Abstract
A cell’s fate is tightly controlled by its microenvironment. Key factors contributing to this microenvironment include physical contacts with the extracellular matrix and neighboring cells, in addition to soluble factors produced locally or distally. Alterations to these cues can drive homeostatic processes, such as tissue regeneration/wound healing, or may lead to pathologic tissue dysfunction. In vitro models of cell and tissue microenvironments are desirable for enhanced understanding of the biology and ultimately for improved treatment. However, mechanisms to exert specific control over cellular microenvironments remains a significant challenge. Genetic modification has been used but is limited to products that can be manufactured by cells and release kinetics of therapeutics cannot easily be controlled. Herein we describe a non-genetic approach to engineer cells with an intracellular depot of phenotype altering agent/s that can be used for altering cell fate via intracrine-, paracrine-, and endocrine-like mechanisms. Specifically, we show that human mesenchymal stem cells (MSCs) can be engineered with poly lactide-co-glycolic acid (PLGA) particles containing dexamethasone, which acts on cytoplasmic receptors. The controlled release properties of these particles allowed for sustained intracellular and extracellular delivery of agent to promote differentiation of particle carrying cells, as well as neighboring cells and distant cells that do not contain particles.
Keywords: Mesenchymal stem cell, Controlled drug release, Cell signaling, Microsphere, Osteogenesis
1. Introduction
Control of cell fate and its extracellular environment is critical for tissue regeneration and cell therapy. During development, for example, cells are instructed by a complex set of microenvironmental cues, comprising soluble mediators and direct contacts with extracellular matrix and neighboring cells that are precisely regulated in time and space [1]. Consequently, when the microenvironmental balance is altered, cells may be activated toward homeostatic responses, such as to regeneration of damaged tissues, or pathologic changes in cell phenotype leading to aberrant cell growth or loss of function. To better understand these processes, engineer tissues, develop in vitro tissue models, and develop cell therapies, one must be able to exert localized control over the cell microenvironment.
Current methods to control cell fate in culture include: i) genetic manipulation of cells to program a desired phenotype, ii) addition of drugs or growth factors to the culture media, and iii) presentation of an engineered extracellular environment. Genetic modification has been used to program cell fate in culture to promote expression of specific cell surface receptors and to drive production of therapeutic peptides and proteins [2–7]. However, these modifications often exhibit a long-term impact on the cells, are limited to agents that can be manufactured by cells, and aside from use of genetic switches, there is an inability to finely tune the release kinetics of these agents. Drugs or growth factors can be added to culture media to mimic a tissue microenvironment, however all cells receive essentially the same signal, and application of soluble factors for controling the fate of transplanted cells is limited to pre-conditioning regimens. Alternatively, scaffolds or 2D/3D micro/nano-engineered substrates are useful to create multiple distinct microenvironments within a single culture system. These types of substrates have been used extensively to study cell-cell interactions, transplant cells, or mimic stem cell niches in vitro through support of cell proliferation, differentiation, or migration via controlled presentation of soluble cues, adhesive interactions, or surface stiffness and topology [8–12]. In addition, cues such as growth factors can be chemically immobilized to the substrate, providing specific locations to modulate cell behavior [13–15]. However, all of these strategies require cells to be on, or in close proximity to the substrate. Engineering substrates to control cell phenotype and function often involves a complex manufacturing methodology and there are several circumstances under which it is desirable to infuse cells in vivo without the use of a carrier or substrate (e.g. systemic cell infusion) [16].
Thus, there is a need to exert control over cells and their microenvironment without genetic modification or the use of an engineered substrate. Such a strategy would be useful to create in vitro models of regenerative or disease microenvironments that recapitulate critical cell-cell signalling events in situ. This approach could also be applied to control the fate of cells following transplantation or control specific in vivo microenvironments without the need for a cell carrier.
Here we propose a method to control the cellular microenvironment through a simple biomaterial-based cell modification approach independent of genetic manipulation or the presence of an artificial substrate. Rather than immobilizing cells on a biomaterial to control the cellular microenvironment, we present a strategy in which readily internalized biodegradable particles containing phenotype altering agents can be used to control cell fate (Fig. 1A). Upon modification of the cells, intracellular and extracellular release of agents was characterized. Assays were developed to test whether the released agents could promote osteogenic differentiation of particle-carrying cells as well as neighboring and distant cells (Fig. 1B). Furthermore, in vitro and in vivo applications of the cell modification approach are discussed.
Fig. 1.
Controlling cell fate through internalized biodegradable particles. (A) Schematic illustration of functionalizing cells with biodegradable particles to generate cells with internalized particles. (B) The encapsulated agent can control the cell and neighboring microenvironment in three distinct ways. The release of the agent can control the fate of the (i) particle-carrying cell through intracrine-like signaling, (ii) neighboring cell, through paracrine-like signaling, (iii) and distant cells through endocrine-like signaling.
2. Materials & methods
2.1 Mesenchymal stem cell culture and characterization
Primary human MSCs were obtained from the Texas A&M Health Science Center, College of Medicine, Institute for Regenerative Medicine at Scott & White Hospital which has a grant from NCRR of the NIH, Grant # P40RR017447. MSCs were derived from healthy consenting donors and thoroughly characterized as previously described [17]. MSCs were maintained in α-MEM expansion media (Invitrogen) supplemented with 15% Fetal Bovine Serum (Atlanta Biologicals), 1% (v/v) L-Glutamine (Invitrogen), and 1% penicillin:streptomycin solution (Invitrogen). Cells were cultured to 70–80% confluence before passaging. All experiments were performed using MSCs at passage number 3–6 where cells expressed high levels of MSC markers CD90 and CD29 (>99% cells), and did not express hematopoietic markers CD34 or CD45 (0% of cells) as observed from flow cytometry analysis.
