Abstract
MRF4 belongs to the basic helix-loop-helix class of transcription factors and it and other members of its family profoundly influence skeletal muscle development. Less is known about the role of these factors in aging. Since MRF4 is preferentially expressed in sub-synaptic nuclei, we postulated it might play a role in maintenance of the neuromuscular junction. To test this hypothesis, we examined the junctional regions of 19-20 month old mice and found decreased levels of SV2B, a marker of synaptic vesicles, in MRF4-null mice relative to controls. There was a corresponding decrease in grip strength in MRF4-null mice. Taken together, these data suggest that the intrinsic muscle factor MRF4 plays an important role in maintenance of neuromuscular junctions. (117/120 words)
Keywords: Endplate, synapse, neuromuscular junction, basic helix-loop-helix transcription factor, muscle regulatory factor, skeletal muscle, aging, denervation, MRF4
Introduction
The elderly, especially those over 60 years of age, suffer from sarcopenia, a loss of muscle mass, strength, and function [1-5]. There are a number of etiological factors that contribute to sarcopenia, including a decrease in testosterone in men and growth hormone in men and women, a decrease in muscle protein synthesis, an increase in inflammatory factors that contribute to muscle catabolism, and progressive denervation leading to a subsequent loss of muscle fibers [1-5]. This last process, age-related denervation, results from progressive denervation/reinnervation that increases the size of motor units with aging [1-3]. As aging progresses, some motorneurons die, destroying the synaptic connections in the muscle fibers that it innervated. A process of reinnervation from the branches of neighboring motorneurons replaces failing synaptic contacts, but as a consequence of this process, motor units become progressively larger with aging and myofibers of different types (e.g., type IIB, type I) become grouped together [3,6,7]. Eventually, the motor units cannot become larger and there is increased loss of motorneurons and motor units [8-11]. Although the death of motorneurons is clearly an important contributor to this process, lack of trophic factors from underlying muscle fibers may also contribute significantly to this overall pathology.
The idea that the neuromuscular junction (NMJ) can be strengthened by intrinsic muscle factors is not new. Recent work indicates that a number of intrinsic factors can stabilize neuromuscular connectivity [12-14], or conversely, disrupt it [15]. Some of these intrinsic muscle factors are regulated by an important family of muscle transcription factors, the basic helix-loop-helix (bHLH) transcription factors [14]. This family is comprised of MyoD, myf-5, myogenin, and MRF4 [16]. Developmentally, MRF4, myf-5, and MyoD all have roles in the earliest muscle development, myogenin has a unique role in the formation of myotubes, and MRF4 is the primary bHLH factor expressed in adult muscle [16, 17]. MyoD- and MRF4-null mice have defects in their NMJs, indicating a role for these factors in synaptic development [18-20].
In spite of the NMJ defects observed in the MRF4-null mice, they are reported to exhibit a normal phenotype except for defects in the lower ribs, including rib fusions and bifurcations [17]. The goal of the present study was to determine if a phenotypic deficit in strength and synaptic connectivity emerged in aged MRF4-null mice. Here we find a mild but significant loss of strength and SV2B, a marker of synaptic vesicles, at NMJs in aged MRF4-null mice, suggesting an age-related loss of vesicles in the pre-synaptic terminal of MRF4-null mice.
Methods
Animal care, aging and strength measurements
The MRF4-null mice were a gift from Dr. Eric Olson (University of Texas Southwestern Medical Center) and were genotyped and propagated as reported previously [19]. To establish an aged colony, wild-type or MRF4-null females were maintained in cages, with 2-4 mice per cage. Males were not used due to problems with fighting when multiply housed.
Mouse forelimb grip strength was measured using a Grip Strength Meter from Columbus Instruments (Columbus, OH). Each mouse was grasped firmly by its tail between the thumb and the index finger and the middle finger placed in a curled position at the base of the tail to steady the mouse. It was then lowered and allowed to grasp the pull bar with its front paws, and pulled slowly backward from the meter in the horizontal plane. The mouse releases the bar at its peak strength and this tension is measured by the meter in Newtons. This process is repeated three times for each mouse and the results averaged as the grip strength for that animal.
Mice were euthanized with CO2 inhalation followed by cervical dislocation prior to tissue harvest. Animal protocols were used in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Immunostaining and Confocal Analysis
Tibialis anterior (TA) and soleus muscles were dissected and fixed 20 minutes with 4% paraformaldehyde in phosphate buffered saline (PBS), washed twice for 20 minutes in PBS, and cryoprotected in 15% sucrose in PBS at 4°C overnight. Following cryoprotection, muscles were snap frozen in liquid nitrogen and stored at − 80°C until use.
