Abstract
Objectives:
In recent years, older patients are being referred for esophagectomy, and the associated morbidity and mortality is not well defined. Advances in minimally invasive techniques now allow minimally invasive esophagectomy (MIE) to be performed that may minimize the morbidity of this procedure. The objective of this report was to summarize our experience with MIE in the elderly.
Methods:
From February 1997 through February 2001, 41 patients (14 women, 27 men) 75 years of age or older (mean age 78, range 75 to 89) underwent esophagectomy (28 for adenocarcinoma, 7 squamous, 6 Barrett's with high-grade dysplasia).
Results:
Esophagectomy was performed in a minimally invasive fashion in 41 patients. No open conversions were necessary. The median ICU stay was 1 day (range 1 to 34). The median hospital stay was 7 days (range 5 to 50). Major morbidity occurred in 19% of the cases and included 1 persistent air leak, 1 case of pneumonia with acute respiratory failure, 1 tracheal tear, 1 chylothorax, and 1 myocardial infarction. Three anastomotic leaks and 1 small bowel perforation occurred. All were recognized early and treated surgically. No perioperative mortalities took place.
Conclusion:
In our center, MIE was performed in elderly patients with an acceptable morbidity, low mortality, and reduced length of hospital stay compared with that in previous reports.
Keywords: Minimally invasive esophagectomy, Elderly, Esophageal cancer
INTRODUCTION
Despite advances in surgical technology, intensive care medicine, and nutritional supplementation, the morbidity associated with esophagectomy remains high. In elderly populations, this morbidity has been reported to be increased.1–3 With the aging population and the increasing incidence of esophageal cancer, surgeons are being referred more elderly patients. Advances in minimally invasive techniques now allow minimally invasive esophagectomy (MIE) to be performed that may minimize the morbidity of this procedure.4 The objective of this report was to summarize our experience with MIE in the elderly.
MATERIALS AND METHODS
From February 1997 through August 2001, 41 patients over the age of 75 underwent minimally invasive esophagectomy. Demographics and indications are summarized in Table 1. Preoperative assessment in all the patients included functional status evaluation, chest Xray, electrocardiogram, arterial blood gas analysis, pulmonary function tests, and biochemical and hematological tests. A complete cardiopulmonary workup was performed when it was indicated from the patient's history or when symptoms and signs of chronic lung or heart disease were present. Patients suffering from unstable coronary artery disease were treated and stabilized before operation.
Table 1.
Demographics and Patient Data
| No.of Patients | Males/Females | Indications | Previous Thoracic or Abdominal Surgeries | Neoadjuvant Chemoradiation |
|---|---|---|---|---|
| 41 | 27/14 | 28 Adenocarcinoma | 13 Patients | 10 Patients |
| 6 Squamous cell ca | ||||
| 4 Barrett's with high-grade dysplasia | ||||
Preoperative staging included esophagoscopy, bronchoscopy, endoscopic ultrasound, and chest and abdominal computerized tomography. In cases of larger tumors (T3 or suspected T4), laparoscopic staging was performed before esophagectomy. Eligible patients with N1 disease were offered neoadjuvant therapy on protocol.
Surgical Technique
The approach most commonly used at the University of Pittsburgh is the combined laparoscopic and thoracoscopic esophagectomy. This technique is described below.
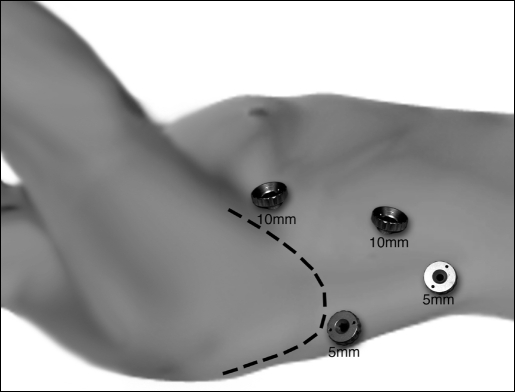
VATS Mobilization: After intubation with a double lumen tube for single lung ventilation, the patient is positioned in the left lateral decubitus position. Four thoracoscopic ports are placed (Figure 1). The camera port (10 mm) is placed at the seventh intercostal space, midaxillary line. A 5-mm port is placed at the eighth or ninth intercostal space posterior to the posterior axillary line for the ultrasonic coagulating shears (US Surgical, Norwalk, CT). Two additional 5-mm ports are placed, 1 posterior to the tip of the scapula and 1 at the fourth intercostal space at the anterior axillary line for retraction and countertraction during the esophageal dissection. Next, a single retracting suture, (O-Surgitek, US Surgical, Norwalk, CT) is placed near the central tendon of the diaphragm and brought out of the inferior anterior chest wall through a 1-mm skin incision. This will provide downward traction on the diaphragm to allow good exposure of the distal esophagus.