2.2 PLGA Microparticle Fabrication
Rhodamine 6G dye (Sigma) or the osteogenic differentiation agent, dexamethasone (DEX), were encapsulated in poly (lactic-co-glycolic) acid (PLGA) particles using a single emulsion encapsulation technique. Briefly, 100 mg of 50:50 PLGA(carboxylic acid end group) was dissolved in 2 mL dichloromethane. DEX or dye was then added to the PLGA solution and mixed thoroughly. For complete dissolution of DEX, 10% methanol was added to dichloromethane. The PLGA solution was then added to 20 mL of 1% (w/v) polyvinylalcohol solution in deionized water and emulsified using a sonicator at 30W for 60 seconds. The solution was then stirred overnight at room temperature on a magnetic stirrer to allow extraction and evaporation of the organic solvent. The remaining solution was centrifuged and rinsed with PBS to isolate particles and lyophilized. Particle size was determined by dynamic light scattering and confirmed by scanning electron microscopy. To determine the encapsulation efficiency, briefly, 10 mg of DEX-PLGA particles were dissolved in anhydrous dimethyl-solfoxide (DMSO) followed by quantification of DEX with a UV-vis spectrophotometer at 251 nm. Blank PLGA particles without any DEX served as control. DEX was reliably encapsulated in DEX-PLGA particles with an efficiency of 71±13.5% (e.g. from an initial 10mg of DEX, ~7.1mg ±1.35 was typically entrapped within the PLGA particles).
2.3 Modifying MSCs with PLGA microparticles
To improve particle uptake, PLGA microparticles were incubated with 50 μg/mL poly-L-lysine for 3 hrs before incubation with MSCs. PLGA particle suspensions with concentrations of 0.1 mg/mL and 0.5 mg/mL in PBS were added to 90% confluent layers of MSCs in a 24 well plate for 10 min after which the PBS was removed and complete media was added. The MSCs were allowed to internalize particles for 24 hrs at 37 °C. To characterize particle internalization and stability of internalized particles, MSCs were loaded with DiO containing PLGA particles and characterized with a Zeiss LSM510 laser scanning confocal microscope equipped with a 63X water dipping objective. After a 24 hr incubation, the cells were fixed with 3.7% formaldehyde at room temperature and stained with 5 μg/mL of propidium iodide (PI) solution or 5 μl/mL DiL Vybrant cell stain solution for 10 min to visualize the cells. The cells were visible through the red fluorescence channel and the particles were visible through the green fluorescence channel. The internalization of the particles was examined from 3-D re-constructed Z-stack confocal microscopy images and a particle was considered internalized if it was localized within the plane of the nucleus, yet inside the borders of the cell membrane. The percentage of internalized particles was calculated from the number of particles present inside the cell compared to the total number of particles associated with cells in the field of view for ten random fields. For transmission electron microscopy, particle modified cells were prepared as described above, fixed, and analyzed by the W.M Keck Microscopy Facility at the Whitehead Institute. Specifically, the cells were fixed in 2.5% gluteraldehyde, 3% paraformaldehyde with 5% sucrose in 0.1M sodium cacodylate buffer (pH 7.4), pelletted, and post fixed in 1% OsO4 in veronal-acetate buffer. The cell pellet was stained in block overnight with 0.5% uranyl acetate in veronal-acetate buffer (pH 6.0), then dehydrated and embedded in Spurrs resin. Sections were cut on a Reichert Ultracut E microtome with a Diatome diamond knife at a thickness setting of 50 nm, stained with uranyl acetate, and lead citrate. The sections were examined using a FEI Tecnai spirit at 80KV and photographed with an AMT CCD camera. The viability, adhesion kinetics and proliferation of particle-modified MSCs and unmodified MSCs were examined using our previously reported experimental methodology [18]. Briefly, the viability of the cells was examined immediately after modification (time 0) and after the cells were incubated within 6-well plates for 48 hrs using a trypan blue exclusion assay. Cell adhesion kinetics were quantified by measuring the number of adherent cells on the tissue culture surface after 10, 30, and 90 min. Proliferation of modified and unmodified MSCs was quantified by plating cells in T25 flasks at low density and counting the number of cells in the flask for an 8 day period with light microscopy at 10X for ten random fields. Multi-lineage differentiation potential of the particle modified MSCs and unmodified MSCs was examined by incubating cells with osteogenic and adipogenic induction media followed by respective colorimetric staining [18]. Cells were assayed for osteogenic differentiation and adipogenic differentiation using cell membrane associated alkaline phosphatase activity and Oil Red O staining, respectively.
2.4 In vitro release experiment from particle modified MSCs
0.1 mg/mL, 0.5mg/mL, or 1mg/mL PLGA microparticles with entrapped rhodamine dye were incubated with MSCs for 24 hrs at 37 °C. The media was then discarded and the cells were rinsed with PBS and supplied fresh media to create a baseline for the dye release measurements. On days 2, 4, 7, 10 media was collected and the quantity of dye released was measured using a fluorescence spectrophotometer with excitation and emission wavelengths of 540 and 625 nm, respectively. Preliminary characterization of the particle-modification approach showed that 0.1mg/mL particles were efficiently internalized by cells and resulted in adequate cell loading, therefore this concentration was used for the remainder of the experiments. To quantify the amount of dexamethasone released, MSCs were incubated with 0.1 mg/mL DEX-PLGA particles for 24 hrs at 37 °C. On day 2, 4, 6, 10, 14, 18, and 22, 1 mL of media was collected and replenished with fresh media. The released DEX was determined using ultraviolet (UV) spectrophotometer at 251 nm. Cells with no particles and cells with blank particles (no DEX) served as controls.