Longitudinal sections (10μm) were cut on a Microm cryostat and mounted on chilled glass slides for staining. For assessment of MRF4 localization, sections were stained with antibody to MRF4 (provided by Dr. Andres Buonanno) counterstained with a fluorescein-conjugated anti-rabbit antibody, an all nuclear To-Pro stain (Molecular Probes), and rhodamine-conjugated α-bungarotoxin (αBTX from Molecular Probes) to mark acetylcholine receptors (AChR) at NMJs. For assessment of NMJs, sections were stained with an antibody to neurofilament, which marks axons, an antibody to SV2B, which marks synaptic vesicles in pre-synaptic nerve terminals, and αBTX to mark the postsynaptic AChR. Stained sections were analyzed by confocal microscopy using an Olympus Fluoview (20X, air 0.7 NA and 60X oil 1.4 NA objectives; Olympus, Tokyo, Japan). The images shown are displayed as single plane projections of confocal stacks of images that capture both presynaptic and postsynaptic elements of the NMJ.
In aged animals, especially MRF4-null, there was reduced staining of SV2B. To quantify this reduction, NMJs throughout each muscle were surveyed for staining of SV2B versus αBTX in superimposed fields. Most NMJs were white, indicating complete overlap for the stains for the nerve terminal and the underlying postsynaptic receptors. However, especially in MRF4-null aged mice, there were some NMJs that were primarily red, indicating that there pronounced stain for the postsynaptic AChR but reduced stain for the SV2B marker. These NMJs were considered to have “dim SV2B staining” and were generally adjacent to NMJs with a normal appearance. The total number of “dim” synapses was taken as a percentage of all synapses for purposes of quantification. Muscles from young control or MRF4-null mice did not exhibit any “dim” synapses, and therefore this observation was not considered to be an artifact of the staining and imaging process.
Results
Sub-synaptic MRF4 expression decreases with aging
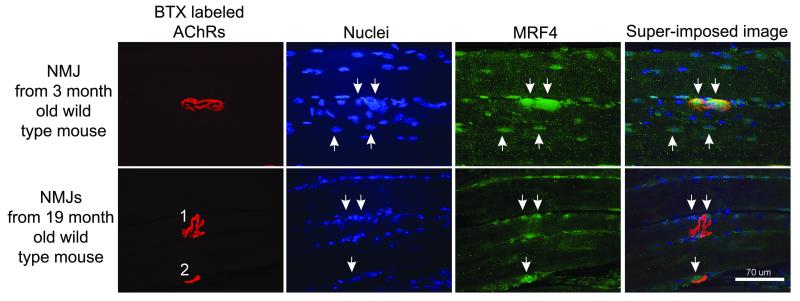
We and others have shown previously that MRF4 is enriched in sub-synaptic nuclei in the muscles of young adult animals [19, 21, 22]. As shown in Fig. 1, this finding is confirmed in young mice, in that there is brighter staining of the sub-synaptic nuclei with the MRF4 antibody, while lower levels are expressed in other nuclei throughout the muscle. In older mice, this sub-synaptic enrichment is greatly reduced, although there is residual MRF4 expressed in nuclei throughout the muscle. We postulated that this reduced expression of MRF4 at the NMJ might impair synaptic function in old age and these deficits might be greater in MRF4-null mice.
Figure 1. MRF4 staining of neuromuscular junctions declines during aging.
Shown are the staining of a representative neuromuscular junction (NMJ) from a young mouse (3 months old) and 2 NMJs from an old mouse (19 months old). NMJs are labeled by staining of acetylcholine receptors (AChRs) with α bungarotoxin (BTX). MRF4 staining in young muscle yielded a stereotyped pattern with a large region of very bright staining that encompassed the region containing multiple nuclei below the NMJ (downward arrows, upper row). In addition, MRF4 stained a subset of nuclei outside the NMJ (two examples, upward arrows, upper row). Two old NMJs are shown in the bottom row of images. While NMJ 2 had a region of bright MRF4 staining encompassing the NMJ (downward arrow near NMJ 2), no comparable region of MRF4 staining was present at NMJ1. Some nuclei near NMJ 1 were positive for MRF4 (downward arrows).
