Abstract
A study of meningococcal carriage dynamics was performed with a cohort of 190 first-year students recruited from six residential halls at Nottingham University, United Kingdom. Pharyngeal swabs were obtained on four occasions between November 2008 and May 2009. Direct plating and culture on selective media were succeeded by identification and characterization of meningococci using PCR-based methodologies. Three serogroup Y clones and one serogroup 29E clone were highly prevalent in particular residential halls in November 2008, which is indicative of rapid clonal expansion since the start of the academic year. Persistent carriage of the same meningococcal strain for at least 5 to 6 months was observed in 45% of carriers, with infrequent evidence of antigenic variation in PorA. Sequential carriage of heterologous meningococcal strains occurred in 36% of carriers and involved strains with different capsules and antigenic variants of PorA and FetA in 83% of the cases. These clonal replacement strains also exhibited frequent differences in the presence and antigenic structures of two other surface proteins, NadA and HmbR. This study highlights the low level of antigenic variation associated with persistent carriage but, conversely, the importance of alterations in the repertoire of antigenic variants for sequential carriage of meningococcal strains. Rapid clonal expansion of potentially pathogenic strains in residential halls has implications for the implementation of public health interventions in university populations.
Neisseria meningitidis is an obligate, human-specific commensal and pathogen of importance due to its impact on human health (8). This organism can persist in the human nasopharynx without causing clinical symptoms in a state known as carriage (4). Invasion of the pharyngeal tissues and subsequent proliferation in the blood and cerebrospinal fluids are infrequent but lead to diseases, such as septicemia and meningitis, which are associated with significant levels of morbidity and mortality (8).
Previous carriage studies have estimated the rate of meningococcal carriage in the wider population at 10 to 11% (7), with young adults exhibiting the highest rates of between 10 and 35% (10). Rates of >50% have been detected in the semienclosed, close-contact populations of army training camps and university halls of residence (9, 21). Circulating strains can be characterized by serological or molecular typing methods. The main molecular technique, multilocus sequence typing (MLST) (20), requires sequencing of seven housekeeping genes and enables identification of both specific clones (sequence types [STs]) and groups of related clones termed clonal complexes (CCs). Outer membranes of meningococci contain numerous proteins whose abundances range from high to low, termed major and minor surface proteins, respectively. Molecular typing of two of these proteins, PorA (23) and FetA (26), has detected associations between particular CCs and specific antigenic variants of these proteins, suggesting that immune responses are responsible for structuring meningococcal populations (5). These studies have also revealed a complex relationship between carriage and disease, with some CCs being infrequent in carriers but commonly associated with disease (10). Critically, the number and closeness of contacts are considered risk factors for acquisition of both carriage and disease isolates (19).
Longitudinal studies of meningococcal carriage in specific volunteers are important for characterization of the length of the carrier state and the impact of host immune responses on carriage. Stable carriage, lasting 5 to 6 months, was observed in 43 to 69% of individuals by Caugant et al. (11). Other studies have estimated average stable carriage rates of 7 to 9 months, with modeling indicating that carriage may last for up to 29 months (12, 27). Examinations of carriage in teenagers and military recruits using molecular typing techniques have shown that most carriers (85%) are persistently colonized by a single strain, with clonal replacement occurring in only 0.7 to 13% of carriers (11, 15). Ala'Aldeen et al. (1) observed a higher level of clonal replacement in a university student population, with 43% of carriers harboring strains with different pulsed-field gel electrophoresis (PFGE) profiles. An association between persistent carriage and elicitation of strain-specific humoral responses has also been detected using longitudinal carriage studies (18). In general, few of the longitudinal carriage studies have utilized molecular typing methods, hampering interpretation of results and evaluation of the impact of persistent carriage on antigenic variation of surface antigens.