2.5 Examination of Osteogenic Differentiation
To evaluate osteogenic differentiation, cell membrane associated ALP activity was examined after 21 days by aspirating the culture media and rinsing the cells followed by fixation with 3.7% formaldehyde solution for 10 min at room temperature and rinsing. After 45 min incubation in 0.06% Red Violet LB salt solution in Tris HCl, DMF and Naphthol AS MX-PO4, the wells were rinsed 3 times with distilled water and visualized with light microscopy. Osteogenic differentiation was identified by red staining for alkaline phophatase. To visualize individual cells, the nuclei of the cells were stained with 100 μL of DAPI solution (1 μg/mL in PBS) after treatment with 100 μL of 0.1% TRITON X solution in PBS. To quantify the percentage of MSCs stained positively for alkaline phosphatase, ImageJ® software was used. Some cultures stained for ALP were further examined for the presene of mineralization via the Von Kossa stain. Briefly, plates were rinsed 3–4 X in ddH2O, and stained with 2.5% silver nitrate for 30 min. After rinsing 3–4 X in ddH2O, plates were incubated in sodium carbonate formaldehyde for 1–2 min, rinsed, air dried, and examined by light microscopy.
2.6 Differentiation of particle modified cells
Microparticles containing DEX were incubated with MSCs for 24 hr followed by rinsing to remove free particles and the media was replaced with β-glycerolphosphate (G) and Ascorbic Acid (A) containing media. Cells grown in α-MEM complete media served as a negative control, while cells grown in media supplemented with DEX, G and A served as a positive control for osteogenic differentiation. Additional controls included media containing only G or A and cells containing empty PLGA particles (no DEX). Cultures were maintained for 21 days and then assessed for osteogenic differentiation by ALP staining as described above.
2.7 Differentiation of neighboring and distant cells
To assess the potential of MSCs modified with DEX-PLGA microparticles to induce osteogenic differentiation of adjacent unmodified MSCs, a model assay was developed. MSCs modified with DEX-PLGA particles were mixed with equal number of unmodified MSCs and plated at a density of 300,000 cells per well in a 6 well plate. The media was supplemented with β-glycerolphosphate (G) and Ascorbic Acid (A). Cells grown in α-MEM complete media served as a negative control, while cells grown in media supplemented with DEX, G and A served as a positive control. Other controls included media containing only G or A and cells containing empty PLGA particles. Cultures were maintained for 21 days and then assessed for osteogenic differentiation as described above. To assess the potential of DEX-PLGA microparticle modified MSCs to induce osteogenic differentiation of unmodified MSCs at a distant site, two model assays were employed. First, MSCs containing DEX-PLGA microparticles were plated into 6-well culture plates and unmodified MSCs were plated in separate 6-well culture plates. The media added to DEX-PLGA modified MSCs was supplemented with G and A. Media from the particle modified MSCs was transferred to wells containing unmodified MSCs every third day and fresh media with β-glycerolphosphate (G) and Ascorbic Acid (A) was replenished. Cells grown in α-MEM complete media served as a negative control, while cells grown in media supplemented with DEX, G and A served as a positive control. Other controls included media containing only G or A and cells containing empty PLGA particles. Cultures were maintained for 21 days and then assessed for osteogenic differentiation as described above. To rule out the possibility that the observed induction of osteogenesis was mediated by factors secreted by the differentiating DEX-PLGA modified MSCs, the experiment was repeated using fibroblasts (in place of MSCs) modified with DEX-PLGA particles. Towards the same goal, the impact of transferring media from >21 day osteogenic cultures of MSCs (without particles) to a separate culture dish containing unmodified cells was assessed. Second, MSCs containing DEX-PLGA microparticles were plated on the bottom well of a transwell plate. Unmodified MSCs were then plated on the membrane of the transwell and the media was supplemented with β-glycerolphosphate (G) and Ascorbic Acid (A). Cells grown in α-MEM complete media (without osteogenic factors) served as a negative control, while cells grown in media supplemented with DEX, G and A served as a positive control. Other controls included media containing only G or A and cells containing PLGA particles without DEX. Cultures were maintained for 21 days and then assessed for osteogenic differentiation as described above.
2.8 Effect of Cryopreservation
To examine the effect of cryopreservation on DEX release and ability to influence the cellular microenvironment, the DEX-PLGA particles were incubated with MSCs for 24 hr followed by trypsinization with 1X trypsin-EDTA solution. The particle modified cells were frozen in complete cell culture media supplemented with 5% dimethyl sulfoxide at −140°C. After 10 days the cells were thawed, plated, and the release of DEX was examined in addition to repeating the osteogenic differentiation experiments described above.
3. Results and discussion
To exert control over cells without genetic modification or engineered substrates, we conceived of a strategy utilizing a controlled drug delivery approach. Specifically, we envisioned that cells could be modified with a depot containing drugs or differentiation factors that could impact the modified cells and their cellular microenvironment through diffusion or transport of agents out of the carrier cell. Although strategies for modifying the surface of cells with nanoparticles exists, achieving stability beyond minutes or hours requires chemical modification of the cell surface [19, 20]. To develop an approach that does not require chemical modification of the cell, we considered utilizing biodegradable particles which are readily internalized by multiple cell types. Particles formulated with poly(lactide-co-glycolide) (PLGA) enable a nontoxic and efficient system for sustained intracellular delivery of multiple therapeutic agents directly to the cytoplasm through rapidly escaping the degradative endo-lysosomal compartment [21]. PLGA is a polyester that hydrolyzes into biologically compatible and metabolizable moieties (lactic acid and glycolic acid). While small molecules such as dexamethasone (DEX), a commonly utilized osteogenic differentiation factor, can freely cross the membrane of cells such as MSCs to engage intracellular receptors [22,23], many exogenously supplied large or acidic molecules (i.e. added to the culture media) have limited ability to transverse membranes unless the membranes are permeabilized [24, 25]. For agents that cannot passively transverse the cell membrane, active processes including gap junctions and permeability glycoproteins can be utilized [26, 27]. Thus, we hypothesized that particle based carriers could be used to deliver high intracellular concentrations of agents leading to either passive or active transport across the cell membrane to impact the extracellular environment. For proof of concept of this approach, we focused on small molecules that have been shown to freely cross the cell membrane including dexamethasone and rhodamine dye.