There is increased loss of the synaptic vesicle marker, SV2B, in aged MRF4-null mice
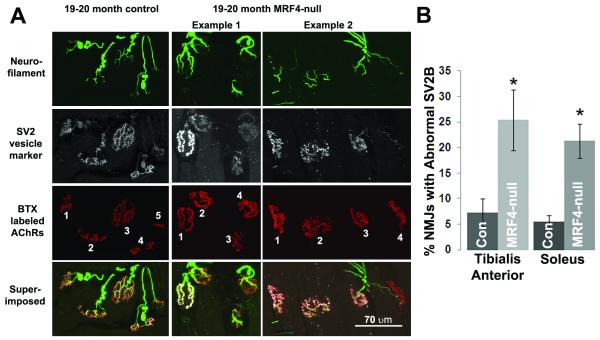
The tibialis anterior (TA) muscles from six aged control and six aged MRF4-null mice were examined in the region of their NMJs for both presynaptic and postsynaptic elements (Fig. 2). The NMJs from the control mice are normal in their alignment of SV2B and BTX staining. This is most easily seen in the superimposed view. When the NMJ is considered normal, it is white throughout every branch of the terminal arbor, suggesting that SV2B staining is present at normal levels opposed to the postsynaptic receptors. In contrast, some of the NMJs from the aged MRF4-null mice show an obvious loss of synaptic vesicle marker, SV2B, either almost completely (NMJs 4 in both examples 1 and 2) or to a severe degree (in NMJs 2 and 3 from example 1). In the superimposed views of these NMJs, much of the arbor is red, reflecting a loss of the vesicular marker and possibly the vesicles from that region of the presynaptic terminal. NMJ 4 from example 2 may also be denervated, with a sprout from the adjacent nerve extending towards it, but overall the degree of denervation for both controls and MRF4-null was low at this age.
Figure 2. Absence of MRF4 worsens breakdown of NMJs during aging.
A, Shown are 1 field of NMJs from an aged control mouse and 2 different fields from aged MRF4 null mice. Neurofilament staining of axons entering NMJs, SV2 staining of synaptic vesicles and α bungarotoxin (BTX) staining of acetylcholine receptors (AChRs) are all normal in this aged control mouse. NMJ 5 of this control mouse is on the edge and was not scored. In example 1 from a 20 month old MRF4 null muscle are 4 NMJs, all innervated by nerve as indicated by bright neurofilament staining. NMJ 1 has normal SV2 staining, but NMJs 2-4 have little SV2 staining, suggesting a marked reduction in the number of synaptic vesicles. In example 2 from a 19 month old mouse NMJs 1-3 have normal staining, but NMJ 4 has almost no SV2 staining. The neurofilament staining entering NMJ 4 consists of a thin line that is suggestive of a sprout that is reinnervating the NMJ (arrow). B, Quantification of the percentage of NMJs with “dim” SV2B staining was carried out in both aged control and MRF4-null muscles. A total of 20/269 NMJs in the control mice and 65/255 NMJs in the MRF4-null mice had reduced SV2B in TA muscles. To confirm that this phenomenon was not muscle type specific, three control and three MRF4-null soleus muscles were examined by the same approach with similar results. The asterisks indicate p < 0.05.
To determine if there was muscle-type specificity in this loss of SV2B, the soleus muscles from three control and three MRF4-mice were examined by the same approach, and as shown quantitatively in Fig. 2B, the loss of SV2B was similar in TA and soleus muscles in the MRF4-null mice compared to controls. Taken together, this data indicates that the intrinsic muscle factor, MRF4, contributes a stabilizing influence on presynaptic components in aged animals.
There is a corresponding diminution in muscle strength in aged MRF4-null mice
The breakdown of the synapse observed in aged MRF4-null animals by immunostaining was of sufficient magnitude that it suggested that there may be a functional effect on muscle strength. To test this possibility, the aged control and MRF4-null mice were screened using a grip strength meter that tested the maximum forelimb strength of the animal. As shown in Fig. 3, the average maximal force generated by the control mice was 2.30 newtons versus 1.95 newtons for the MRF4-null, a reduction of 15%. This reduction in muscle strength is approximately the same as the 18% increase in NMJs lacking SV2B in MRF4-null animals relative to controls (Fig. 2B).
Figure 3. Aged Female MRF4-null Mice Have Reduced Grip Strength Relative to Controls.