Figure 1.
Video-assisted thoracoscopic port sites.
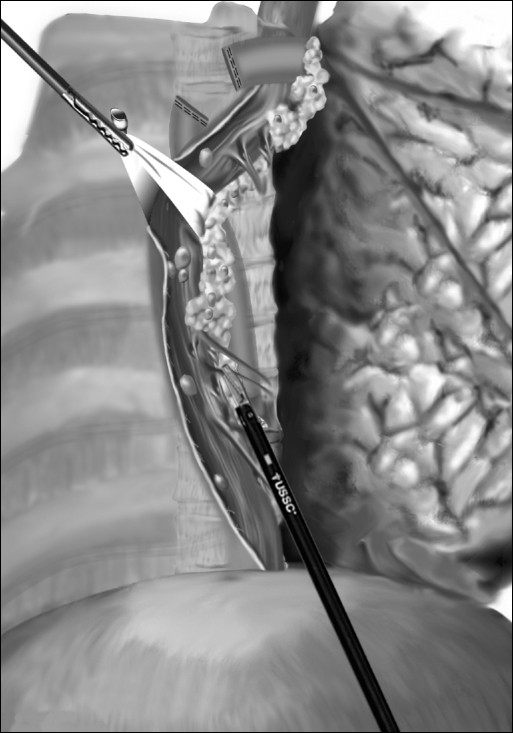
The inferior pulmonary ligament is divided up to the level of the inferior pulmonary vein. The mediastinal pleura overlying the esophagus is divided to expose the entire thoracic esophagus. The azygos vein is divided using the Endo GIA stapler (US Surgical, Norwalk, CT). Circumferential mobilization of the esophagus is undertaken with all surrounding lymph nodes and periesophageal tissue and fat. To facilitate traction and exposure, a Penrose drain is placed around the esophagus (Figure 2). Following mobilization of the esophagus from the thoracic inlet to the diaphragmatic reflection, a single 28 F chest tube is inserted through the camera port. The other ports are closed, and the patient is turned to the supine position.
Figure 2.
Thoracoscopic mobilization of the esophagus.
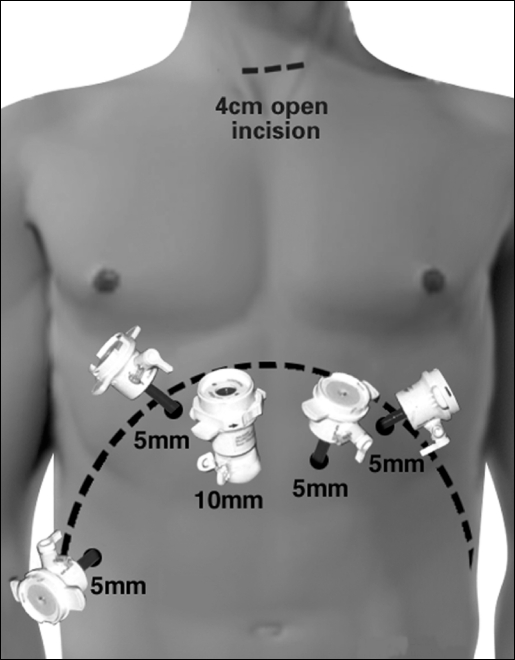
Laparoscopy and Neck Incision: The patient is placed in the supine position. The surgeon stands on the patient's right and the assistant on the left. Five abdominal ports are placed similarly to the approach used for laparoscopic Nissen fundoplication (Figure 3). The left lobe of the liver is retracted upward with a Diamond flex retractor (Genzyme, Tucker, GA) to expose the esophageal hiatus and held in place with a self-retaining system (Mediflex, Velmed, Inc., Wexford, PA). The gastrohepatic ligament is divided, exposing the right crus of the diaphragm. The stomach is mobilized with division of the short gastric vessels with the ultrasonic coagulating shears. The gastrocolic omentum is divided with preservation of the right gastroepiploic arcade. The stomach is retracted superiorly, and the left gastric vessels are identified and divided with the Endo GIA stapler.
Figure 3.
Abdominal port sites for laparoscopy.
