Abstract
Since the etiologies and clinical outcomes of bacteremia in children with Plasmodium falciparum infections, particularly in areas of holoendemic malaria transmission, are largely unexplored, blood cultures and comprehensive clinical, laboratory, hematological, and nutritional parameters for malaria-infected children (aged 1 to 36 months, n = 585 patients) were investigated at a rural hospital in western Kenya. After the exclusion of contaminant microorganisms, the prevalence of bacteremia was 11.7% in the cohort (n = 506), with nontyphoidal Salmonella spp. being the most common isolates (42.4%). Bacteremia was found to occur in a significantly higher proportion of females than males and was associated with elevated blood glucose concentrations and lowered malaria parasite and hemoglobin (Hb) levels compared to those in abacteremic participants. In addition, the incidences of respiratory distress and severe malarial anemia (SMA; Hb level of <6.0g/dl) were nonsignificantly greater in children with bacteremia. Mortality was 8.5-fold higher in children with bacteremia. Multivariate logistic regression analyses revealed that bacteremia was significantly associated with reduced incidences of high-density parasitemia (HDP; ≥10,000/μl) and increased incidences of malnutrition (i.e., underweight; weight-for-age Z score of <−2 using the NCHS system). Since previous studies showed that bacteremia caused by Gram-negative organisms is associated with enhanced anemia and mortality, multivariate logistic regression was also performed separately for randomly age- and gender-matched children with bacteremia caused by Gram-negative organisms (n = 37) and for children found to be abacteremic (n = 74). These results revealed that the presence of bacteremia caused by Gram-negative organisms was significantly associated with reduced HDP, enhanced susceptibility to respiratory distress, SMA (Hb level of <6.0 g/dl), and being underweight (Z score, <−2). Data presented here from a region of holoendemic P. falciparum transmission demonstrate that although bacteremia is associated with reduced malaria parasitemia, a number of unfavorable clinical outcomes, including malnutrition, respiratory distress, anemia, and mortality, are elevated in children with bacteremia, particularly in cases of Gram-negative origin.
Invasive bacteria are important causes of bacteremia, pneumonia, meningitis, and septicemia in African children (5, 16, 20, 28, 36). A previous study performed at Kilifi District Hospital, coastal Kenya, showed that community-acquired bacteremia is a common cause of acute pediatric admissions that results in substantial mortality in infants and young children (5). Additional investigations established that diarrhea, vomiting, dehydration, malnutrition, HIV-1 infection, female gender, fever (lasting >7 days), age (<5 yrs), malaria parasitemia, and the presence of intraleukocytic hemozoin were important predictors of bacteremia in children residing in areas where Plasmodium falciparum is endemic (1, 8, 11, 15, 20). Moreover, children with P. falciparum malaria who acquire invasive bacteria have enhanced hepatomegaly and splenomegaly and an increased risk of severe malarial anemia (SMA), cerebral malaria (CM), respiratory distress, and mortality (4, 9, 15, 36). In areas of P. falciparum holoendemicity where malnutrition, HIV-1, and helminthic infections are prevalent, bacteremia is associated with greater severity of anemia (8–10). These findings are important, since severe anemia is a leading cause of morbidity and mortality in children residing in areas where P. falciparum transmission is holoendemic (24, 27). However, despite the importance of bacteremia in the pathogenesis of malaria and other childhood illnesses, most laboratories in rural sub-Saharan Africa, where the greatest at-risk populations reside, are often unable to perform bacterial cultures (26). Since the facilities and qualified personnel required to perform microbial procedures are often absent in rural settings, most available data on bacteremia originate from urban and referral health facilities.
Consistent with the lack of facilities in rural health care settings, secondary bacterial infections in children with malaria were postulated more than 10 years ago as potential risk factors for the development of severe anemia and for the high rates of mortality of hospitalized patients and of patients posthospitalization at Siaya District Hospital (SDH), western Kenya. However, the ability to perform blood cultures was not available at that time to confirm this hypothesis (38). As part of our studies investigating the pathogenesis of pediatric anemia, and the impact of common copathogens on anemia outcomes in regions of P. falciparum holoendemicity, we performed a case series analysis describing the clinical, hematological, parasitological, and nutritional factors associated with bacteremia in children (aged 1 to 36 months) presenting at the hospital with P. falciparum infections (n = 585 patients) from March 2004 to January 2006.
(Portions of this work were presented at the 56th Annual Meeting of the American Society for Tropical Medicine and Hygiene [ASTM], 4 to 8 November 2007, Philadelphia, PA; abstract 20.)
MATERIALS AND METHODS
Study participants and experimental design.