Forelimb grip strength was measured in aged (19-20 month old) female mice as described in the methods. The MRF4-null mice were significantly weaker in this test, with a mean grip strength of 1.95 ± 0.06 Newtons versus 2.3 ± 0.07 Newtons for the controls. Asterisks indicate p < 0.01. N = 14, control and n = 11, MRF4-null.
Young control and MRF4-null mice have normal NMJs
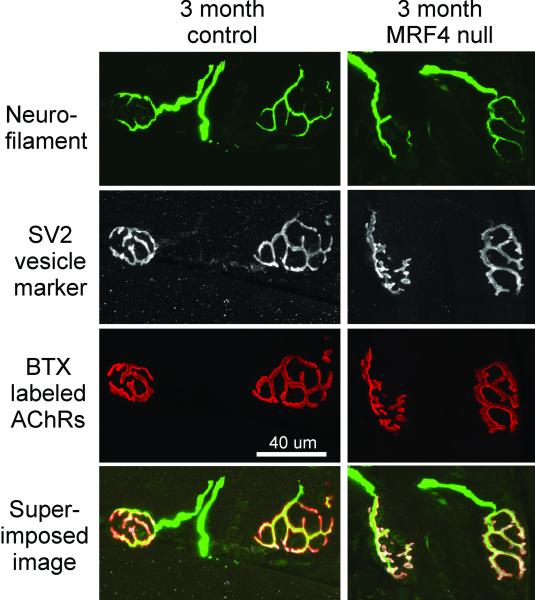
To determine if the effect of MRF4 was exerted through a developmental effect rather than an aging effect, we screened young control and MRF4-null animals in the same type of immunostaining analysis used for the aged animals (Fig. 4). In a total of 99 NMJs, both presynaptic and postsynaptic elements are perfectly aligned in young animals, including robust SV2B staining throughout each branch of the terminal arbor. The neurofilament stain shows that the nerve comes into the NMJs, where the SV2B completely stains the entire presynaptic terminal perfectly opposed to the postsynaptic receptor field, stained by αBTX. These data suggest that lack of MRF4 exerts an effect post-developmentally.
Figure 4. Absence of MRF4 has no discernable effect on NMJs from young animals.
Shown are 1 field of NMJs from a young control mouse and 1 field from a young MRF4-null mouse. Neurofilament staining of axons entering NMJs, SV2 staining of synaptic vesicles and α bungarotoxin (BTX) staining of acetylcholine receptors (AChRs) are all normal.
Discussion
This study suggests that the intrinsic muscle transcription factor, MRF4, has a positive role in maintaining nerve terminals in aged mice. Loss of MRF4 increases the loss of the synaptic vesicle marker SV2B and causes a parallel reduction in grip strength. We hypothesize that the two findings are related such that the reduction in strength is due to an age-related loss of synaptic vesicles from a subset of NMJs that causes failure in synaptic transmission. Further experiments are necessary to test this hypothesis, including electron microscopy studies to confirm actual loss of vesicles and not just the vesicular marker, SV2B. The loss of SV2B staining does not appear to be a developmental effect since NMJs of young animals have normal SV2B staining (Fig. 4).
Previous work suggested that the MRF4-null mice had reduced expression of the NaV 1.4 sodium channels at NMJs, but other features examined were normal [19]. There was an increase in expression of the other three bHLH factors in the MRF4-null mice [19], perhaps compensating for the loss of MRF4 for most functions, although expression of the NaV 1.4 Na+ channel is preferentially driven by MRF4 and thus very sensitive to the lack of this factor [23]. Like the MRF4-null mice, the MyoD-null mice initially appeared phenotypically normal, but recent ultrastructural analyses of these mice have revealed unexpected deficits in the structure of their NMJs [18]. Taken together, these studies suggest the need for further analyses of the role of the bHLH factors in building and maintaining the NMJ throughout the life of these animals. Although much work has focused on the role of the motorneuron in driving development and maintenance of the NMJ [24], it is also clear that muscle intrinsic factors have a role in development [25], and possibly in synaptic maintenance.
It will be important to identify the factors that the bHLH factors regulate. A recent report shows the importance of microRNA-206 in delaying the onset of ALS and promoting synaptic regeneration [14]. The gene that produces this microRNA has three motifs for bHLH binding in its promoter region and thus may be one target of MRF4 [14]. This microRNA promotes muscle regeneration, at least in part, through the fibroblast growth factor pathway [14]. Thus, one mechanism through which intrinsic muscle factors may stabilize motorneurons is via secretion of trophic factors.