3.1 Engineering MSCs with PLGA particles
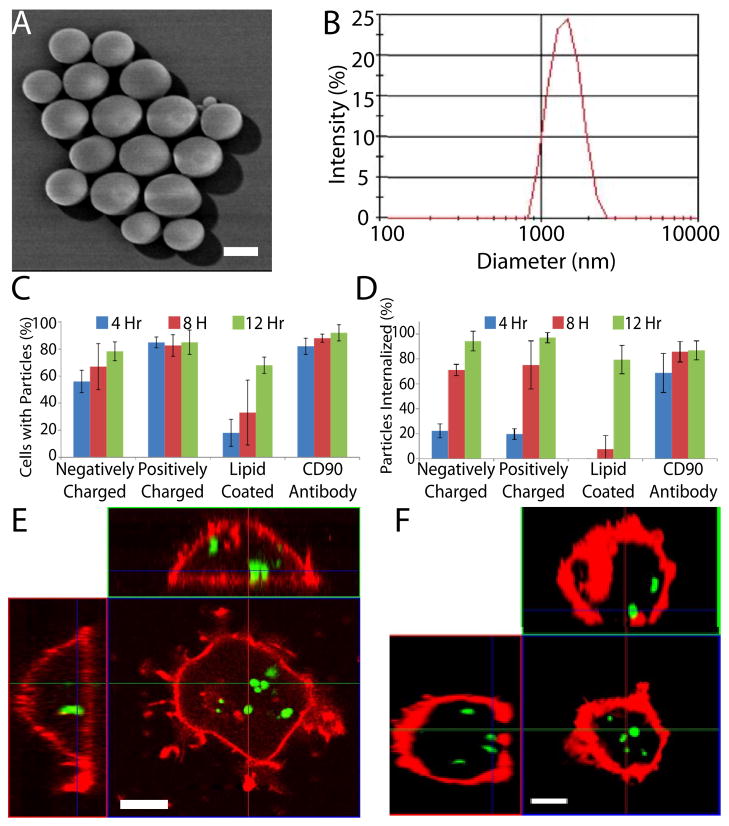
Although MSCs readily internalize nano-sized particles [28], small particles (<1μm) that are typically endocytosed [29] have been shown in other cell types to be rapidly exocytosed unless they are conjugated to the cell membrane [21, 30–32]. To reduce the potential for exocytosis, PLGA particles with a diameter of 1–2μm were fabricated (Fig. 2A & B) and found to be internalized by MSCs irrespective of the surface chemistry, likely via phagocytosis [29] (Fig. 2C). However, the kinetics of internalization was increased by modifying the surface with a positive charge or with an antibody directed towards an MSC surface antigen (e.g. CD90) (Fig. 2C). Thus positively charged particles were selected for further experimentation. Confocal microscopy demonstrated that ~95% of the PLGA particles were internalized following a 12 hr incubation (Fig. 2D). Additionally, internalization of particles was confirmed with transmission electron microscopy (Fig. S1. A). Importantly, in contrast to previous reports of nanoparticle exocytosis, the 1–2μm particles were stable inside the cell for at least 7 days (Fig. 2E & F). Furthermore, modification of MSCs with PLGA particles did not impact cell phenotype including viability, adhesion, proliferation (Fig. S2) and multilineage differentiation potential (Fig. S3).
Fig. 2.
Particle morphology, size, uptake and stability. (A) Scanning electron microscope image of PLGA particles reveals particles are spherical with a smooth pin-hole free surface (Scale bar: 1 μm). (B) Representative distribution of particle diameter as determined by dynamic light scattering. (C) Particle interaction/binding with cells was moderately affected by changes in surface chemistry, yet after 12 hr the majority of cells contained bound particles regardless of surface chemistry. (D) Kinetics of particle internalization as a function of particle surface chemistry. (E,F) Stability of internalized particles within DiD stained MSCs (red) as analyzed by confocal microscopy. Representative orthogonal confocal images (E) 1 day, and (F) 7 days after incubation with DiO loaded PLGA particles (green). (Scale bar: 10 μm)
Following the development of particles that were readily and stably internalized by MSCs, we sought to examine the potential for agents encapsulated within the particles to be released into the intracellular and extracellular milieu using rhodamine dye as a model small molecule (mol. wt. 479). Intracellular accumulation of rhodamine dye was examined over a 10 day period through permeabilization of the cells at different time points following rinsing to remove residual culture media. Dye was released in an initial burst within the first 2 days followed by relatively constant release (Fig. 3A). To examine the potential for rhodamine to be transported into the extracellular miliu, we sampled the media throughout the culture period with a fluorescence spectrophotometer and compared this result to a particle suspension without cells. Remarkably, we detected increasing concentrations of rhodamine over time in the culture media indicating transport from the intracellular to the extracellular milieu. Release of rhodamine from particles without cells showed a characteristic initial burst release with over 40% of encapsulated rhodamine being released within the first day followed by steady sustained release (Fig. 3B). In contrast, rhodamine was released from internalized PLGA depots at a constant rate, with 40% of entrapped rhodamine released by day 5 and 100% by day 10 (Fig. 3B). Importantly the rate of rhodamine delivery was easily tuned by changing the concentration of particles added to the cultures (Fig. S4). This demonstrates the potential of engineering cells with particles to achieve sustained targeted release of membrane permeable agents to the carrier cell and its microenvironment.
Fig. 3.
Rhodamine intracellular accumulation and extracellular release from MSCs. (A) To quantify the intracellular accumulation of rhodamine over time, MSCs loaded with 0.1 mg/mL or 0.5 mg/mL of rhodamine-PLGA particles were permeabilized with 5 mg/mL of L-lysine at 4 hr, 2 days, 4 days, 7 days, or 10 days, the permeabilized cells were discarded, and the dye concentration in the lysate was assessed with UV-Spectrophotometry. (B) Kinetics of rhodamine dye released into the culture media from MSCs modified particles versus a suspension of PLGA particles without cells. 200 μl of a 0.1mg/mL rhodamine-PLGA particle solution was added to the MSCs leading to internalization of ~19 μg and release was examined in 500 μl of media. To examine release of dye from particles without cells, conditions were normalized to the experimental group with ~19 μg of particles suspended in 500 μl of PBS.
