutive laparoscopic cholecystectomy, only 14 unsuspected malignant tumors of the extrahepatic biliary tree were found (0.35%). The limits of the preoperative workup and the difficult diagnosis of biliary tract carcinoma during laparoscopic cholecystectomy, has led to the present retrospective study and several significant recommendations.
Keywords: Gallbladder carcinoma, Laparoscopic cholecystectomy
INTRODUCTION
Tumors of the gallbladder, both malignant and benign are rare. Gallbladder carcinoma (GBC) represents 1% to 3% of all malignant tumors and ranks fifth among gastrointestinal tumors.1, 2 In the United States, gallbladder cancer results in approximately 2400 deaths per year, while in Italy 600 deaths per year are reported.3
Gallbladder carcinoma is found in 0.2% to 5% of patients undergoing cholecystectomy, and gallstones are found in 70% to 98% of patients with gallbladder carcinoma.3 According to Diehl et al,4 the risk of developing cancer in patients with untreated cholelithiasis is estimated to be between 0.2 and 0.5% over a 20-year period.5-6 The risk of GBC in patients with chronic cholecystitis and cholelithiasis is 7 times greater than that in the normal population.7
An incidence of 1% to 2% of incidental gallbladder carcinoma was reported before the laparoscopic era.4, 8 Early diagnosis is difficult because of the absence of specific symptoms and the frequent association with chronic cholecystitis and gallstones.
To date, laparoscopic cholecystectomy (LC) is the gold standard in the surgical treatment of symptomatic cholelithiasis and other benign gallbladder diseases.9–12 The incidental finding of GBC during LC causing a conversion to open surgery has been reported to be less than 0.1%.11 The tremendous increase in the number of cholecystectomies performed yearly is likely to result in an increase of the diagnosis of GBC. The increased risk of abdominal wall recurrences and intraperitoneal tumor cell dissemination in case of occult GBC diagnosed after LC is a matter of concern. Although, Suzuki et al13 reported a long-term survival rate in patients with occult GBC after LC, which compared favorably to that observed after open cholecystectomy (OC), this issue is still debated. In particular, guidelines for the treatment of occasional (intraoperative diagnosis) or occult (postoperative histologic diagnosis) carcinoma of the extrahepatic biliary tree in patients undergoing LC are lacking.
The aim of this study was to review the data of 2 large series of unselected patients who underwent LC in order to assess (1) the incidence of occasional and occult gallbladder carcinoma, (2) the impact of laparoscopy on the diagnosis and treatment of unexpected extrahepatic biliary tree carcinomas, (3) future guidelines that could be considered when the problem is encountered.
MATERIALS AND METHODS
The clinical records of 3900 patients who underwent LC from July 1990 to December 1998, at 2 university hospitals (VII Patologia Chirurgica Università di Roma “La Sapienza,” Italy and Departamento de Cirugia Hospital Universitario de Getafe, Madrid, Spain) were retrospectively reviewed. The preoperative workup included routine laboratory tests, electrocardiogram (ECG), chest Xray, and abdominal ultrasonography performed less than 3 months before surgery. Patients with occasional (intraoperative = Group A) or occult (postoperative = Group B) gallbladder or common bile duct (CBD) carcinoma entered the study group. The operative procedures, imaging, histopathologic findings, and postoperative outcome were reviewed.
In all patients, a 12 mm Hg CO2 pneumoperitoneum was used. Reusable and disposable instrumentation were used. Intraoperative cholangiogram was carried out when one of the following conditions was present: previous or current jaundice and/or acute pancreatitis; elevated serum levels of bilirubin, alkaline phosphatase and amylase (1.5 times normal value); stones or bile duct dilation (diameter > 6 mm) at preoperative ultrasound scan. Histologic examination of gallbladders with cancer was performed, using step-wise tissue sections at intervals of 5 mm. The tissue sections were stained with hematoxylin-eosin and examined following the criteria of the Manual for Staging of Cancer from the American Joint Committee on Cancer (Table 1).14 Patients with GBC underwent routine follow-up every 3 months by an oncologist or attending surgeon. Follow-up data were obtained in June 2000.