A longitudinal carriage study was performed at Nottingham University between November 2008 and May 2009. A high rate of meningococcal carriage (47 to 62%) was detected in this cohort at all time points, with disease-associated serogroup Y clones forming the major component of the meningococcal population (D. A. A. Ala'Aldeen et al., submitted for publication). In this report, we present an analysis of the distribution of these meningococcal clones across the residential halls and describe the length/nature of meningococcal carriage in specific individuals, with particular emphasis on the frequency of changes in surface antigens and dynamics of transmission.
MATERIALS AND METHODS
Recruitment of students and collection of isolates.
A cohort of 190 Nottingham University students was recruited in November 2008 at five sites (A to E) covering six catered residential halls (site D recruitment covered two halls, D1 and D2). The study was approved by the Nottingham University Medical School Ethics Committee, and written informed consent was obtained from all volunteers. Samples were collected in November 2008 and at three subsequent time points (December, February, and May 2009). Cotton swabs were used to sample the posterior pharynx, but not tonsils, of each volunteer. All swabbing was carried out by experienced medical professionals. Swabs were immediately spread onto one half of a chocolate GC selective agar plate (Oxoid). This inoculum was spread out with a loop onto the other half of the GC plate in order to obtain single colonies. Plates were then incubated at 37°C with 5% CO2 overnight. Colonies with a morphology suggestive of Neisseria meningitidis were tested for a positive oxidase test. One representative colony was picked from each of these plates and subcultured overnight on chocolate agar plates (Oxoid) prior to preparation of DNA extracts, storage in glycerol broth at −80°C, and further characterization. Up to 19 morphologically similar colonies were also picked from each plate and subjected to subculture and DNA extraction.
Characterization of isolates.
DNA was extracted from one isolate per carrier per time point using a DNeasy purification kit (Qiagen). Isolates were confirmed as meningococci in PCR assays by amplification of two meningococcal genes, ctrA and crgA, using the following primers: ctrA-for (5′GCTGCGGTAGGTGGTTCAA), ctrA-rev (5′TTGTCGCGGATTTGCAACTA), crgA-for (5′GCTGGCGCCGCTGGCAACAAAATTC), and crgA-rev (5′CTTCTGCAGATTGCGGCGTGCCGT) (25). PorA types were determined for each isolate by amplification of the porA gene and automated sequencing of these PCR products with two porA-specific primers, porA-for-seq (5′CCGCACTGCCGCTTGCGG) and porA-rev-seq (5′CGCATATTTAAAGGCATA), followed by querying of the relevant database (http://pubmlst.org/neisseria/) with the DNA sequence. FetA types were obtained in a similar fashion using the primers fetA-for-seq (5′TTCAACTTCGACAGCCGCCTT) and fetA-rev-seq (5′TTGCAGCGCGTCRTACAGGCG). Multilocus sequence typing (MLST) was performed as described elsewhere (17) and using the Neisseria multilocus sequence typing website ((http://pubmlst.org/neisseria/). PCR-based serogrouping utilized previously described primers specific for capsular biosynthetic genes (synD, siaDB, siaDC, siaDW-135, and siaDY for serogroups A, B, C, W-135, and Y, respectively) or alleles of ctrA (29E, H, X, and Z) (3, 25). PCR-based detection of the nadA, hpu, hmbR, and opc genes was performed with the following primers: nadA-for (5′TCGACGTCCTCGATTACGAAGGC), nadA-rev (5′TGGCTGTGGTCAGTACTTTGGATGG), opc-for (5′GAGAATAACAATTCGTTGTA), opc-revS (5′CTCATTAGCGGTTTGAAGCTCTTGTGCAG), hmbR-RF3 (5′TGCCAACCTCTTTTACGAATGG), hmbR-RF4 (5′GCTACTGAACACGTCGTTCC), hpuAC (5′ATGCGATGAAATACAAAGCCC), hpuA350Rev (5′GGATGAAAGGGCGTATTGCGC), and P26.85 (5′GGGAAACGCTTGGGCGATGG). The variable regions (VRs) of hmbR were amplified and sequenced with primers hmbR2834 (5′CAACGGATGGCTGTCCAAAC) and hmbR3424 (5′ACGCGTAACCGTGAATGGAC). These sequences were then queried against a new database (http://neisseria.org/nm/typing/hmbR) to obtain allele numbers for each of the three VRs of this protein. Variations between isolates and strains in the VRs of PorA, FetA, or HmbR were classified as minor if the change was within a VR family or major if it was between two VR families.