3.2 Controlling the fate of particle engineered cells, neighboring cells, and distant cells
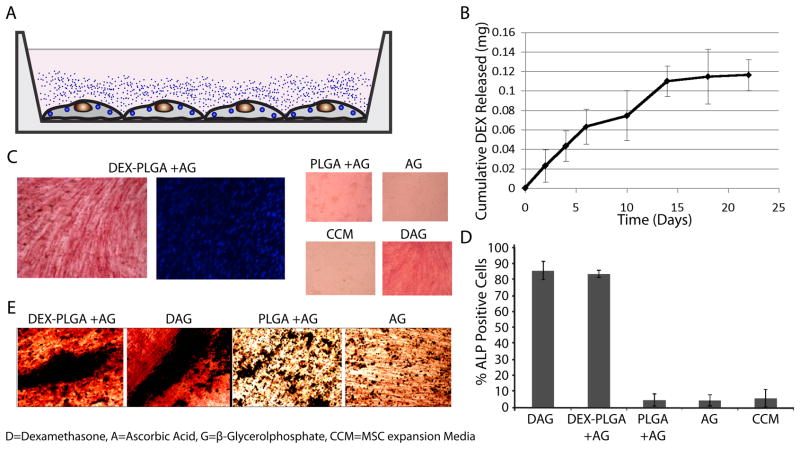
MSCs are multipotent cells capable of self-renewal that can give rise to a number of unique, differentiated mesenchymal cell types including osteoblasts, chondrocytes, and adipocytes. To examine the potential to control MSC phenotype we utilized an osteogenesis assay where differentiation of MSCs to osteoblasts can easily be detected through the characteristic expression of alkaline phosphatase (ALP) [33]. MSCs differentiate into osteogenic cells in the presence of the glucocorticoid, dexamethasone (DEX) that passively diffuses across the cell membrane [22,23], but only produce mineralized extracellular matrix in the presence of ascorbic acid (A) and phosphate ions (e.g. from β-glycerol-phosphate (G)) [33]. Instead of placing DEX into media, we incorporated DEX into PLGA microparticles that were internalized by MSCs (Fig. 4A). Quantification of dexamethasone in media above modified cells demonstrated that DEX was transported from the particle engineered MSCs to the extracellular environment for up to 2 weeks (Fig. 4B). The media was supplemented with A and G and after 21 days, osteogenic differentiation was detected via ALP staining (Fig. 4C). MSCs with blank particles, and MSCs in the presence of A and G alone did not stain positive for ALP (Fig. 4D). Approximately 80% of the MSCs engineered with DEX containing particles in the presence of A and G stained positive for ALP, which was comparable to the ALP staining of MSCs (without particles) in complete ostegenic media. In addition, co-staining cultures with ALP and Von Kossa revealed the formation of bone nodules in DEX-PLGA cultures (Fig. 4E). Since DEX binds to intracellular glucocorticoid receptors [22,23], these results demonstrate that DEX released from PLGA microparticles induced osteogenic differentiation of particle modified MSCs as previously shown with nanoparticles [34,35]. Thus microparticles that do not readily undergo exocytosis, as nanoparticles do [21], can be used to deliver phenotype altering agents such as dexamethasone to intracellularly control the fate of particle modified cells.
Fig. 4.
Intracrine-like signaling leads to osteogenic differentiation of DEX-PLGA particle modified MSCs. (A) Schematic of DEX release into culture media from adherent MSCs modified with DEX-PLGA particles. (B) Release kinetics of DEX from MSCs incubated with 0.1mg/mL DEX-PLGA particles into media at 37 °C for 21 days. (C) Osteogenic differentiation of DEX-PLGA modified MSCs and controls were assessed via alkaline phosphatase staining (ALP, red), nuclei were counterstained with DAPI (blue). (D) Quantification of ALP staining. (E) Bone nodules were identified via positive dual staining for Von Kossa and ALP in DEX and internalized DEX-PLGA particle containing cultures supplemented with A and G but not in the absence of DEX or DEX-PLGA particles.
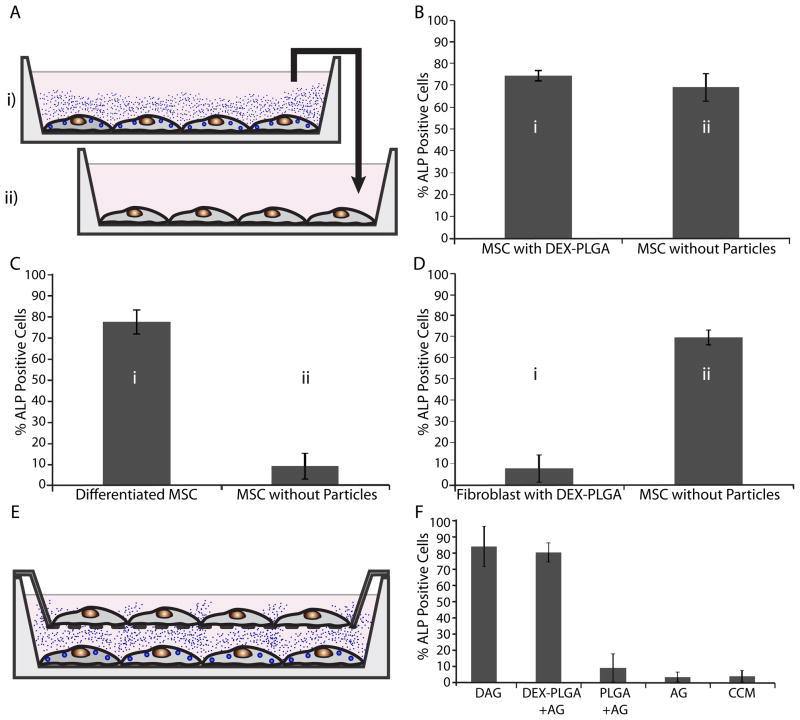
Given that DEX can be transported across the MSC membrane into the extracellular environment following internalization of DEX loaded microparticles, we envisioned particle engineered cells could be used to control the phenotype of neighboring cells in a paracrine-like manner. For an in vitro model, the previous experiment was repeated, with only half of the MSCs containing DEX-PLGA particles (Fig. 5A). Specifically, MSCs and DEX-PLGA modified MSCs were mixed in a 1:1 ratio and plated in a 6-well plate. Strikingly, following differentiation conditions, the majority of cells within the coculture with DEX-PLGA particles stained positive for ALP (Fig. 5B). Given that cell adhesion and proliferation properties of the PLGA modified and unmodified cells were similar (Fig. S2), these results are likely not due to differences in adhesion and proliferation between the two populations of cells. Thus, this data suggests that DEX released from particle modified MSCs can control the fate of adjacent cells.