Study participants with P. falciparum malaria (n = 585 patients; 295 males and 290 females, aged 1 to 36 months) were recruited at Siaya District Hospital (SDH), a rural health facility in Siaya District, equatorial western Kenya. Bacterial infections and P. falciparum malaria are coendemic in this region, with infants and young children less than 3 years of age being the most affected groups (3, 18, 24, 30). Common causes of childhood illnesses among SDH patients are malaria, respiratory, and gastrointestinal infections (32, 33). A detailed description of the study site and anemia in the pediatric population can be found in our previous report (30). After the parent/guardian of the child provided written informed consent to participate in the study, a questionnaire was used to collect demographic and clinical information, including the signs and symptoms of the present illness. Children with a history of prior hospitalization (for any reason) and/or CM, an infrequent occurrence in western Kenya (6, 29), were not enrolled in the study.
Venous blood samples (<3.0 ml) were collected in EDTA-containing tubes at the time of enrollment, prior to initiation of supportive care or other treatment interventions. Blood samples were used for malaria diagnosis, hematological measurements, HIV testing, and bacterial culture. Since HIV-1 is a common copathogen in this region and influences childhood anemia status (5, 31), all study participants were screened for HIV-1. Pre- and posttest HIV counseling was provided to the parents/guardians of all participating children. HIV-1 status was determined using two serological methods (Unigold [Trinity Biotech Plc., Bray, Ireland] and Determine [Abbott Laboratories, Tokyo, Japan]), and positive serological results were confirmed by proviral DNA PCR, as described in our previous study (31). HIV-1-positive (PCR-positive) study participants were included in the analyses.
Based on bacterial cultures (see below), children were further characterized as bacteremia negative or bacteremia positive. Children with malaria and bacteremia were treated according to Kenyan Ministry of Health (MoH) guidelines, which included the use of an artemether and lumefantrin combination drug (Coartem) for nonsevere malaria, intravenous quinine for severe malaria, and broad-spectrum antibiotics for bacterial infection. Written informed consent was obtained from the parents/guardians of all the participating children prior to enrollment. The study was approved by the scientific and ethical review committee of the Kenya Medical Research Institute and the institutional review boards of the University of Pittsburgh and the University of New Mexico.
Clinical and nutritional assessment and mortality.
A history of the present illness was obtained from each child's parent/guardian and recorded on a standardized questionnaire. All children were assessed for clinical findings indicative of anemia and markers of anemia severity (23, 37) by clinical officers and clinicians. Fever was defined as axillary temperature of >37.5°C. Respiratory distress was defined as the presence of any of the following signs: alar flaring, chest retraction, use of accessory muscles during respiration, or abnormally deep, acidotic breathing (23). Glucose levels were obtained with an Accu-Chek compact glucometer (Roche Diagnostics, Indianapolis, IN).
Nutritional status was determined upon enrollment. Weight (kg), height (cm), head (cm), and mid-upper-arm circumference (MUAC; cm) measurements were obtained to determine whether patients were underweight (weight-for-age Z score, <−2) and to measure wasting (weight-for-height Z score, <−2) and stunting (height-for-age Z score, <−2) (30). Weight was obtained using a Salter scale to the nearest 0.01 kg for children weighing <10 kg and to the nearest 0.1 kg for children weighing >10 kg. Height measurements were recorded to the nearest 0.1 cm while children were in a recumbent position for those <2 years of age and while children were in a standing position for those >2 years of age. Anthropometric measures of nutritional status were calculated using the National Center for Health Statistics (NCHS) reference standards with EpiInfo, version 3.3 (Centers for Disease Control and Prevention, Atlanta, GA).
Children were also assessed for 7-day postenrollment mortality. For those children who were discharged from the hospital prior to the assessment of mortality within the 7-day span, the parents/guardians of all children were asked to report to the hospital 1 week postenrollment to have their child's health status evaluated. For cases in which children did not report to the hospital, members of the study team traveled to the child's residence and inquired about the child's health status.
Bacterial cultures.
Approximately 1.0 ml of venipuncture blood was collected aseptically into sterile pediatric Isolator microbial tubes (Wampole Laboratories, Princeton, NJ) for bacterial cultures. Blood samples were inoculated directly onto chocolate agar plates and incubated for 18 h at 37°C in 5% CO2, followed by subculture for 18 to 24 h in an inverted position. If no growth was obtained, subcultures were incubated for an additional 4 days. Plates were inspected daily for signs of microbial growth. Bacterial colonies were identified by Gram staining, colonial characteristics and appearances, and biochemical tests. API biochemical galleries (bioMérieux, Louvres, France) and/or agglutination serology were used to confirm the presence of suspected blood-borne bacterial pathogens.
Malaria diagnosis.
Thick and thin blood films were used to determine P. falciparum parasitemia in peripheral circulation. Blood films were prepared from venous blood, stained with 3% Giemsa, and examined by oil immersion microscopy for malaria parasites. The number and species of asexual Plasmodium parasites were determined per 300 leukocytes, and the parasite density was calculated based on the total leukocyte count for each individual.