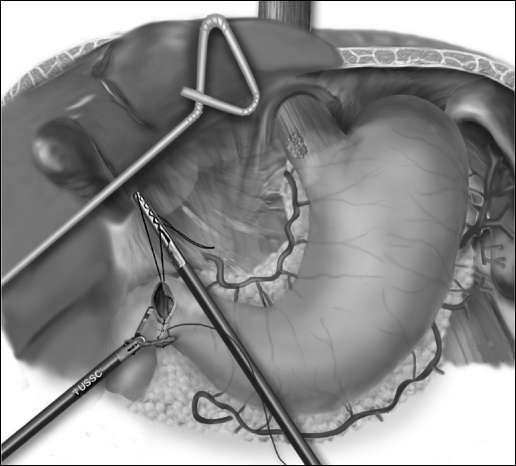
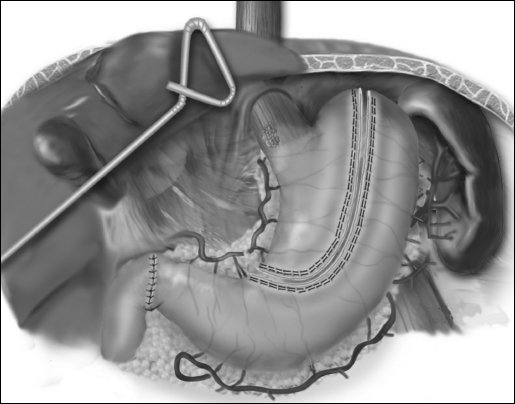
Pyloroplasty is then performed using the ultrasonic shears to open the pylorus and the Endo Stitch (US Surgical, Norwalk, CT) to close the pylorus transversely (Figure 4). The lessor curve and lymph nodes and omentum are dissected en bloc with the stomach. A gastric tube is constructed by dividing the stomach starting on the lesser curve, preserving the right gastric vessels using the 4.8-mm stapler (Endo GIA II, US Surgical, Norwalk, CT) (Figure 5). The gastric tube is then attached to the esophageal and gastric specimen with 2 sutures (Endo Stitch, US Surgical, Norwalk, CT). An additional stitch is placed on the anterior proximal gastric tube to facilitate orientation and prevent twisting as the tube is brought up into the neck. A laparoscopic jejunostomy tube is placed by first attaching a limb of proximal jejunum to the anterior abdominal wall with the Endo Stitch. A needle catheter kit (Compact Biosystems, Minneapolis, MN) is placed percutaneously into the peritoneal cavity under direct laparoscopic vision and directed into the loop of the jejunum. The guidewire and catheter are threaded into the loop of the jejunum. The jejunal puncture area is tacked completely to the anterior abdominal wall for a distance of several centimeters. The phrenoesophageal ligament is opened as the last laparoscopic step, to minimize loss of pneumoperitoneum into the mediastinum. The right and left crura are also partially divided to prevent gastric tube outlet obstruction. Once the gastric tube is pulled into the mediastinum, it is tacked inferiorly to the hiatus to prevent subsequent thoracic herniation.
Figure 4.
Laparoscopic pyloroplasty.
Figure 5.
Laparoscopic gastric tubularization.
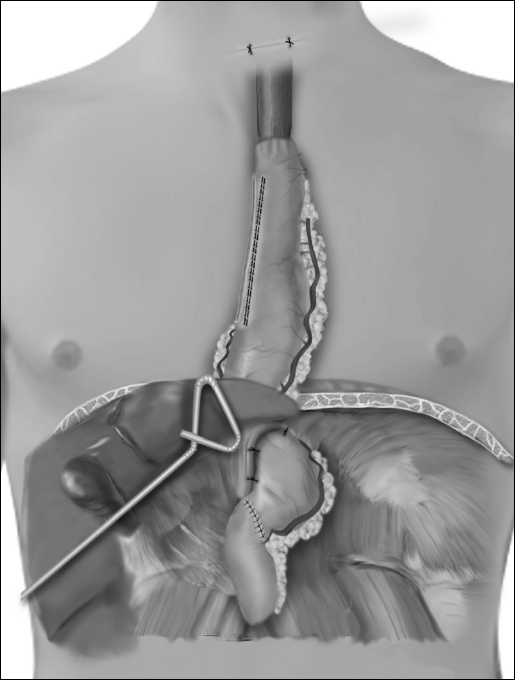
A 4- to 6-cm horizontal neck incision is made just above the suprasternal notch, and the cervical esophagus is exposed. Finger dissection is continued down into the mediastinum until the thoracic dissection plane is encountered. The cervical esophagus is divided and the esophago-gastric specimen pulled out of the neck incision. As traction is applied to the specimen in the neck, another surgeon guides the specimen in its proper alignment into the mediastinum. The specimen is removed from the field. An anastomosis is performed between the esophagus and gastric tube. The completed reconstruction is shown in Figure 6.