Fig. 5.
Paracrine-like activity of modified MSCs. (A) Schematic illustration of DEX- PLGA modified MSCs controlling the fate of neighboring MSCs without particles (black arrows). (B) Osteogenic differentiation of DEX-PLGA modified MSCs and neighboring MSCs seeded in a 1:1 ratio quantified through ALP staining. D=Dexamethasone, A=Ascorbic Acid, G=β-Glycerolphosphate, CCM=MSC expansion media
Next we examined the potential for extracellular release of DEX from particle modified cells to promote differentiation of unmodified MSCs in a different culture dish (endocrine-like signalling). On every third day, conditioned media was transferred from particle modified cells (supplemented with G and A) to the unmodified cells and after 21 days stained to detect ALP activity (Fig. 6A). ALP staining of the unmodified cells incubated in conditioned media from DEX-PLGA modified cells was comparable to the DEX-PLGA modified MSCs (Fig. 6B). Importantly, no detectable ALP staining was observed when the media was transferred from MSCs engineered with blank PLGA particles (supplemented with G and A) and from unmodified MSCs (supplemented with G and A) to a separate dish contatining unmodified MSCs. To ensure that the released DEX was responsible for induction of osteogenic differentiation and that this was not due to a factor released from the differentiating MSCs, additional experiments were performed. Specifically, media transferred from unmodified MSC cultures following 21 days of osteogenic differentiation (supplemented with DEX, G, and A) resulted in no detectable ALP staining(Fig. 6C). In a separate experiment, lung microvascular fibroblasts with internalized DEX-PLGA particles were used in place of MSCs. Media transferred from the DEX-PLGA modified fibroblast cultures to unmodified MSCs (supplemented with G and A) induced osteogenic differentiation of the MSCs to the same degree as media transferred from DEX-PLGA modified MSCs (Fig. 6D). These two controls demonstrate that the DEX released from the particle modified cells was responsible for inducing osteogenic differentiation of the unmodified MSCs in a different culture dish in an endocrine-like manner.
Fig. 6.
Endocrine-like activity of modified MSCs. (A) Schematic illustration of programming cell fate of distant cells (without particles in well ‘ii’) by transferring conditioned media from well ‘i’, containing DEX-PLGA modified MSCs, differentiated MSCs, or DEX-PLGA modified fibroblasts to well ‘ii’. (B) Osteogenic differentiation of DEX-PLGA modified MSCs and distant cells quantified through ALP staining. (C) Osteogenic differentiation of MSCs treated with conditioned media from differentiated MSCs without DEX-PLGA particles. (D) Osteogenic differentiation of MSCs treated with conditioned media from DEX-PLGA modified fibroblasts. (E) Schematic illustration of DEX-PLGA modified MSCs controlling the fate of MSCs (without particles) separated by a transwell membrane 2 mm above the surface. (F) Osteogenic differentiation of unmodified MSCs atop transwell membrane quantified through ALP staining. D=Dexamethasone, A=Ascorbic Acid, G=β-Glycerolphosphate, CCM=MSC expansion media
To determine if engineered endocrine-like signalling could promote differentiation in a more relevant assay, we investigated the ability of adhered DEX-PLGA modified MSCs to impact the fate of cells on a distant transwell membrane in the same culture environment. We incubated MSCs with DEX-PLGA particles on the bottom surface of a transwell dish, and unmodified MSCs on a filter surface that was 2mm above in the presence of A and G (Fig. 6E). Cells were stained to detect ALP activity after 21 days in culture. DEX-PLGA modified MSCs were shown to induce the differentiation of ~80% of the unmodified MSCs on the transwell membrane (Fig. 6F). This demonstrates that agents released from particle modified cells can impact the fate of distant cells without cell contact.
3.3 Controlling cell fate after cryopreservation
To assess the potential for particle modified MSCs to retain their DEX releasing properties following cryopreservation, cells containing DEX-PLGA particles were stored for 10 days at −140° C. Upon thawing and replating, the particle modified MSCs differentiated into osteogenic cells via intracellular release of DEX, as indicated by positive alkaline phosphatase staining (Fig. S5C) and induced osteogenic differentiation of distant unmodified MSCs, comparable to non-cryopreserved DEX-PLGA modified cells (Fig. S5D) Thus particle engineered MSCs can be cryopreserved without loss of activity.