Detection of carriage of multiple strains.
For a subset of carriers, DNA was extracted from an additional 19 isolates per carrier per time point. Isolates were confirmed as meningococci by amplification of the crgA and ctrA genes. In some cases, PCR-based serogrouping was also performed on these isolates. A PCR-based approach was utilized to determine whether the additional isolates exhibited the same PorA type as the initial isolate. Amplification was performed with a primer specific for one of the relevant variable regions (porA-VR2-N50rev [5′GAGAGTAGGCTGACTTTTAGG], specific for P1.2; porA-VR2-N425rev [5′GTAGGCGGCTGATTTTGC], specific for P1.10-10; porA-VR1-N54for [5′CTAACGGTGTGCAAGGCAATC], specific for P1.21; or porA-VR1-N428for [5′CACAAGGTCAGACGGGCAATAAAG], specific for P1.18-1) and either porA-rev-seq or porA-for-seq.
RESULTS
Variations in meningococcal carriage rates between residential halls at Nottingham University.
A longitudinal study of meningococcal carriage was performed with a cohort of students recruited from six residential halls in Nottingham University during the 2008-2009 academic year. Sampling was initiated in November 2008 approximately 5 weeks after the start of the term, when relatively high carriage rates were expected. The overall carriage rate at our initial time point was 47% (89/190), which increased to 62% (39/63) over the course of the study (Table 1) (Ala'Aldeen et al., submitted for publication). Interestingly, 51% (18/35) of volunteers who were noncarriers at the initial time point were still noncarriers after 6 months. Meningococcal carriage rates were not uniform across the residential halls (Table 1). An extremely high rate (78%) was observed in hall C at the initial time point and took 6 months to drop to below 60%. In halls A, B, and E, carriage rates increased from 39 to 48% to 67 to 69% during the course of the study so that the numbers of carriers exceeded the numbers of noncarriers by May 2009. A significant dropout rate (Table 1) was experienced during the course of this study due to a combination of factors (e.g., absence of volunteers on the day of sampling, all samples being provided on a voluntary basis with no financial inducements for participation, and resistance to provision of blood samples, which were collected concomitantly with swabbing). None of these factors are likely to have introduced a bias toward retention of carriers versus noncarriers in the study. However, the lower numbers of volunteers at later time points reduced the statistical significance of differences in the overall carriage rate and, for specific clones, between time points for each hall, particularly halls D1/D2. The dropout rate also reduced detection of persistent carriage (see below).
Table 1.
Numbers of carriers and noncarriers in each residential hall
| Collection time | Carriage status | No. (%)a in hall: |
||||
|---|---|---|---|---|---|---|
| A | B | C | D1/D2 | E | ||
| November 2008 | Noncarrier | 19 (58) | 22 (61) | 7 (22) | 28 (68) | 25 (52) |
| Carrier | 14 (42) | 14 (39) | 25 (78) | 13 (32) | 23 (48) | |
| Total | 33 | 36 | 32 | 41 | 48 | |
| December 2008 | Noncarrier | 13 (50) | 11 (52) | 4 (25) | 7 (64) | 10 (59) |
| Carrier | 13 (50) | 10 (48) | 12 (75) | 4 (36) | 7 (41) | |
| Total | 26 | 21 | 16 | 11 | 17 | |
| February 2009 | Noncarrier | 6 (43) | 9 (47) | 4 (27) | 4 (80) | 11 (52) |
| Carrier | 8 (57) | 10 (53) | 11 (73) | 1 (20) | 10 (48) | |
| Total | 14 | 19 | 15 | 5 | 21 | |
| May 2009 | Noncarrier | 5 (31) | 4 (31) | 6 (43) | 4 (80) | 5 (33) |
| Carrier | 11 (69) | 9 (69) | 8 (57) | 1 (20) | 10 (67) | |
| Total | 16 | 13 | 14 | 5 | 15 | |
Dropout rates for the December, February, and May time points were 22, 58, and 52%, respectively, for hall A; 39, 47, and 64% for hall B; 50, 53, and 56% for hall C; 73, 88, and 88% for halls D1/D2; and 65, 56, and 69% for hall E.