Hematological investigations.
Complete blood counts were performed with a Beckman Coulter Ac-T diff2 machine (Beckman Coulter, Inc., Miami, FL). Sickle-cell status was determined by alkaline cellulose acetate electrophoresis with Titan III plates according to the manufacturer's protocols (Helena BioSciences, Oxford, United Kingdom). Briefly, hemolysates prepared from blood samples or Hemo AFSC controls were dispensed onto the acetate paper, and hemoglobin (Hb) variants were separated by electrophoresis with an alkaline buffer at pH 8.6. The plates were then stained using Ponceau S stain, and Hb types were scored using the Hemo AFSC control. Glucose-6-phosphate dehydrogenase (G6PD) deficiency was determined by a fluorescent spot test (Trinity Biotech Plc., Bray, Ireland). Briefly, blood was hemolyzed and spotted onto a filter paper. Assay solution containing glucose-6-phosphate and oxidized NADP (NADP+) was added, and samples were excited with UV light at 340 nm. Based on the presence or absence of fluorescence emissions, the samples were scored as normal (high emission), intermediate (moderate emission), or deficient (no emission).
Statistical analyses.
Data analyses were conducted with SPSS, version 15.0 (SPSS, Inc., Chicago, IL). The association between categorical variables was assessed by Fisher's exact tests. Paired comparisons of continuous variables were performed by Mann-Whitney U test. Multivariate logistic regression analyses were used to evaluate the association between bacteremia and the predictor variables in children with malaria, controlling for age, gender, G6PD deficiency, sickle-cell status, and HIV-1 status. Only those variables associated with bacteremia at a significance level (P value) of <0.100 in the univariate analysis were included in the multivariate models. All tests were two-tailed, and P values of <0.050 were considered statistically significant.
RESULTS
Etiologies of bacteremia.
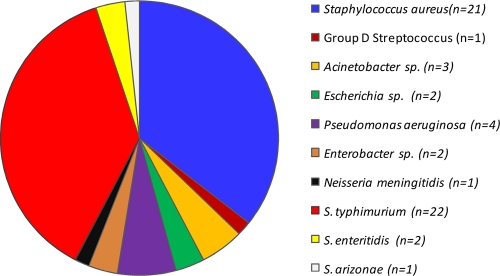
During the study period (March 2004 to January 2006), we enrolled 585 children with P. falciparum malaria. Blood cultures were performed with samples from all enrolled children to identify bacterial pathogens. The prevalence of contaminant microorganisms (i.e., coagulase-negative Staphylococcus, Bacillus, and Micrococcus species) in the cohort was 13.5% (79 of 585 children). After exclusion of contaminants, the overall prevalence of bacteremia was 11.7% (59 of 506) (Fig. 1). The prevalence of bacteremia of Gram-positive origin in the cohort was 4.3% (22 of 506 children), while that of Gram-negative origin was 7.3% (37 of 506) (Fig. 1). Enterobacteriaceae spp. were the most common Gram-negative isolates in the study population (5.7%; 29 of 506) (Fig. 1) with nontyphoidal Salmonella spp. (NTS) accounting for nearly all Enterobacteriaceae spp. (86.2%; 25 of 29) (Fig. 1).
Fig. 1.
Proportion of bacterial pathogens identified for children with P. falciparum malaria upon presentation at the hospital. Data are presented as the proportion (%) of subjects. S. typhimurium, Salmonella enterica serovar Typhimurium; S. enteritidis, Salmonella enteritidis; S. arizonae, Salmonella arizonae.
Demographic and clinical characteristics.
Since previous studies have shown that bacteremia exacerbates the severity of malaria in African children (8, 9, 15, 36), we investigated the demographic and clinical parameters for malaria-infected children with (n = 59 children) and without (n = 447) bacteremia (Table 1). Bacteremia was associated with a higher proportion of the female gender (P value, 0.018), elevated blood glucose concentrations (P value, 0.015), and reduced median (P value, <0.001) and geometric mean (P value, 0.005) malaria parasitemias (Table 1). Consistent with the lower parasitemias, bacteremic individuals also had a reduced prevalence of high-density parasitemia (HDP; ≥10,000 parasites/μl, P value, 0.001) (Table 1). There was no difference between HIV-1 prevalences in children with and without bacteremia (P value, 0.957) (Table 1). Evaluation of mortality within 7 days postenrollment revealed that bacteremic children had a higher mortality rate (P value, 0.069) (Table 1).
Table 1.