Figure 6.
Completed laparoscopic thoracoscopic operation.
Postoperative Care
All patients were observed in the ICU for the first 24 hours after surgery. A nasogastric suction tube was passed through the anastomosis and left in place until the water-soluble contrast swallow. Patients were extubated postoperatively as soon as their central temperature and respiratory function permitted. Postoperatively, patients received enteral nutrition through the jejunostomy feeding tube on postoperative day 2. ICU stay, length of stay, morbidity, and mortality were recorded.
RESULTS
Minimally invasive esophagectomy was performed in 41 patients (27 males and 14 females). The average age was 78 years (range 75 to 89). Laparoscopic-thoracoscopic esophagectomy was performed in 33 patients, laparoscopic-only in 3, and laparoscopic with right thoracotomy in 5. Cervical anastomoses were performed in all cases. Thirteen patients (32%) had undergone prior surgery involving the abdominal or thoracic cavity.
The indications for esophagectomy included carcinoma (n = 35) and Barrett's high-grade dysplasia (n = 6) (Table 1). Ten patients received preoperative chemotherapy. No open conversions were necessary. The median operative time was 7.5 hours (range 4.0 to 13.6). The size of the tumor did not significantly affect operative times. The median ICU stay was 1 day (range 1 to 34). The median hospital stay was 7 days (range 5 to 50). Major morbidity occurred in 19% of the cases (Table 2). Three anastomotic leaks in the neck were managed by conservative measures. One patient suffered a tracheal tear on postoperative day 6 during reintubation for respiratory distress. The reintubation injury was due to malposition of the stylet of the endotracheal tube. This was repaired by right thoracotomy and was followed by a prolonged hospital stay of 50 days, but ultimately the patient recovered. One patient had a persistent air leak from an injured right lung and was treated with small wedge resection, and 1 patient had a small bowel perforation attached to the jejunostomy site, which was repaired by a segmental resection and anastomosis.
Table 2.
Perioperative Morbidity
| Complication | No.of Patients | Management |
|---|---|---|
| Persistent air leak | 1 | Thoracotomy |
| Pneumonia with acute respiratory failure | 1 | Prolonged intubation and ICU support |
| Tracheal tear | 1 | Immediate surgical repair |
| Chylothorax | 1 | Thoracotomy and ligation of thoracic duct |
| Myocardial infarction | 1 | Cardiac intensive care unit and catheterization with reperfusion |
| Small bowel perforation | 1 | Relaparotomy |
| Anastomotic leaks | 3 | Locally controlled |
The final pathology of the cancer patients included stage (4), I (2), IIA (16), IIB (10), III (12), and IV (1); and 10% of the surgical margins were negative on frozen section in all cases. The average number of lymph nodes removed with the specimen was 16 (range 10 to 51). Approximately 60% of the dissected nodes were from the laparoscopic dissection, and 40% were from the chest. The 6 patients with Barrett's high-grade dysplasia were all alive at 20-month follow-up. The cancer group (35 patients) had an overall survival of 81% at a median follow-up of 20 months.
DISCUSSION
The diagnosis of esophageal cancer in the elderly, given the known increased morbidity associated with this population,5 raises the question of whether a major operation is indicated. More elderly patients are being referred for esophagectomy.6 A few surgeons have reported their experience with open esophagectomy in elderly patients. Jougon7 described a series of 89 patients, 70 years of age or older, and compared it with a group of patients younger than 70 years of age. His results suggest that esophagectomy can be performed in select elderly patients with acceptable morbidity (24.7%) and mortality (7.8%).7 In a similar study, Chino8 reported on 45 patients over 80 years old with esophageal cancer. Only 13 had surgical resection, with a 60% morbidity that consisted primarily of cardiovascular and respiratory complications. Poon9 had a large series of 167 elderly patients (70 years or more) who underwent esophagectomy for carcinoma of the esophagus. The perioperative mortality rate was 7.2%, with 80.5% perioperative morbidity. Nguyen10 in his comparison study in between open to minimally invasive esophagectomies reported no significant difference in the incidence of postoperative respiratory complications.