Distribution of meningococcal clones across the residential halls.
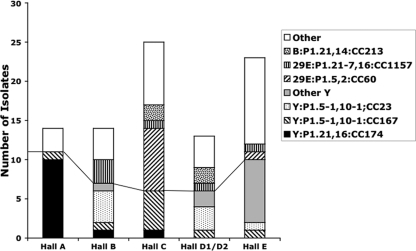
Meningococcal isolates were subject to PCR-based typing systems in order to identify the number and distribution of clones. More than 50% of all isolates belonged to any one of four PorA types (i.e., P1.5-1,10-1, P1.21,16, P1.5,2, and P1.21-7,16) and to four clonal complexes (i.e., CC23, CC60, CC167, and CC174). When the distribution of these clones in the six halls of residence was examined, specific clones were partially restricted to certain halls of residence (Fig. 1; see Table S1A in the supplemental material). Hall A was dominated by two CC174 clones, Y:P1.21,16:F3-7:ST1466 and Y:P1.21,16:F3-7:ST8510, with 71% of isolates (10/14) being from these two clones, which differ by a single nucleotide in fumC of the MLST genes (data not shown). Two major clones were present in hall B, with 29% and 21% of carriers being colonized with Y:P1.5-1,10-1:F4-1:ST1655 (CC23) and 29E:P1.21-7,16:ST1157 (CC1157), respectively. It is notable that three students in this hall acquired carriage of the CC23 clone at the December time point, which is indicative of continuing spread of this clone. Hall C was dominated by two clones, at frequencies of 20% for Y:P1.5-1,10-1:F1-3:ST767 (CC167) and 32% for 29E:P1.5,2:F1-7:ST1383 (CC60). The three other halls of residence were not dominated by specific clones, but in all cases serogroup Y clones were at high levels in carriers, i.e., 46% for halls D1/D2 and 43% for hall E (see Table S1A in the supplemental material).
Fig. 1.
Distribution of clones in each hall of residence. This graph shows the numbers of isolates for each of the major clones detected in November 2008 and their distribution across the catered residence halls of Nottingham University. Groups below the line are serogroup Y isolates.
Examination of longitudinal changes in proportions of clones in each hall was hampered by incomplete rerecruitment of students (Table 1). The only trends were reductions between the first and last time points in carriage of Y:P1.21,16:F3-7:CC174 in hall A (from 71 to 27%) and of 29E:P1.5,2:F1-7:CC60 in hall C (from 32 to 12.5%). A rise in carriage was observed for Y:P1.5-1,10-1:F1-4/F1-8:CC167 in hall C, from 20 to 37.5%. A shift in the spread of the major clones was also apparent, with 75% (9/12) of acquired strains being from these clones in December but only 35% (6/17) in May (see Table S2A in the supplemental material).
Persistence of meningococcal carriage.