Demographic, clinical, and laboratory characteristics of study participantsa
| Category | Result for indicated group |
P value | |
|---|---|---|---|
| Bacteremia negative | Bacteremia positive | ||
| No. of participants | 447 | 59 | |
| Demographic and clinical characteristics | |||
| No. female (%) | 204 (45.6) | 37 (62.7) | 0.018b |
| Age (mo) | 10.1 (6.3–15.8) | 9.5 (5.9–13.7) | 0.263c |
| Axillary temp (°C) | 37.5 (36.6–38.4) | 37.9 (36.8–38.7) | 0.055c |
| No. with axillary temp >37.5°C (%) | 199 (44.7) | 33 (56.9) | 0.093b |
| Blood glucose (mmol/liter) | 5.1 (4.5–5.9) | 5.7 (4.8–6.6) | 0.015c |
| No. with hypoglycemia (%) | 6 (1.2) | 0 (0.0) | 0.996b |
| No. with respiratory distress (%) | 12 (2.7) | 4 (6.8) | 0.092b |
| No. with splenomegaly (%) | 64 (14.3) | 11 (18.6) | 0.319b |
| No. with lymphadenopathy (%) | 25 (5.1) | 3 (5.1) | 0.921b |
| Hematological characteristics | |||
| No. of leukocytes (103/μl) | 11.6 (8.9–15.6) | 12.3 (8.7–17.6) | 0.498c |
| No. of lymphocytes (103/μl) | 5.5 (4.0–8.0) | 6.0 (4.0–8.5) | 0.699c |
| No. of monocytes (103/μl) | 1.0 (0.7–1.6) | 1.0 (0.6–1.7) | 0.895c |
| No. of granulocytes (103/μl) | 4.5 (2.9–6.7) | 4.8 (2.9–7.4) | 0.390c |
| Hemoglobin level (g/dl) | 6.7 (5.3–8.7) | 5.9 (4.8–7.1) | 0.031c |
| No. of RBCs (106/μl) | 3.2 (2.3–4.0) | 2.8 (2.2–3.5) | 0.053c |
| No. of platelets (103/μl) | 157 (108–229) | 160 (118–232) | 0.939c |
| No. with thrombocytopenia (%) | 190 (45.2) | 23 (40.4) | 0.571b |
| Parasitological characteristics | |||
| Parasite density/μl | 20,826 (5,767–55,162) | 6,515 (1,121–26,486) | <0.001c |
| Geometric mean parasite density/μl | 14,872 | 5,162 | 0.005d |
| No. with HDP (%) | 322 (72.0) | 29 (49.2) | 0.001b |
| Coinfection and outcome | |||
| No. with HIV-1 infection (%) | 22 (4.9) | 3 (5.1) | 0.957b |
| No. with SMA (%) | 170 (38.0) | 30 (50.8) | 0.066b |
| Mortalitye (no. of participants) (%) | 2 (0.4) | 2 (3.4) | 0.069b |
Data are presented as medians (first quartile [Q1] and Q3) or proportions, unless otherwise indicated. Hypoglycemia, blood glucose level of <2.2 mmol/liter; HDP, high-density parasitemia (≥10,000 parasites/μl); thrombocytopenia, platelet count of <150 × 103/μl; RBCs, number of red blood cells (106/μl); SMA, severe malarial anemia (Hb level of <6.0 g/dl). Values in boldface are statistically significant.
Fisher's exact test.
Mann-Whitney U test.
Student's t test.
Death within 7 days postenrollment.
Hematological indices in children with bacteremia.
A number of studies from Malawi and coastal Kenya have shown that bacteremia is associated with marked hematological alterations and increased severity of anemia in children presenting at the hospital with malaria (8–10, 15, 36). Although bacteremia was not associated with significant differences in leukocytic and platelet indices, the Hb level (P value, 0.031) and red blood cell (RBC) count (P value, 0.053) were lower in bacteremic individuals (Table 1). SMA, using a modified definition that takes into account Hb distributions within this particular geographic context according to age and gender (i.e., Hb levels of <6.0 g/dl) (24) was also more prevalent in bacteremic individuals (P value, 0.066) (Table 1).
Nutritional parameters in children with bacteremia.
Previous investigations showed that reduced weight-for-age values (being underweight) and MUACs are associated with increased susceptibility to bacteremia in infants and children from rural Africa (1, 22). Thus, the association between nutritional status and bacteremia was examined. As shown in Table 2, the only nutritional parameters that emerged as different between the groups were reduced height (P value, 0.045) and an increased prevalence of weight-for-age Z scores of <−2 (underweight; P value, 0.098) in children with bacteremia.
Table 2.