In our experience, we have seen a reduction in pulmonary complications and length of hospital stay. In the current series, we defined elderly as greater than 75 years of age. At the University of Pittsburgh Medical Center, we have already preformed more than a 170 minimally invasive esophagectomies. Our surgical technique and experience have been reported recently,11 and the report emphasized the multiple advantages and good outcome from this procedure. In previous reports, preoperative complications were noted in up to 90% of the patients.12–14 Our study showed a perioperative complication rate of 26.7% with no perioperative mortality. Major morbidity occurred in 19%; 2 patients had a medical cardiopulmonary complication, which is lower than the overall reported medical morbidity for esophagectomies. Four patients had surgical complications that included 1 persistent air leak from injury to the right lung, 1 tracheal tear due to difficult intubation, 1 small bowel perforation in the feeding jejunostomy site, and 1 injury to the thoracic duct resulting in chylothorax. The complications were treated surgically with an uneventful follow-up. All these are well documented as surgical complications of esophagectomies and occurred within the same percentages as that in other reported series.11
It has already been shown that patients who undergo thoracoscopic esophagectomy had less postoperative pain and more complete recovery of vital capacity than do those who undergo open surgery.12 Our initial experience with minimally invasive esophagectomies confirmed the feasibility and safety of this technique,13 and led us to approach an older group of patients with probably a greater operative risk. Our results with the elderly population required advanced laparoscopic and thoracoscopic surgical skills and represent the overall advantages of less pain and rapid recovery following minimally invasive esophagectomy.
Footnotes
Presented at the 10th International Congress and Endo Expo, Society of Laparoendoscopic Surgeons Annual Meeting, December 5-8, 2001, New York, New York, USA.
References:
- 1. Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–2053 [PubMed] [Google Scholar]
- 2. Eckardt VF, Kanzler G, Bernhard G. Life expectancy and cancer risk in patients with Barrett's esophagus: a prospective controlled investigation. Amer J Med. 2001;111(1):33–37 [DOI] [PubMed] [Google Scholar]
- 3. Linn BS, Linn MW, Wallen N. Evaluation of results of surgical procedures in the elderly. Ann Surg. 1982;195:90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohr DN. Estimation of surgical risk in the elderly: a correlative review. J Am Geriatr Soc. 1983;31:99–102 [DOI] [PubMed] [Google Scholar]
- 5. Kinugasa S, Tachibana M, Yoshimura H, et al. Esophageal resection in elderly esophageal carcinoma patients: improvement in postoperative complications. Ann Thorac Surg. 2001;71(2): 414–418 [DOI] [PubMed] [Google Scholar]
- 6. Reed CE. Who should undergo esophagectomy? Ann Thorac Surg. 1997;63:1225–1226 [DOI] [PubMed] [Google Scholar]
- 7. Jougon JB, Ballester M, Duffy J, Dubrez J, Delaisement C, Velly JF, Couraud L. Esophagectomy for cancer in the patient aged 70 years and older. Ann Thorac Surg. 1997;63(5):1423–1427 [DOI] [PubMed] [Google Scholar]
- 8. Chino O, Makuuchi H, Machimura T, et al. Treatment of esophageal cancer in patients over 80 years old. Surg Today. 1997;27(1):9–16 [DOI] [PubMed] [Google Scholar]
- 9. Poon RT. Law SY. Chu KM. Branicki FJ. Wong J. Esophagectomy for carcinoma of the esophagus in the elderly: results of current surgical management. Ann Surg. 1998;227(3): 357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen NT, Follette DM, Wolfe BM, Schneider PD, Roberts P, Goodnight JE., Jr Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg. 2000;135(8):920–925 [DOI] [PubMed] [Google Scholar]
- 11. van La, nschot JJ, Hulscher JB, Buskens CJ, Tilanus HW, Kate FJ, Obertop H. Hospital volume and hospital mortality for esophagectomy. Cancer. 2001;91(8):1574–1578 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen NT, Schauer P, Luketich JD. Minimally invasive esophagectomy for Barrett's esophagus with high-grade dysplasia. Surgery. 2000;127(3):284–290 [DOI] [PubMed] [Google Scholar]
- 13. Fernando HC, Christie NA, Luketich JD. Thoracoscopic and laparoscopic esophagectomy. Semin Thorac Cardiovasc Surg. 2000;12(3):195–200 [DOI] [PubMed] [Google Scholar]
- 14. Luketich JD, Schauer PR, Christie NA, et al. Minimally invasive esophagectomy. Ann Thorac Surg. 2000;70(3):906–911 [DOI] [PubMed] [Google Scholar]