Table 1.
TNM Staging of Gallbladder Cancer (AJCC)
| Tumor | Node | Metastasis | |
|---|---|---|---|
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| T3 | N0-1 | M0 | |
| Stage IVA | T4 | N2 | M0 |
| Stage IVB | T1-4 | N0-2 | M1 |
Tis, Carcinoma in situ; T1, tumor invades mucosa or muscle layer; T2, tumor invades perimuscular connective tissue; T3, tumor perforates the serosa and/or directly invades 1 adjacent organ; T4, tumor extends more than 2 cm into the liver and/or into 2 or more adjacent organs; N0, no regional lymph node metastasis; N1, metastasis in cystic duct, peri choledochal and/or hilar lymph nodes; N2, metastasis in peripancreatic, periduode- nal, periportal, celiac, and/or mesenteric lymph nodes; M0, no distant metastasis; M1, distant metastasis.
RESULTS
A total of 14 patients (0.35%), 3 men and 11 women, mean age 60.8 years (range 37 to 73) with occasional or occult carcinoma of the biliary tree entered the study. In these patients, the indications for surgery were symptomatic cholelithiasis in 11, acute cholecystitis in 2, and gallbladder and CBD stones in one. Preoperative ultrasound (US) showed gallstones in all patients, a nonspecific thickening of the gallbladder wall in 8, choledocolithiasis in 1, and dilated CBD (maximum diameter 9 mm) in 1. In the last patient, an intraoperative cholangiogram showed stenosis of the choledocus at the cystic duct confluence. The patient with CBD stones underwent preoperative endoscopic sphincterotomy with successful clearance of the choledocus. The LC was delayed 15 days due to the onset of hyperamylasemia and evidence of pancreatitis at ultrasound following the endoscopic procedure.
Occasional carcinoma occurred in 8 patients (Group A) (Table 2). Malignancy was suspected during the procedure because of overt macroscopic features of cancer. In 6 patients, firm adhesions and/or parietal thickening were present, and in 2 patients concomitant multiple peritoneal nodules on the hepatoduodenal ligament and on the right diaphragm were found. In all cases, intraoperative frozen sections were performed allowing the diagnosis of GBC in 5 patients, of metastatic peritoneal nodules and GBC in 2 patients, and of CBD carcinoma in 1 case.
Table 2.
Group A (n=8)
| Sex | Age | Site ofTumor | Treatment | Histology | Follow-up (M) |
|---|---|---|---|---|---|
| M | 70 | CBD | LC Conversion: DCP | T1N0M0 | Alive (62) |
| F | 54 | GB | LC Conversion: Extended cholecystectomy | T3N0M0 | Disease free (90) |
| F | 55 | GB | Laparoscopy + peritoneal biopsy | TxNxM1 | Exitus (6) |
| M | 56 | GB | LC Conversion: Extended cholecystectomy | T3N1M0 | Exitus (6) |
| F | 59 | GB | Laparoscopy + peritoneal biopsy | TxNxM1 | Exitus (8) |
| F | 64 | GB | LC Conversion: Extended cholecystectomy | T2N1M0 | Disease free (15) |
| F | 73 | GB | LC Conversion: Extended cholecystectomy, CBD resection, hepaticojejunostomy | T4N1M1 | Exitus (24) |
| F | 73 | GB | Laparoscopy Conversion: Extended cholecystectomy | T4N1M0 | Exitus (10) |
In 6 patients, the procedure was converted to open surgery. In 4 patients, cholecystectomy, wedge resection of the gallbladder bed, and “en bloc” dissection of the regional lymph nodes were performed (extended cholecystectomy). One patient, with GBC of the infundibulum infiltrating the CBD, underwent cholecystectomy, common bile duct resection, portal lymphadenectomy, omentectomy, and Roux-en-Y hepaticojejunostomy (pT4 N1 M1). In the patient with CBD carcinoma, cholecystectomy, common bile duct resection, portal lymphadenectomy, and Roux-en-Y hepaticojejunostomy were performed. At the definitive histologic examination, tumor involvement of the distal CBD margin was present. The patient was reoperated on and a Whipple “curative”" resection was performed 5 weeks later. In the remaining 2 patients with advanced peritoneal disease, no further surgery was carried out. Histopathologic staging is described in Table 2.