Nutritional parametersa
| Characteristic | Result for indicated group |
P value | |
|---|---|---|---|
| Bacteremia negative | Bacteremia positive | ||
| No. of participants | 447 | 59 | |
| Height (cm) | 70.5 (65.0–76.0) | 67.5 (64.5–73.0) | 0.045b |
| Weight (kg) | 7.8 (6.5–9.2) | 7.5 (6.1–8.5) | 0.136b |
| No. underweight (WAZ < −2) (%) | 137 (30.6) | 24 (40.7) | 0.098c |
| No. with wasting (WHZ < −2) (%) | 113 (25.3) | 17 (28.8) | 0.517c |
| No. with stunting (HAZ < −2) (%) | 80 (17.9) | 8 (13.6) | 0.579c |
| MUAC (cm) | 13.5 (12.5–14.6) | 13.5 (12.2–14.5) | 0.505b |
| No. with MUAC-for-age Z score < −2 (%) | 106 (23.7) | 13 (22.0) | 0.856c |
| No. with MUAC-for-ht Z score < −2 (%) | 106 (23.7) | 12 (20.3) | 0.997c |
| Head circumference (cm) | 44.0 (42.5–46.0) | 44.0 (42.0–45.0) | 0.200b |
| No. with head circumference Z score < −2 (%) | 47 (10.5) | 5 (8.5) | 0.820c |
Data are presented as medians (Q1 and Q3) or proportions, unless otherwise indicated. WAZ, weight-for-age Z score; WHZ, weight-for-height Z score; HAZ, height-for-age Z score; MUAC, mid-upper-arm circumference. The value in boldface is statistically significant.
Mann Whitney U test.
Fisher's exact test.
Association of bacteremia with clinical outcomes.
To identify the clinical outcomes associated with bacteremia in children with malaria, multivariate logistic regression analyses were performed, controlling for the potential confounding effects of age, gender, G6PD deficiency, sickle-cell status, and HIV-1 status (Table 3). Bacteremia was associated with decreased HDP (odds ratio [OR], 0.34; 95% confidence interval [CI], 0.19 to 0.63; P value, 0.001) and greater risk of respiratory distress (OR, 3.08; 95% CI, 0.90 to 10.49; P value, 0.073), nutritional deficiency (weight-for-age Z score, <−2 [underweight]; OR, 2.12; 95% CI, 1.11 to 4.04; P value, 0.022), and mortality within 7 days postenrollment (OR, 5.84; 95% CI, 0.76 to 45.01; P value, 0.090). Since previous investigations showed that bacteremia caused by Gram-negative organisms was associated with enhanced SMA, hypoglycemia, respiratory distress, and mortality in children with malaria (2, 4, 9, 15, 36), additional multivariate analyses that included only children with bacteremia caused by Gram-negative organisms (n = 37), randomly age and gender matched with abacteremic children (n = 74), were performed. This modeling revealed that bacteremia caused by Gram-negative organisms was associated with protection against HDP (OR, 0.31; 95% CI, 0.13 to 0.74; P value, 0.008), and increased susceptibility to respiratory distress (OR, 17.28; 95% CI, 1.75 to 170.84; P value, 0.015), SMA (OR, 2.30; 95% CI, 1.01 to 5.29; P value, 0.048), a weight-for-age Z score of <−2 (underweight; OR, 7.96; 95% CI, 3.05 to 20.77; P value, <0.001), and mortality within 7 days postenrollment (OR, 10.29; 95% CI, 0.65 to 164.12; P value, 0.099).
Table 3.
Clinical, hematological, and nutritional predictors of bacteremiaa
| Characteristic | Result for: |
|||
|---|---|---|---|---|
| All bacteremic children |
Gram-negative bacteremic children |
|||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Axillary temp > 37.5°C | 1.47 (0.81–2.70) | 0.208 | 1.45 (0.64–3.29) | 0.375 |
| HDP | 0.34 (0.19–0.63) | 0.001 | 0.31 (0.13–0.74) | 0.008 |
| Respiratory distress | 3.08 (0.90–10.49) | 0.073 | 17.28 (1.75–170.84) | 0.015 |
| SMA | 1.62 (0.89–2.98) | 0.116 | 2.30 (1.01–5.29) | 0.048 |
| Underweight | 2.12 (1.11–4.04) | 0.022 | 7.96 (3.05–20.77) | <0.001 |
| Mortality | 5.84 (0.76–45.01) | 0.090 | 10.29 (0.65–164.12) | 0.099 |
All bacteremic children (n = 59) were modeled against all of the abacteremic children (n = 447), but the children with bacteremia caused by Gram-negative organisms (n = 37) were matched for age and gender against randomly selected abacteremic children (n = 74). Data are presented as the odds ratio (OR) (95% confidence interval [CI]). HDP, high-density parasitemia (≥10,000 parasites/μl); SMA, severe malarial anemia (Hb level, <6.0 g/dl). Values in boldface are statistically significant.
DISCUSSION
Accumulating evidence from sub-Saharan Africa demonstrates that invasive bacterial infections are important concurrent infections in pediatric populations with severe malaria and other childhood illnesses (5, 9, 20, 28). Results presented here demonstrate that bacteremia is also a common cause of childhood illnesses in children in western Kenya who present at the hospital with malaria. This is the first study describing the etiologies of bacteremia in a pediatric population from western Kenya with P. falciparum malaria. The elevated rate of bacteremia in the study population may be due to the high prevalence of respiratory and gastrointestinal tract infections, as well as malnutrition, in the study area (7, 18, 32, 33).
