Abstract
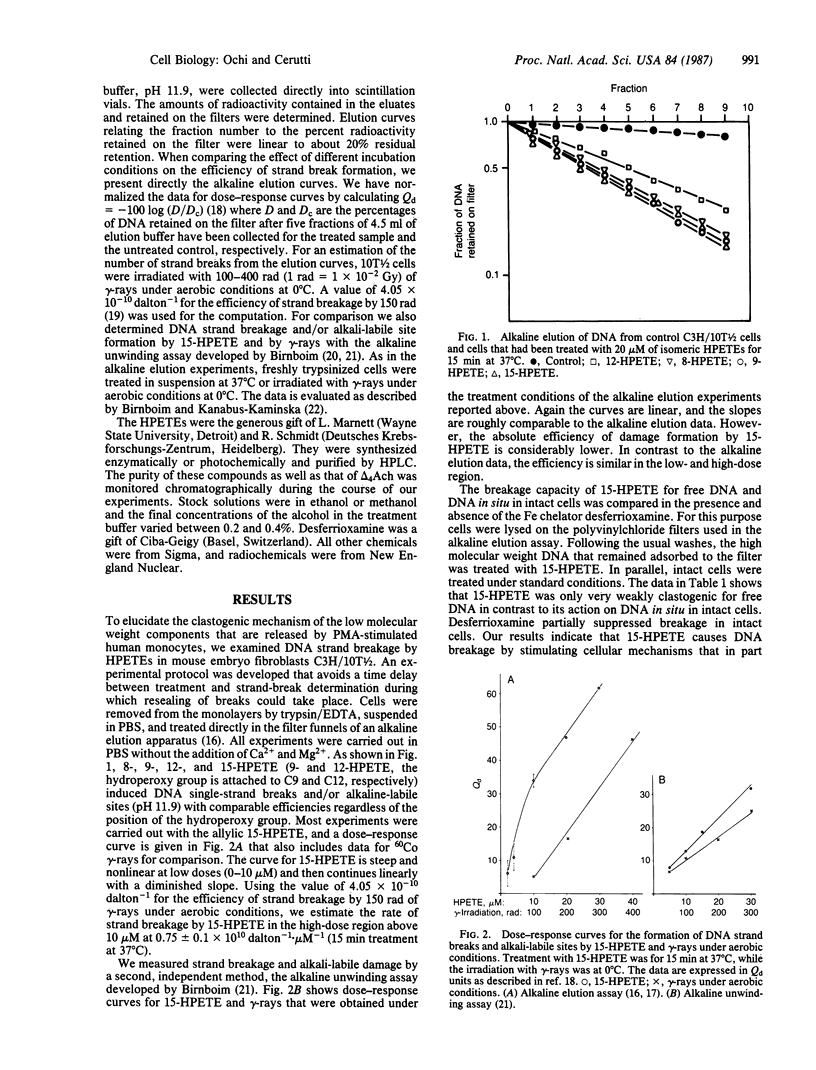
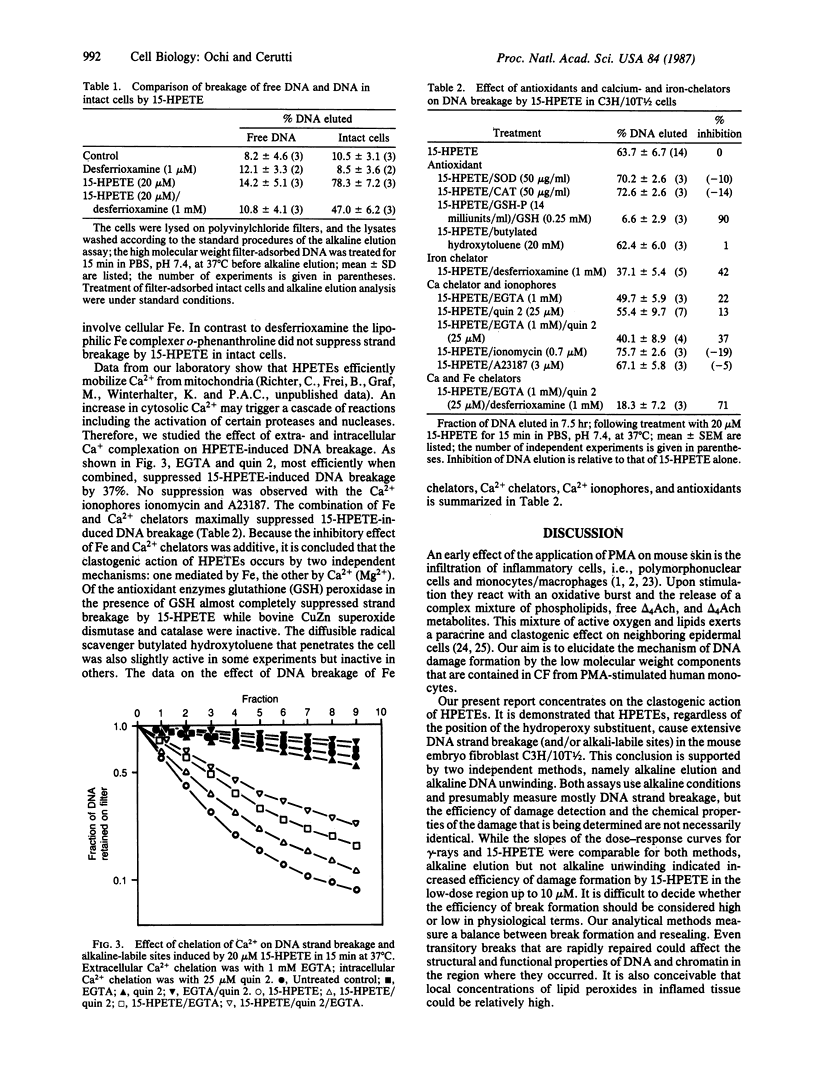
Phorbol 12-myristate 13-acetate induces the release of a low molecular weight clastogenic factor from monocytes. Hydroperoxy-5,8,11,13-icosatetraenoic acids represent major components of clastogenic factor. We report that several isomeric hydroperoxy-5,8,11,13-icosatetraenoic acids efficiently induce DNA strand breakage and/or alkali-labile sites in the mouse embryo fibroblasts C3H/10T1/2. Fe chelation by desferrioxamine suppresses breakage by approximately equal to 42% indicating the participation of Fe-catalyzed radical reactions. An additional 37% inhibition is observed upon addition of the Ca2+ chelators EGTA and quin-2. This result suggests that hydroxyperoxy-5,8,11,13-icosatetraenoic acid may activate a Ca2+-dependent nuclease. The addition of the antioxidant enzymes CuZn-superoxide dismutase and catalase had no effect, while glutathione peroxidase suppressed strand breakage by 90%. To our knowledge, our results yield a first insight into the mechanism of action of monocyte clastogenic factor and the role of inflammation in tumor promotion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C. DNA strand breakage in human leukocytes exposed to a tumor promoter, phorbol myristate acetate. Science. 1982 Mar 5;215(4537):1247–1249. doi: 10.1126/science.6276978. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Birnboim H. C., Kanabus-Kaminska M. The production of DNA strand breaks in human leukocytes by superoxide anion may involve a metabolic process. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6820–6824. doi: 10.1073/pnas.82.20.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Erickson L. C., Kohn K. W. Non-enzymatic DNA strand breaks induced in mammalian cells by fluorescent light. Biochim Biophys Acta. 1978 Aug 23;520(1):11–20. doi: 10.1016/0005-2787(78)90003-5. [DOI] [PubMed] [Google Scholar]
- Bull A. W., Nigro N. D., Golembieski W. A., Crissman J. D., Marnett L. J. In vivo stimulation of DNA synthesis and induction of ornithine decarboxylase in rat colon by fatty acid hydroperoxides, autoxidation products of unsaturated fatty acids. Cancer Res. 1984 Nov;44(11):4924–4928. [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Dix T. A., Marnett L. J. Metabolism of polycyclic aromatic hydrocarbon derivatives to ultimate carcinogens during lipid peroxidation. Science. 1983 Jul 1;221(4605):77–79. doi: 10.1126/science.6304879. [DOI] [PubMed] [Google Scholar]