Occult carcinoma occurred in 6 patients (Group B) (Table 3). Three patients underwent CT scan for staging, and a second-look open procedure was performed 4 weeks later. In 1 patient, extended cholecystectomy, as previously described, was carried out. At histology, lymph nodes and the hepatic gallbladder bed were free from tumor involvement (T1 N0 M0). In the second patient, locoregional carcinomatosis was found, and no further surgery was performed. The third patient had tumor infiltration of the portal vein not diagnosed by preoperative imaging. No further surgery was carried out. In the remaining 3 patients, no additional therapy was undertaken: 2 patients over 65 years of age had a T1 tumor; 1 patient in the T2 stage refused the open procedure.
Table 3.
Group B (n=6)
| Sex | Age | Histology | Operation | Follow-up (M) |
|---|---|---|---|---|
| M | 44 | T1 | Extended cholecystectomy | Disease free (48) |
| F | 72 | T1 | - | Disease free (23) |
| F | 53 | T2 | - | Disease free (109) |
| F | 37 | T2 | Laparotomy + peritoneal biopsy | Exitus (13) |
| F | 70 | T1 | - | Alive (7) |
| F | 71 | T4 | Laparotomy + biopsy | Exitus (24) |
No deaths occurred in either group. Morbidity included, in a patient of group A who underwent extended cholecystectomy, a subhepatic abscess.
The patient who underwent completion surgery with the Whipple procedure developed jaundice 15 months after surgery. Hepaticojejunostomy recurrence was diagnosed by percutaneous transhepatic cholangiography and brushing, and 2 biliary stents were positioned. External beam radiotherapy and a chemotherapy regimen were carried out. At 63-month follow-up, the patient is alive and free of extrahepatic recurrences.
In Group B, 1 patient with T1 adenocarcinoma who did not undergo a second open procedure, had a single liver metastasis at segment IV 12 months after surgery. The patient underwent curative liver resection.
The average survival rate at mean follow-up of 31.7 months was 37.5% in group A and 66.6% in group B, respectively. The average survival rate of the patients of both groups who underwent extended cholecystectomy at mean follow-up of 32.1 months was 50%, while that of the patients of both groups who did not receive resective surgery was 28.6 at 27.1-month follow-up.
DISCUSSION
Over the past 10 years, LC has become the treatment of choice for benign gallbladder diseases. During the same period of time, a 2-fold increase has occurred in the number of cholecystectomies performed yearly in industrialized countries (from 29 to 59.3%).15 The problem of unsuspected GBC in patients undergoing LC has attracted increasing attention. Incidental discovery of GBC in LC patients has been reported since early 1990. In 1991, Drouard et al16 first described trocar site recurrences of GBC after LC. Other authors17–19 have suggested that LC might favor the dissemination of unsuspected carcinomas affecting the prognosis of these patients.
