Abstract
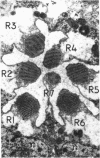
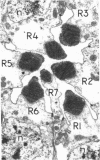
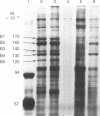
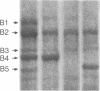
The ninaC gene is one of eight nina (neither inactivation nor afterpotential) genes identified from mutations that drastically reduce the amount of rhodopsin in the compound eye of Drosophila melanogaster. The gene has been cytogenetically localized to the 27E-28B region of the second chromosome. NaDodSO4/PAGE analysis of eye proteins of flies carrying one, two, or three copies of the ninaC region shows that two eye-specific proteins of molecular weight 170,000 and 130,000 display a strong dependence on the dosage of the ninaC gene, although the dependence is evident only when the dosage is decreased and not when it is increased. All mutations in the ninaC gene studied to date have pronounced effects on these two polypeptides. These results suggest that the ninaC locus encodes these two polypeptides. Ultrastructural studies show that the polypeptides encoded by ninaC are very likely to be important components of the cytoskeletal structure of rhabdomeral microvilli.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blest A. D., Stowe S., Eddey W. A labile, Ca2+-dependent cytoskeleton in rhabdomeral microvilli of blowflies. Cell Tissue Res. 1982;223(3):553–573. doi: 10.1007/BF00218476. [DOI] [PubMed] [Google Scholar]
- Brown H. M., Cornwall M. C. Spectral correlates of a quasi-stable depolarization in barnacle photoreceptor following red light. J Physiol. 1975 Jul;248(3):555–578. doi: 10.1113/jphysiol.1975.sp010988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S. C., Hotta Y. [Two-dimensional electrophoretic analysis of tissue specific proteins of Drosophila melanogaster (author's transl)]. Tanpakushitsu Kakusan Koso. 1979;24(12):1336–1343. [PubMed] [Google Scholar]
- Hillman P., Hochstein S., Minke B. A visual pigment with two physiologically active stable states. Science. 1972 Mar 31;175(4029):1486–1488. doi: 10.1126/science.175.4029.1486. [DOI] [PubMed] [Google Scholar]
- Johnson E. C., Pak W. L. Electrophysiological study of Drosophila rhodopsin mutants. J Gen Physiol. 1986 Nov;88(5):651–673. doi: 10.1085/jgp.88.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrivee D. C., Conrad S. K., Stephenson R. S., Pak W. L. Mutation that selectively affects rhodopsin concentration in the peripheral photoreceptors of Drosophila melanogaster. J Gen Physiol. 1981 Nov;78(5):521–545. doi: 10.1085/jgp.78.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L. S., Ganguly R., Ganguly N., Manning J. E. The selection, expression, and organization of a set of head-specific genes in Drosophila. Dev Biol. 1982 Dec;94(2):451–464. doi: 10.1016/0012-1606(82)90362-1. [DOI] [PubMed] [Google Scholar]
- Lindsley D. L., Sandler L., Baker B. S., Carpenter A. T., Denell R. E., Hall J. C., Jacobs P. A., Miklos G. L., Davis B. K., Gethmann R. C. Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics. 1972 May;71(1):157–184. doi: 10.1093/genetics/71.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tousa J. E., Baehr W., Martin R. L., Hirsh J., Pak W. L., Applebury M. L. The Drosophila ninaE gene encodes an opsin. Cell. 1985 Apr;40(4):839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Pak W. L., Conrad S. K., Kremer N. E., Larrivee D. C., Schinz R. H., Wong F. Photoreceptor function. Basic Life Sci. 1980;16:331–346. doi: 10.1007/978-1-4684-7968-3_24. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H. R. An ordered membrane-cytoskeleton network in squid photoreceptor microvilli. J Mol Biol. 1982 Jul 5;158(3):435–456. doi: 10.1016/0022-2836(82)90208-x. [DOI] [PubMed] [Google Scholar]
- Scavarda N. J., O'tousa J., Pak W. L. Drosophila locus with gene-dosage effects on rhodopsin. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4441–4445. doi: 10.1073/pnas.80.14.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinz R. H., Lo M. V., Larrivee D. C., Pak W. L. Freeze-fracture study of the Drosophila photoreceptor membrane: mutations affecting membrane particle density. J Cell Biol. 1982 Jun;93(3):961–967. doi: 10.1083/jcb.93.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Steven A. C., Roop D. R. The molecular biology of intermediate filaments. Cell. 1985 Sep;42(2):411–420. doi: 10.1016/0092-8674(85)90098-4. [DOI] [PubMed] [Google Scholar]
- Stephenson R. S., O'Tousa J., Scavarda N. J., Randall L. L., Pak W. L. Drosophila mutants with reduced rhodopsin content. Symp Soc Exp Biol. 1983;36:477–501. [PubMed] [Google Scholar]
- Zuker C. S., Cowman A. F., Rubin G. M. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985 Apr;40(4):851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]