- Emerit I., Cerutti P. A. Tumour promoter phorbol-12-myristate-13-acetate induces chromosomal damage via indirect action. Nature. 1981 Sep 10;293(5828):144–146. doi: 10.1038/293144a0. [DOI] [PubMed] [Google Scholar]
- Emerit I., Cerutti P. Clastogenic action of tumor promoter phorbol-12-myristate-13 acetate in mixed human leukocyte cultures. Carcinogenesis. 1983 Oct;4(10):1313–1316. doi: 10.1093/carcin/4.10.1313. [DOI] [PubMed] [Google Scholar]
- Emerit I., Levy A., Cerutti P. Suppression of tumor promoter phorbolmyristate acetate-induced chromosome breakage by antioxidants and inhibitors of arachidonic acid metabolism. Mutat Res. 1983 Aug;110(2):327–335. doi: 10.1016/0027-5107(83)90149-5. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Witz G., Amoruso M., Troll W. Protease inhibitors antagonize the activation of polymorphonuclear leukocyte oxygen consumption. Biochem Biophys Res Commun. 1979 Jun 13;88(3):854–860. doi: 10.1016/0006-291x(79)91487-6. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Fürstenberger G., Kittstein W., Besemfelder E., Hull W. E., Hagedorn H., Opferkuch H. J., Marks F. Generation of the arachidonic acid metabolite 8-HETE by extracts of mouse skin treated with phorbol ester in vivo; identification by 1H-n.m.r. and GC-MS spectroscopy. Carcinogenesis. 1986 Mar;7(3):449–455. doi: 10.1093/carcin/7.3.449. [DOI] [PubMed] [Google Scholar]
- Hirschi M., Netrawali M. S., Remsen J. F., Cerutti P. A. Formation of DNA single-strand breaks by near-ultraviolet and gamma-rays in normal and Bloom's syndrome skin fibroblasts. Cancer Res. 1981 May;41(5):2003–2007. [PubMed] [Google Scholar]
- Hunter J. A., Finkbeiner W. E., Nadel J. A., Goetzl E. J., Holtzman M. J. Predominant generation of 15-lipoxygenase metabolites of arachidonic acid by epithelial cells from human trachea. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4633–4637. doi: 10.1073/pnas.82.14.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn K. W., Erickson L. C., Ewig R. A., Friedman C. A. Fractionation of DNA from mammalian cells by alkaline elution. Biochemistry. 1976 Oct 19;15(21):4629–4637. doi: 10.1021/bi00666a013. [DOI] [PubMed] [Google Scholar]
- Lands W. E. Biological consequences of fatty acid oxygenase reaction mechanisms. Prostaglandins Leukot Med. 1984 Jan;13(1):35–46. doi: 10.1016/0262-1746(84)90100-8. [DOI] [PubMed] [Google Scholar]