The disadvantages of LC in cases of incidental GBC derive from the following considerations: possible adverse effects of CO2 pneumoperitoneum (chimney effect); peritoneal seeding after bile spillage and/or tumor cell spillage due to accidental perforation of the gallbladder; desquamation of cancer cells due to excessive gallbladder manipulation; direct tumor contact with the abdominal wall during extraction of the specimen with consequent subcutaneous seeding of cancer cells.20
Experimental studies on the effect of the pneumoperitoneum on tumor cell growth and abdominal wall metastasis has yielded controversial results.21–24 Moreover, intraperitoneal dissemination of incidental GBC after gasless LC has been reported.25, 26
Gallbladder perforation and bile spillage occur in up to 30% of cases during LC.27 In the study of Suzuki et al,13 18 of 41 patients with unsuspected GBC had intraoperative bile spillage, and in 4 of the 7 patients who developed late abdominal recurrences, bile spillage occurred. Tumor cell dissemination into the peritoneal cavity, sub-cutaneous seeding due to either contaminated instruments, direct tumor contact, or both are well known surgeon-related factors that interfere with the natural history of the tumor.28
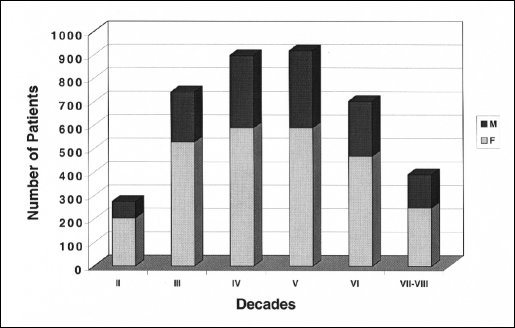
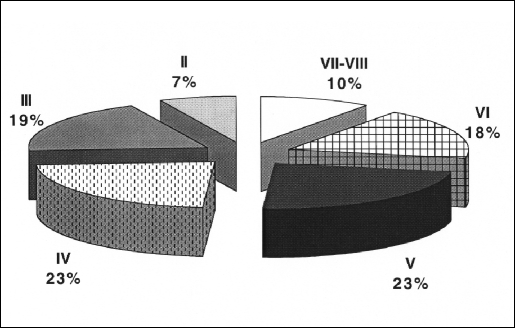
The following considerations can be drawn from this study: (1) In a large series of unselected consecutive LC, only 14 occult and occasional malignant tumors of the extrahepatic biliary tree were found, with an incidence of 0.35%. These data confirm that the incidence of unexpected GBC in the laparoscopic era is significantly lower than that in reported large series of OC (0.35% vs. 1% to 2%).8 These results could be due to the increased compliance of patients to LC, the reduced interval between the diagnosis of cholelithiasis and the surgical treatment and the lower mean age of patients undergoing surgery. In the present series, 49% of patients were under 50 years of age (Figures 1 and 2). (2) The usual preoperative workup has limitations in cases of chronic cholecystitis. However, a macroscopic differential diagnosis between chronic cholecystitis and GBC may also be very difficult. Intraoperative frozen sections in cases of suspected malignancies and routine postoperative histology of the gallbladder are mandatory. In fact, in 5 group A patients it was possible to perform a radical resection giving a chance of cure of incidental adenocarcinomas. The survival rate in the present series was similar to that reported in larger open series,4,8,29 suggesting that laparoscopy may have no adverse effects on the natural history of unexpected extrahepatic biliary tree carcinomas (Table 2). (3) The occurrence of occult carcinomas in 6 cases confirms that the diagnosis of GBC in patients with either cholelithiasis, cholecystitis, or both can be extremely difficult even during LC. In 1 patient with occult carcinoma (pT1), the evidence of the locoregional carcinomatosis at laparoscopic reexploration 4 weeks later could be related to bile spillage. However, in spite of the absence of any intraoperative protection of the trocar site, no cases of trocar seeding have been observed at mean follow-up of 31.7 months. At any rate, trocar recurrences should not be considered as isolated localized phenomenon, but rather a manifestation of the aggressive behavior of the GBC.
Figure 1.
Decade and sex distribution in 3900 patients undergoing laparoscopic cholecystectomy.
Figure 2.
Percentage of patients for each decade.
The results of this study confirm that LC itself does not increase the risk of abdominal wall recurrences. General agreement exists that patients with laparoscopically removed GBC at an early stage (pTis pT1) do not need additional surgical therapy.4, 8,30–32 On the contrary, patients with occult GBC invading the muscular layer (pT2) benefit by extended cholecystectomy.
On the basis of the present retrospective study we recommend (1) routine histologic examination of the removed gallbladder; (2) in all suspicious cases that multiple frozen sections of both gallbladder and suspected peritoneal metastases be obtained; (3) in cases of intra-operative diagnosis, conversion to an open procedure be done only if the surgeon is confident doing major hepatobiliarypancreatic surgery; (4) in cases of conversion or second-look, the resection of trocar sites in not necessary; (5) in cases of bile spillage during LC when there is GBC present, a second-look laparoscopic procedure is indicated to exclude carcinomatosis.