The most common Gram-positive isolate in the children was Staphylococcus aureus. The results for children presenting at the hospital are similar to those of recent investigations showing that S. aureus was the most frequent cause of community-acquired bacteremia caused by Gram-positive organisms in infants and young children in Nigeria and Mozambique, respectively (17, 34). Consistent with previous studies of areas of sub-Saharan Africa where malaria is endemic, which showed that NTS are the most common invasive bacteria associated with elevated pediatric morbidity and mortality (4, 9, 15, 36), NTS were the most common isolates obtained from children with malaria presenting at SDH. In addition, bacteremia was associated with 6-fold-higher mortality within 7 days postenrollment. Consistent with previous studies showing increased case fatality rates for young children with community-acquired bacteremia of Gram-negative origin, bacteremia of Gram-negative origin was nonsignificantly associated with elevated levels of mortality (∼10-fold increase) in the 7 days postenrollment (2, 14).
Results here for children presenting at the hospital in a region where P. falciparum transmission is holoendemic differ from those of a previous study conducted in an area of malaria transmission hyperendemicity, Kilifi District, Kenya, in which there was a high prevalence of community-acquired Streptococcus pneumoniae bacteremia among children <5 years of age (5). Although it is difficult to directly compare rates of community-acquired bacteremia by using the population that presented at the hospital in the current study, based on the fact that these rates may not be representative of those in the community, one plausible explanation for the absence of Streptococcus pneumoniae-positive cultures in our study may be one of the enrollment criteria: children presenting with symptoms of respiratory tract infection, a condition commonly associated with malaria in the region, were excluded from enrollment (35).
A number of previous studies of African children showed that bacteremia was associated with more profound anemia in children with and without malaria (9, 10, 15, 36). The findings presented here, showing significant reductions in Hb levels in bacteremic children, are consistent with these observations. In addition, multivariate modeling revealed that SMA was an independent predictor of bacteremia of Gram-negative origin. This result parallels those of previous studies, in which bacteremia of Gram-negative origin was associated with increased development of SMA (9, 15).
The lack of significant associations between bacteremia and both leukocytes and neutrophils is, in part, consistent with previous studies performed at Kilifi District Hospital illustrating a lack of association between leukocytes and bacteremia (19). Possible explanations for these findings may be related to the fact that elevated leukocytes and neutrophils (>98% of the granulocyte fraction) are common hematological derangements for cases of both bacteremia and malaria; therefore, they may not be further elevated in the presence of coinfection with the two pathogens.
Respiratory distress is associated with enhanced mortality in children with bacterial sepsis and malaria monoinfection (12, 23). The prevalence of respiratory distress was ∼3-fold higher in the bacteremic children than in the nonbacteremic children with malaria. In addition, respiratory distress was a significant predictor of bacteremia of Gram-negative origin, suggesting that although respiratory distress is common to malaria, it is even more prominent when children with malaria are coinfected with bacteria. This finding is important, since respiratory distress in children with malaria is associated with significantly elevated rates of mortality (2).
In our cohort, the proportion of bacteremic children markedly decreased as parasite density increased. These results are similar to those of previous studies from rural Mozambique and The Gambia (2, 21, 28) showing a lower parasitic prevalence and lower mean parasite densities in malaria-infected children with bacteremia than those for the abacteremic controls. Multivariate modeling of the cohort presented here revealed that bacteremia was associated with a 66% reduction in the development of HDP. These findings appear to indicate that the bacteremic patients are sick from the bacterial infection in the presence of incidental malaria. This explanation is consistent with studies of community-acquired bacteremia in Kilifi, Kenya, showing that bacterial sepsis with incidental parasitemia is common in febrile children (5). These observations can also be attributed to the fact that the bulk of bacteremic patients in the these studies had lower hemoglobin levels and were younger than the abacteremic patients; hence, they are likely to experience asymptomatic malaria and have lower parasite densities, as previously reported for anemic (13) and young (6) children residing in areas of Ghana and western Kenya, respectively, where malaria is endemic.
Multivariate analyses also showed an association between a weight-for-age Z score of <−2 (underweight) and an increased risk of bacteremia. These results are similar to those of previous studies, which showed that malnutrition is associated with increased rates of bacteremia in infants and young children in rural sub-Saharan Africa (1, 8, 22). Our results also support previous investigations demonstrating that being underweight is an accurate nutritional screening tool that predicts disease severity in pediatric patients (22, 25). In addition, the findings presented here confirm results of our previous studies (30) and those of others (7), showing that underweight children are common in western Kenya.