- Lewis J. G., Adams D. O. Induction of 5,6-ring-saturated thymine bases in NIH-3T3 cells by phorbol ester-stimulated macrophages: role of reactive oxygen intermediates. Cancer Res. 1985 Mar;45(3):1270–1275. [PubMed] [Google Scholar]
- Lewis J. G., Hamilton T., Adams D. O. The effect of macrophage development on the release of reactive oxygen intermediates and lipid oxidation products, and their ability to induce oxidative DNA damage in mammalian cells. Carcinogenesis. 1986 May;7(5):813–818. doi: 10.1093/carcin/7.5.813. [DOI] [PubMed] [Google Scholar]
- Marnett L. J., Reed G. A. Peroxidatic oxidation of benzo[a]pyrene and prostaglandin biosynthesis. Biochemistry. 1979 Jul 10;18(14):2923–2929. doi: 10.1021/bi00581a001. [DOI] [PubMed] [Google Scholar]
- McWilliams R. S., Cross W. G., Kaplan J. G., Birnboim H. C. Rapid rejoining of DNA strand breaks in resting human lymphocytes after irradiation by low doses of 60Co gamma rays or 14.6-MeV neutrons. Radiat Res. 1983 Jun;94(3):499–507. [PubMed] [Google Scholar]
- Pryor W. A. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- Reed G. A., Brooks E. A., Eling T. E. Phenylbutazone-dependent epoxidation of 7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene. A new mechanism for prostaglandin H synthase-catalyzed oxidations. J Biol Chem. 1984 May 10;259(9):5591–5595. [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Triplett L. L., Yotti L. P., Trosko K. E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981 Aug 28;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- Taylor L., Menconi M. J., Polgar P. The participation of hydroperoxides and oxygen radicals in the control of prostaglandin synthesis. J Biol Chem. 1983 Jun 10;258(11):6855–6857. [PubMed] [Google Scholar]
- Yoshihara K., Tanigawa Y., Burzio L., Koide S. S. Evidence for adenosine diphosphate ribosylation of Ca2+, Mg2+-dependent endonuclease. Proc Natl Acad Sci U S A. 1975 Jan;72(1):289–293. doi: 10.1073/pnas.72.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman R., Cerutti P. Active oxygen acts as a promoter of transformation in mouse embryo C3H/10T1/2/C18 fibroblasts. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2085–2087. doi: 10.1073/pnas.81.7.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello Filho A. C., Meneghini R. Protection of mammalian cells by o-phenanthroline from lethal and DNA-damaging effects produced by active oxygen species. Biochim Biophys Acta. 1985 Oct 30;847(1):82–89. doi: 10.1016/0167-4889(85)90156-9. [DOI] [PubMed] [Google Scholar]