Contributor Information
Gianfranco Silecchia, Department of Surgery, “P. Stefanini,” Policlinico “Umberto I,” University of Rome “La Sapienza” Rome, Italy..
Luigi Raparelli, Department of Surgery, “P. Stefanini,” Policlinico “Umberto I,” University of Rome “La Sapienza” Rome, Italy..
Jose' Maria Jover Navalon, Department of Surgery, Hospital Universitario de Getafe, Carretera Toledo, Getafe, Madrid, Spain..
Ana Serantes Gomez, Department of Surgery, Hospital Universitario de Getafe, Carretera Toledo, Getafe, Madrid, Spain..
Mariano Moreno Azcoita, Department of Surgery, Hospital Universitario de Getafe, Carretera Toledo, Getafe, Madrid, Spain..
Nicola Basso, Department of Surgery, “P. Stefanini,” Policlinico “Umberto I,” University of Rome “La Sapienza” Rome, Italy..
References:
- 1. Holmes HL, Mark JBD. Carcinoma of the gallbladder. Surg Gynecol Obstet. 1971;133:561. [PubMed] [Google Scholar]
- 2. Jones RS. Carcinoma of the gallbladder. Surg Clin North Am. 1990;70:1419–1428 [DOI] [PubMed] [Google Scholar]
- 3. Robustelli della Cuna G, Santoro A, Gennari L. Neoplasie dell'apparato digerente. In: Manuale di Oncologia Medica. Milan: Masson; 1991:703–705 [Google Scholar]
- 4. Diehl A, Aggaewal S, Berry M. Ultrasonography of carcinoma of the gallbladder: an analysis of 80 cases. J Clin Ultrasound. 1990;18:715–720 [PubMed] [Google Scholar]
- 5. Yamaguchi K, Tsuneyoshi M. Subclinical gallbladder carcinoma. Am J Surg. 1992;163:382–386 [DOI] [PubMed] [Google Scholar]
- 6. Bergdahl L. Gallbladder carcinoma first diagnosed at microscopic examination of gallbladders removed for presumed benign disease. Ann Surg. 1980;191:19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aretxabala X, Roa I, Burgos L, et al. Gallbladder cancer in Chile: a report on 54 potentially resectable tumors. Cancer. 1992;69:60–65 [DOI] [PubMed] [Google Scholar]
- 8. Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder - an appraisal of a radical second operation after simple cholecystectomy. Cancer. 1988;62:1422–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wherry DC, Rob CG, Marohn MR, Rich NM. An external audit of laparoscopic cholecystectomy performed in medical treatment facilities of the Department of Defense. Ann Surg. 1994;220:625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMahon AJ, Russell IT, Ramsay G, et al. Laparoscopic and minilaparotomy cholecystectomy: a randomized trial comparing postoperative pain and pulmonary function. Surgery. 1994;115:533–539 [PubMed] [Google Scholar]
- 11.The Southern Surgeons Club A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med. 1991;324:1073–1078 [DOI] [PubMed] [Google Scholar]
- 12. Cuschieri A, Dubois F, Mouiel J, et al. The European experience with laparoscopic cholecystectomy. Am J Surg. 1991;161:385–387 [DOI] [PubMed] [Google Scholar]
- 13. Suzuki K, Kimura T, Ogawa H. Long-term prognosis of gallbladder cancer diagnosed after laparoscopic cholecystectomy. Surg Endosc. 2000;14:712–716 [DOI] [PubMed] [Google Scholar]
- 14.American Joint Committe on Cancer Manual for Staging of Cancer. 4th ed. Philadelphia: JB Lippincott Co; 1992 [Google Scholar]
- 15. Legorreta AP, Silber JH, Costantino GN, Kobylinski RW, Katz SL. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA. 1993;270:1429–1432 [PubMed] [Google Scholar]