Conclusion
In summary, aged MRF4-null mice have an increased degree of NMJ pathology relative to aged controls. Correspondingly, they are also weaker. Given that MRF4 may be upstream of many factors that positively influence synaptic function, it may be a good therapeutic target for future work in restoring or maintaining NMJs.
Acknowledgments
This work was supported by NIH grants number AR46477 (SDK) and NS040826 (MMR)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–24. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 2.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 3.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854:92–101. doi: 10.1111/j.1749-6632.1998.tb09895.x. [DOI] [PubMed] [Google Scholar]
- 5.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–9. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 6.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol (Berl) 1991;81:377–81. doi: 10.1007/BF00293457. [DOI] [PubMed] [Google Scholar]
- 7.Stalberg E, Fawcett PR. Macro EMG in healthy subjects of different ages. J Neurol Neurosurg Psychiatry. 1982;45:870–8. doi: 10.1136/jnnp.45.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. J Neurol Neurosurg Psychiatry. 1972;35:845–52. doi: 10.1136/jnnp.35.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown WF, Strong MJ, Snow R. Methods for estimating numbers of motor units in biceps-brachialis muscles and losses of motor units with aging. Muscle Nerve. 1988;11:423–32. doi: 10.1002/mus.880110503. [DOI] [PubMed] [Google Scholar]
- 10.Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36:174–82. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45(5):397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- 12.Payne AM, Zheng Z, Messi ML, Milligan CE, Gonzalez E, Delbono O. Motor neurone targeting of IGF-1 prevents specific force decline in ageing mouse muscle. J Physiol. 2006;570:283–94. doi: 10.1113/jphysiol.2005.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, et al. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–9. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliot JL, et al. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, Kraner S, et al. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at endplates. Neuron. 1999;24:567–83. doi: 10.1016/s0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 16.Arnold HH, Braun T. Genetics of muscle determination and development. Curr Top Dev Biol. 2000;48:129–64. doi: 10.1016/s0070-2153(08)60756-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Behringer RR, Olson EN. Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev. 1995;9:1388–99. doi: 10.1101/gad.9.11.1388. [DOI] [PubMed] [Google Scholar]
- 18.Macharia R, Otto A, Valasek P, Patel K. Endplate morphology, fiber-type proportions, and satellite-cell proliferation rates are altered in MyoD -/- mice. Muscle Nerve. 2010;42:38–52. doi: 10.1002/mus.21637. [DOI] [PubMed] [Google Scholar]
- 19.Thompson AL, Filatov G, Chen C, Porter I, Li Y, Rich MM, et al. A selective role for MRF4 in innervated adult skeletal muscle: Na(V) 1.4 Na+ channel expression is reduced in MRF4-null mice. Gene Expr. 2005;12:289–303. doi: 10.3727/000000005783992034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang ZZ, Washabaugh CH, Yao Y, Wang JM, Zhang L, Ontell MP, et al. Aberrant development of motor axons and neuromuscular synapses in MyoD-null mice. J Neurosci. 2003;23:5161–9. doi: 10.1523/JNEUROSCI.23-12-05161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGeachie AB, Koishi K, Andrews ZB, McLennan IS. Analysis of mRNAs that are enriched in the post-synaptic domain of the endplate. Mol. Cell. Neurosci. 2005;30:173–185. doi: 10.1016/j.mcn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi M, Kurahashi H, Noguchi S, Fukudome T, Okinaga T, Tsukahara T, et al. Aberrant endplates and delayed terminal muscle fiber maturation in α-dystroglycanopathies. Hum Mol Genet. 2006;15:1279–1289. doi: 10.1093/hmg/ddl045. [DOI] [PubMed] [Google Scholar]
- 23.Hebert SL, Simmons C, Thompson AL, Zorc CS, Blalock EM, Kraner SD. Basic helix-loop-helix factors recruit nuclear factor I to enhance expression of the NaV 1.4 Na+ channel gene. Biochim Biophys Acta. 2007;1769:649–658. doi: 10.1016/j.bbaexp.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 25.Arber S, Burden SJ, Harris AJ. Patterning of skeletal muscle. Curr. Opin. Neurobiol. 2002;12:100–103. doi: 10.1016/s0959-4388(02)00296-9. [DOI] [PubMed] [Google Scholar]