3.4 Potential for a platform technology
While small molecules such as DEX and rhodamine can freely cross the membrane of cells such as MSCs, it is well known that many exogenously supplied molecules (i.e. added to the culture media) have limited ability to traverse membranes unless the membranes are permeabilized [25]. However, we do not anticipate this to be a significant bottleneck to expanding our results to other agents including small molecules, peptides, and proteins given that many cell types including MSCs possess relevant machinery to facilitate transport of agents from the intracellular to the extracellular environment. For example, MSCs and their subpopulations have been shown to express the plasma membrane protein, P-glycoprotein otherwise known as permeability glycoprotein [36–38], an ATP-dependent efflux pump responsible for multidrug resistance in tumor cells that is also expressed in hematopoietic stem cells and their progeny [26]. Interestingly, P-glycoprotein has the ability to transport multiple types of agents across the cell membrane including steroids, lipids, peptides, and drugs. P-glycoprotein can also be modulated to alter drug efflux [39]. In addition to P-glycoprotein mediated transport of soluble agents, cell-cell communication via soluble cues may occur through gap junctions that permit the movement of small molecules and proteins between cells that are in direct cell contact. This pathway has been exploited for double stranded shRNAs/siRNA delivery [27, 40]. MSCs have been shown to express gap junctions and it has been suggested that this could be used as a means to mediate responses of cells that are in direct cell contact with MSCs [41, 42]. Furthermore, MSCs have been shown to use nanometer scale vesicles called exosomes [43, 44] for transport of multiple intracellular agents to the extracellular environment, as has been shown for other cell types [45, 46]. Thus, the collective activity of these mechanisms theoretically permits the delivery and extracellular transport of a large repertoire of therapeutic agents via internalized biodegradable particles. For example, agents could be used to impact cell survival, proliferation, differentiation, extracellular matrix production, cell death, or secretion of therapeutic peptides and proteins. We envision this intracellular drug depot will be useful for developing cell-based therapies for tissue regeneration, drug delivery and cancer therapeutics and potentially in combination with cell based targeting strategies [18, 47–49].
4. Conclusion
Herein we have developed a strategy to engineer cells with an intracellular depot to impart intracellular and extracellular control of cell fate. In our proof of concept studies we have shown that primary human mesenchymal stem cells (MSCs) can efficiently internalize 1–2 micron sized biodegradable particles containing differentiation factors. The particles remain localized within the cell for at least 7 days while releasing biologically active agents such as dexamethasone. The release kinetics to the extracellular environment can easily be controlled by tuning the number of internalized particles. Remarkably, differentiation factors released from the particles were shown to promote the differentiation of particle-carrying cells (intracrine-like signaling), neighboring cells (paracrine-like signaling), and the differentiation of distant cells (endocrine-like signaling). In addition to use as an in vitro tool to create cell niches in culture where temporal and spatial control of cellular cues is critical, intracellular depots may permit exquisite control over transplanted cells and their microenvironment through impacting cellular phenotype and function. Importantly, this platform does not depend on genetic manipulation or a cell carrier, making it amenable to use in a diverse array of in vitro and in vivo applications.
Supplementary Material
MSC internalization of polydisperse particles. MSCs were incubated with polydisperse DiO loaded PLGA particles, 300 nm - 5 μm, for 24 hr, fixed and prepared for transmission electron microscopy and confocal microscopy. (A) PLGA particles were observed in the intracellular space next to the rough endoplasmic reticulum (Scale bar: 500 nm). (B–D) Three 3D projections of a single confocal z-stack reveals 500 nm to 3 μm sized particles were internalized by MSCs at 24 hr (Scale bar: 10 μm).
Viability, proliferation, and adhesion of modified MSCs. (A) Viability of MSCs engineered with PLGA particles immediately after modification and 48 hr after modification. (B) Proliferation of MSCs engineered with PLGA particles and unmodified MSCs. (C) Adhesion of MSCs engineered with PLGA particles on tissue culture plastic at 10, 30, and 90 min.
Differentiation potential of PLGA modified MSCs. Osteogenesis and adipogenesis 21 days after induction observed by alkaline phosphatase (ALP) and Oil Red O (ORO) staining, respectively. Particle modified MSCs cultured in respective differentiation media showed positive staining for both ORO and ALP. Particle modified MSCs cultured in expansion media, without differentiation factors, showed no ORO or ALP staining.
Extracellular release of a model dye. Sustained and controlled release of dye from MSCs modified with 200 μl of 0.1 mg/mL, 0.5 mg/mL and 1.0 mg/mL rhodamine-PLGA particles into surrounding media at 37 °C over 10 days.
Effect of cryopreservation on DEX-PLGA modified MSCs. MSCs modified with DEX-PLGA particles were frozen at −140 °C for 10 days and then thawed to assess their cell programming capability. (A) Schematic of DEX release into culture media from adherent MSCs modified with DEX- PLGA particles. (B) Schematic illustration of controlling the fate of distant cells (without particles) by transferring conditioned media from well ‘i’, containing DEX-PLGA modified MSCs to well ‘ii’. (C) Osteogenic differentiation of DEX-PLGA modified MSCs quantified through ALP staining. (D) Osteogenic differentiation of distant cells grown in conditioned media from DEX-PLGA modified MSCs quantified through ALP staining. D=Dexamethasone, A=Ascorbic Acid, G=β-Glycerolphosphate, CCM=MSC expansion media
Acknowledgments
This work was supported by National Institute of Health grant HL097172, HL095722 and DE019191 to JMK and by the American Heart Association grant #0970178N to JMK. JAA was supported by National Science Foundation (NSF) Graduate Research Fellowship. We would like to thank Dr. Chenjie Xu of Brigham & Women’s Hospital for his assistance with SEM imaging and Nicki Watson of the Whitehead Institute for help with TEM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic 4 integrin expression. The FASEB Journal. 2007;21:3917–27. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 3.Haider HK, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circulation Research. 2008;103:1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 4.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 5.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 6.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JAJM, Mohapatra G, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–7. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 8.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods. 2006;3:369–75. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 11.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–13. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nature Nanotechnology. 2010 doi: 10.1038/nnano.2010.246. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–51. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 14.Davis M, Hsieh P, Grodzinsky A, Lee R. Custom design of the cardiac microenvironment with biomaterials. Circulation Research. 2005;97:8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater. 2004;3:249–53. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 16.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–16. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar D, Vemula PK, Teo GS, Spelke D, Karnik R, Wee le Y, et al. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug Chem. 2008;19:2105–9. doi: 10.1021/bc800345q. [DOI] [PubMed] [Google Scholar]