In conclusion, the results presented here demonstrate that bacteremia is highly prevalent in children in western Kenya presenting at the hospital with malaria. Although bacteremia was commonly identified for these children, the low volume of blood safely available from small, anemic children required us to perform blood cultures using reduced blood volumes that may reduce the probability of obtaining positive cultures, particularly in those children with low systemic bioburdens. Thus, the overall rate of bacteremia in children presenting at the hospital in western Kenya may be even higher than that reported here. In the overall cohort, bacteremia was associated with reduced HDP and an enhanced risk of being underweight, whereas bacteremia of Gram-negative origin was specifically associated with reduced HDP and an elevated rate of underweight children, along with increased susceptibility to SMA and respiratory distress. These results therefore indicate that infections caused by Gram-negative organisms are an important determinant of malaria severity, particularly in malnourished children in western Kenya who are normally exposed to some of the highest rates of P. falciparum infections. Further studies are required to determine the pathophysiological mechanisms that govern the association between bacteremia and clinical outcomes identified in the present investigation. This study underscores the importance of performing blood cultures with samples from children in sub-Saharan Africa to identify common causes of childhood morbidity and mortality.
ACKNOWLEDGMENTS
We thank the parents, guardians, and children from the Siaya District community for their participation in this study. We are grateful to the University of New Mexico/KEMRI laboratories and the Siaya District Hospital staff for their support during the study. This paper is published with the permission of the Director of the Kenya Medical Research Institute.
This work was supported by grants from the National Institute of Health (AI51305-06 [D.J.P.] and TW05884-06 [D.J.P.]).
T.W., G.C.D., and C.O. performed analyses and conducted the experiments. J.B.H. was involved in the statistical analyses. D.J.P. cowrote the manuscript with T.W. D.J.P. and J.M.O. designed and coordinated the study, along with J.M.V.
There is no conflict of interest for any of the authors due to either commercial or other affiliations.
Footnotes
Published ahead of print on 24 November 2010.
REFERENCES
- 1. Ayoola O. O., Adeyemo A. A., Osinusi K. 2002. Predictors of bacteraemia among febrile infants in Ibadan, Nigeria. J. Health Popul. Nutr. 20:223–229 [PubMed] [Google Scholar]
- 2. Bassat Q., et al. 2009. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop. Med. Int. Health 14:1011–1019 [DOI] [PubMed] [Google Scholar]
- 3. Beier J. C., et al. 1994. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am. J. Trop. Med. Hyg. 50:529–536 [DOI] [PubMed] [Google Scholar]
- 4. Berkley J., Mwarumba S., Bramham K., Lowe B., Marsh K. 1999. Bacteraemia complicating severe malaria in children. Trans. R. Soc. Trop. Med. Hyg. 93:283–286 [DOI] [PubMed] [Google Scholar]
- 5. Berkley J. A., et al. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N. Engl. J. Med. 352:39–47 [DOI] [PubMed] [Google Scholar]
- 6. Bloland P. B., et al. 1999. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission. II. Descriptive epidemiology of malaria infection and disease among children. Am. J. Trop. Med. Hyg. 60:641–648 [DOI] [PubMed] [Google Scholar]
- 7. Bloss E., Wainaina F., Bailey R. C. 2004. Prevalence and predictors of underweight, stunting, and wasting among children aged 5 and under in western Kenya. J. Trop. Pediatr. 50:260–270 [DOI] [PubMed] [Google Scholar]
- 8. Brent A. J., et al. 2006. Salmonella bacteremia in Kenyan children. Pediatr. Infect. Dis. J. 25:230–236 [DOI] [PubMed] [Google Scholar]
- 9. Bronzan R. N., et al. 2007. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J. Infect. Dis. 195:895–904 [DOI] [PubMed] [Google Scholar]
- 10. Calis J. C., et al. 2008. Severe anemia in Malawian children. N. Engl. J. Med. 358:888–899 [DOI] [PubMed] [Google Scholar]
- 11. Cheesbrough J. S., Taxman B. C., Green S. D., Mewa F. I., Numbi A. 1997. Clinical definition for invasive Salmonella infection in African children. Pediatr. Infect. Dis. J. 16:277–283 [DOI] [PubMed] [Google Scholar]
- 12. Chintu C., et al. 2002. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 360:985–990 [DOI] [PubMed] [Google Scholar]
- 13. Crookston B. T., et al. 2010. Exploring the relationship between chronic undernutrition and asymptomatic malaria in Ghanaian children. Malar. J. 9:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enwere G., et al. 2006. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr. Infect. Dis. J. 25:700–705 [DOI] [PubMed] [Google Scholar]