- 16. Drouard F, Delamarre J, Capron JP. Cutaneous seeding of gallbladder cancer after laparoscopic cholecystectomy. N Engl J Med. 1991;325:1316. [DOI] [PubMed] [Google Scholar]
- 17. Fong Y, Brennan MF, Turnbull A, Colt DG, Blumbgart LH. Gallbladder cancer discovered during laparoscopic surgery. Potential for iatrogenic tumor dissemination. Arch Surg. 1993;128:1054–1056 [DOI] [PubMed] [Google Scholar]
- 18. Wibbenmeyer LA, Wade TP, Chen RC, Meyer RC, Turgeon ROP, Andrus CH. Laparoscopic can disseminate in situ carcinoma of the gallbladder. J Am Coll Surg. 1995;181:504–510 [PubMed] [Google Scholar]
- 19. Targarona EM, Pons MJ, Viella P, Trias M. Unsuspected carcinoma of the gallbladder. A laparoscopic dilemma. Surg Endosc. 1994;8:211–213 [DOI] [PubMed] [Google Scholar]
- 20. Copher JC, Rogers JJ, Dalton ML. Trocar site metastasis following laparoscopic cholecystectomy for unsuspected carcinoma of the gallbladder: case report and review of the literature. Surg Endosc. 1995;9:348–350 [DOI] [PubMed] [Google Scholar]
- 21. Bouvy ND, Parquet RL, Leekel H, Bonier HJ. Impact of gasless laparoscopy and laparotomy on peritoneal tumor growth factor and abdominal wall metastases. Ann Surg. 1996;224:694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones DB, Guo LW, Reinhard MK, et al. Impact of pneumoperitoneum on trocar site implantation of colon cancer in hamster model. Dis Colon Rectum. 1995;38:1182–1188 [DOI] [PubMed] [Google Scholar]
- 23. Jacobi CA, Wenger FA, Ordemann J, Gutt C, Sabat R, Muller JM. Experimental study of the effect of intra-abdominal pressure during laparoscopy on tumor growth and port site metastasis. Br J Surg. 1998;85:1419–1422 [DOI] [PubMed] [Google Scholar]
- 24. Neuhaus SJ, Watson DI, Ellis T, et al. Wound metastasis after laparoscopy with different insufflation gases. Surgery. 1998;123:579–583 [DOI] [PubMed] [Google Scholar]
- 25. Sarli L, Costi R, Pietra N, Gobbi S. Incidental gallbladder cancer at laparoscopy: a review of two cases. Surg Laparosc Endosc. 1999;9:414–417 [PubMed] [Google Scholar]
- 26. Othani T, Takano Y, Shiray Y, Hatakeyama K. Early intraperitoneal dissemination after radical resection of unsuspected gallbladder carcinoma following laparoscopic cholecystectomy. Surg Laparosc Endosc. 1998;8:58–62 [PubMed] [Google Scholar]
- 27. Kaminski DL. Invited commentary to Yamaguchi K, Chijiiwa K, Ichimiya H, et al. Gallbladder carcinoma in the era of laparoscopic cholecystectomy. Arch Surg. 1996;131:981–984 [DOI] [PubMed] [Google Scholar]
- 28. Contini S, Dalla Valle R, Zinicola R. Unexpected gallbladder cancer after laparoscopic cholecystectomy. An emerging problem? Reflections on four cases. Surg Endosc. 1999;13:264–267 [DOI] [PubMed] [Google Scholar]
- 29. Suzuki K, Kimura T, Ogawa H. Is laparoscopic cholecystectomy hazardous for gallbladder cancer? Surgery. 1998;123:311–314 [PubMed] [Google Scholar]
- 30. Bouvy ND, Marquet RI, Hamming JF, et al. Laparoscopic surgery in the rat: beneficial effect on body weight and tumor take. Surg Endosc. 1996;10:490–494 [DOI] [PubMed] [Google Scholar]
- 31. Yamaguchi K, Enjoji M. Carcinoma of the gallbladder: a clinicopathology of 103 patients and a newly proposed staging. Cancer. 1988;62:1425–1432 [DOI] [PubMed] [Google Scholar]
- 32. Tsukada K, Hatakeyama K, Kurosaki I, Uchida K, et al. Outcome of radical surgery for carcinoma of the gallbladder recording to the TNM stage. Surgery. 1996;120:816–821 [DOI] [PubMed] [Google Scholar]