- 19.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–41. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng H, Kastrup CJ, Ramanathan R, Siegwart DJ, Ma M, Bogatyrev SR, et al. Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS Nano. 2010;4:625–31. doi: 10.1021/nn901319y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panyam J, Labhasetwar V. Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res. 2003;20:212–20. doi: 10.1023/a:1022219003551. [DOI] [PubMed] [Google Scholar]
- 22.Thompson EB, Lippman ME. Mechanism of action of glucocorticoids. Metabolism. 1974;23:159–202. doi: 10.1016/0026-0495(74)90113-9. [DOI] [PubMed] [Google Scholar]
- 23.Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988;106:2139–51. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison AC, Young MR. Uptake of Dyes and Drugs by Living Cells in Culture. Life Sci. 1964;3:1407–14. doi: 10.1016/0024-3205(64)90082-7. [DOI] [PubMed] [Google Scholar]
- 25.Hong S, Leroueil PR, Janus EK, Peters JL, Kober MM, Islam MT, et al. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug Chem. 2006;17:728–34. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 27.Wolvetang EJ, Pera MF, Zuckerman KS. Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem Biophys Res Commun. 2007;363:610–5. doi: 10.1016/j.bbrc.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Chung TH, Wu SH, Yao M, Lu CW, Lin YS, Hung Y, et al. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28:2959–66. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Gao H, Shi W, Freund LB. Mechanics of receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 2005;102:9469–74. doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahoo SK, Labhasetwar V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol Pharm. 2005;2:373–83. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- 31.Jin H, Heller DA, Sharma R, Strano MS. Size-Dependent Cellular Uptake and Expulsion of Single-Walled Carbon Nanotubes: Single Particle Tracking and a Generic Uptake Model for Nanoparticles. Acs Nano. 2009;3:149–58. doi: 10.1021/nn800532m. [DOI] [PubMed] [Google Scholar]
- 32.Chithrani BD, Chan WCW. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–50. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 33.Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317–30. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 34.Vasir JK, Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Advanced drug delivery reviews. 2007;59:718–28. doi: 10.1016/j.addr.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira JM, Kotobuki N, Tadokoro M, Hirose M, Mano JF, Reis RL, et al. Ex vivo culturing of stromal cells with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles promotes ectopic bone formation. Bone. 2010;46:1424–35. doi: 10.1016/j.bone.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156–65. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- 37.Kim SG, Jeon CH, Suh HS, Choe JY, Shin IH. P-glycoprotein expression in extracellular matrix formation of chondrogenic differentiation of human adult stem cells. Cell Biol Int. 2007;31:1042–8. doi: 10.1016/j.cellbi.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Liu CM, Chang CH, Yu CH, Hsu CC, Huang LL. Hyaluronan substratum induces multidrug resistance in human mesenchymal stem cells via CD44 signaling. Cell Tissue Res. 2009;336:465–75. doi: 10.1007/s00441-009-0780-3. [DOI] [PubMed] [Google Scholar]
- 39.Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009;136:21–9. doi: 10.1016/j.jconrel.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–68. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang JC, Hsu SH, Su HL. The regulation of the gap junction of human mesenchymal stem cells through the internalization of quantum dots. Biomaterials. 2009;30:1937–46. doi: 10.1016/j.biomaterials.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 42.Valiunas V, Doronin S, Valiuniene L, Potapova I, Zuckerman J, Walcott B, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004;555:617–26. doi: 10.1113/jphysiol.2003.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–44. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 46.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–74. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 47.Sarkar D, Vemula PK, Zhao W, Gupta A, Karnik R, Karp JM. Engineered mesenchymal stem cells with self-assembled vesicles for systemic cell targeting. Biomaterials. 2010;31:5266–74. doi: 10.1016/j.biomaterials.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365–72. doi: 10.1038/mt.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko IK, Kean TJ, Dennis JE. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials. 2009;30:3702–10. doi: 10.1016/j.biomaterials.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MSC internalization of polydisperse particles. MSCs were incubated with polydisperse DiO loaded PLGA particles, 300 nm - 5 μm, for 24 hr, fixed and prepared for transmission electron microscopy and confocal microscopy. (A) PLGA particles were observed in the intracellular space next to the rough endoplasmic reticulum (Scale bar: 500 nm). (B–D) Three 3D projections of a single confocal z-stack reveals 500 nm to 3 μm sized particles were internalized by MSCs at 24 hr (Scale bar: 10 μm).
Viability, proliferation, and adhesion of modified MSCs. (A) Viability of MSCs engineered with PLGA particles immediately after modification and 48 hr after modification. (B) Proliferation of MSCs engineered with PLGA particles and unmodified MSCs. (C) Adhesion of MSCs engineered with PLGA particles on tissue culture plastic at 10, 30, and 90 min.
Differentiation potential of PLGA modified MSCs. Osteogenesis and adipogenesis 21 days after induction observed by alkaline phosphatase (ALP) and Oil Red O (ORO) staining, respectively. Particle modified MSCs cultured in respective differentiation media showed positive staining for both ORO and ALP. Particle modified MSCs cultured in expansion media, without differentiation factors, showed no ORO or ALP staining.
Extracellular release of a model dye. Sustained and controlled release of dye from MSCs modified with 200 μl of 0.1 mg/mL, 0.5 mg/mL and 1.0 mg/mL rhodamine-PLGA particles into surrounding media at 37 °C over 10 days.
Effect of cryopreservation on DEX-PLGA modified MSCs. MSCs modified with DEX-PLGA particles were frozen at −140 °C for 10 days and then thawed to assess their cell programming capability. (A) Schematic of DEX release into culture media from adherent MSCs modified with DEX- PLGA particles. (B) Schematic illustration of controlling the fate of distant cells (without particles) by transferring conditioned media from well ‘i’, containing DEX-PLGA modified MSCs to well ‘ii’. (C) Osteogenic differentiation of DEX-PLGA modified MSCs quantified through ALP staining. (D) Osteogenic differentiation of distant cells grown in conditioned media from DEX-PLGA modified MSCs quantified through ALP staining. D=Dexamethasone, A=Ascorbic Acid, G=β-Glycerolphosphate, CCM=MSC expansion media