- 15. Graham S. M., Walsh A. L., Molyneux E. M., Phiri A. J., Molyneux M. E. 2000. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans. R. Soc. Trop. Med. Hyg. 94:310–314 [DOI] [PubMed] [Google Scholar]
- 16. Ikumapayi U. N., et al. 2007. Molecular epidemiology of community-acquired invasive non-typhoidal Salmonella among children aged 2–29 months in rural Gambia and discovery of a new serovar, Salmonella enterica Dingiri. J. Med. Microbiol. 56:1479–1484 [DOI] [PubMed] [Google Scholar]
- 17. Johnson A. W., et al. 2008. Etiologic agents and outcome determinants of community-acquired pneumonia in urban children: a hospital-based study. J. Natl. Med. Assoc. 100:370–385 [DOI] [PubMed] [Google Scholar]
- 18. Lackritz E. M., et al. 1997. Longitudinal evaluation of severely anemic children in Kenya: the effect of transfusion on mortality and hematologic recovery. AIDS 11:1487–1494 [DOI] [PubMed] [Google Scholar]
- 19. Ladhani S., Lowe B., Cole A. O., Kowuondo K., Newton C. R. 2002. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br. J. Haematol. 119:839–847 [DOI] [PubMed] [Google Scholar]
- 20. Lepage P., et al. 1987. Community-acquired bacteraemia in African children. Lancet i:1458–1461 [DOI] [PubMed] [Google Scholar]
- 21. Mabey D. C., Brown A., Greenwood B. M. 1987. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J. Infect. Dis. 155:1319–1321 [DOI] [PubMed] [Google Scholar]
- 22. Maitland K., et al. 2006. Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol? PLoS Med. 3:e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marsh K., et al. 1995. Indicators of life-threatening malaria in African children. N. Engl. J. Med. 332:1399–1404 [DOI] [PubMed] [Google Scholar]
- 24. McElroy P. D., et al. 1999. Analysis of repeated hemoglobin measures in full-term, normal birth weight Kenyan children between birth and four years of age. III. The Asembo Bay Cohort Project. Am. J. Trop. Med. Hyg. 61:932–940 [DOI] [PubMed] [Google Scholar]
- 25. Mei Z., Grummer-Strawn L. M., de Onis M., Yip R. 1997. The development of a MUAC-for-height reference, including a comparison to other nutritional status screening indicators. Bull. World Health Organ. 75:333–341 [PMC free article] [PubMed] [Google Scholar]
- 26. Mulholland E. K., Adegbola R. A. 2005. Bacterial infections: a major cause of death among children in Africa. N. Engl. J. Med. 352:75–77 [DOI] [PubMed] [Google Scholar]
- 27. Obonyo C. O., Vulule J., Akhwale W. S., Grobbee D. E. 2007. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am. J. Trop. Med. Hyg. 77:23–28 [PubMed] [Google Scholar]
- 28. O'Dempsey T. J., et al. 1994. Importance of enteric bacteria as a cause of pneumonia, meningitis and septicemia among children in a rural community in The Gambia, West Africa. Pediatr. Infect. Dis. J. 13:122–128 [DOI] [PubMed] [Google Scholar]
- 29. Okiro E. A., et al. 2009. Age patterns of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar. J. 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ong'echa J. M., et al. 2006. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am. J. Trop. Med. Hyg. 74:376–385 [PubMed] [Google Scholar]
- 31. Otieno R. O., et al. 2006. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS 20:275–280 [DOI] [PubMed] [Google Scholar]
- 32. Paxton L. A., Redd S. C., Steketee R. W., Otieno J. O., Nahlen B. 1996. An evaluation of clinical indicators for severe paediatric illness. Bull. World Health Organ. 74:613–618 [PMC free article] [PubMed] [Google Scholar]
- 33. Perkins B. A., et al. 1997. Evaluation of an algorithm for integrated management of childhood illness in an area of Kenya with high malaria transmission. Bull. World Health Organ. 75(Suppl. 1):33–42 [PMC free article] [PubMed] [Google Scholar]
- 34. Sigauque B., et al. 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr. Infect. Dis. J. 28:108–113 [DOI] [PubMed] [Google Scholar]
- 35. Tornheim J. A., et al. 2007. The epidemiology of hospitalized pneumonia in rural Kenya: the potential of surveillance data in setting public health priorities. Int. J. Infect. Dis. 11:536–543 [DOI] [PubMed] [Google Scholar]
- 36. Walsh A. L., Phiri A. J., Graham S. M., Molyneux E. M., Molyneux M. E. 2000. Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr. Infect. Dis. J. 19:312–318 [DOI] [PubMed] [Google Scholar]
- 37. Warrell D., Molyneux M., Beales P. 1990. Severe and complicated malaria. Trans. R. Soc. Trop. Med. Hyg. 84(Suppl. 2):1–65 [PubMed] [Google Scholar]
- 38. Zucker J. R., et al. 1996. Childhood mortality during and after hospitalization in western Kenya: effect of malaria treatment regimens. Am. J. Trop. Med. Hyg. 55:655–660 [DOI] [PubMed] [Google Scholar]