Longitudinal samples were obtained from 91, 74, and 63 volunteers after 1, 3, and 6 months, respectively. Carriers were separated into persistent carriers, carriers subject to clonal replacement, and carriers subject to clearance. Persistent carriers were defined as being positive at two or more time points for a strain of the same ST with only minor variations in PorA or FetA type (evidence of antigenic variation [see below]). Three carriers (V58, V128 and V185) lost and regained carriage of the same strain, with V58 exhibiting low-level carriage at all time points (<50 colonies), suggesting that carriage may have been at an undetectable level during sampling at the intermediate time point. One of these carriers, V128, showed intermediate carriage of another strain, which is perhaps indicative of carriage of multiple strains. These three carriers were classified as persistent carriers.
Persistent carriage was high over short periods, with 91 and 78% of carriers being colonized with the same clone for 1 to 2 and 3 months, respectively (Table 2). Persistent carriage with the same clone for 5 to 6 months was significantly lower, being observed in 45% of carriers. This drop in persistent carriage was correlated with concomitant rises in clonal replacement and clearance to 36 and 18%, respectively. There were no obvious differences in the length of carriage of particular clones (see Table S2A in the supplemental material). There was, however, a significant difference in the types of spread of the serogroup Y clones. Thus, serogroup Y strains were acquired during clonal replacement in only 17% of carriers (3/18), while 53% (10/19) of new carriers acquired strains of this type (P = 0.04 by Fisher's exact test; odds ratio of 5.6 with a 95% confidence interval [CI] of 1.2 to 25.7 [InStat 2.0]).
Table 2.
Numbers of persistent carriers and carriers subject to clonal replacement or clearance of carriage
| Time points (period) | No. (%) |
|||
|---|---|---|---|---|
| Persistent carriersa | Carriers subject to: |
Total | ||
| Clonal replacementb | Clearance of carriagec | |||
| November to December, December to February (1–2 mo) | 53 (91) | 5 (9) | 0 | 58 |
| November to February, February to May (3 mo) | 36 (78) | 7 (15) | 3 (7) | 46 |
| November to May, December to May (5–6 mo) | 15d (45) | 12 (36) | 6 (18) | 33 |
Presence of a strain with the identical/similar capsule type, PorA type, FetA type, and ST detected at both time points, or assumed if carriage of the persistent clone was detected at a later time point.
Presence of a strain with a different ST or different PorA and FetA types where the ST type has not been determined.
No meningococcal isolates detected.
P values of <0.0001 and 0.004 and odds ratios of 12.7 (95% CI, 4 to 40) and 4.2 (95% CI, 1.6 to 11.2) were obtained in comparisons with the November to December/December to February and November to February/February to May periods of carriage, respectively.
Antigenic variation in PorA during persistent carriage.
Isolates from persistent carriers were analyzed for evidence of minor changes in the amino acid sequences of the VRs of the FetA and PorA proteins. No antigenic variation was detected in FetA, while only three examples of alterations in PorA were detected (Table 3). One alteration was due to a point mutation, while the other two involved changes in the length of trinucleotide repeat tracts. The isolates from carrier V56 were capsule null, while those of V99 were nongroupable, suggesting that absence of capsule may have contributed to immune selection on this antigen. In general, the lack of antigenic variants in the FetA and PorA VRs suggests that antigenic variation occurs infrequently during persistent carriage.
Table 3.
Antigenic variants in variable regions of PorA during persistent carriage in individual carriers
| Volunteer | PorA type (time point[s]a) |
Type of alteration | |
|---|---|---|---|
| Initial | Subsequent | ||
| V56 | P1.7,30-4 (November) | P1.7,30-3 (December) | 9 CTA to 8 CTA |
| V99 | P1.7-2,13-1 (February) | P1.7-2,13 (May) | 4 ACT to 5 ACT |
| V117 | P1.5-1,10-1 (November, December) | P1.5-1,10-29 (February, May) | C to T |
Time points indicate when each strain was isolated from a particular carrier. Note that volunteer V99 was a noncarrier at the November and December time points, such that the initial clone was first detected in February, and that V56 underwent clonal replacement in May (see Table 4).
Clonal replacement is associated with an antigenic shift in multiple antigens.
Both PorA and FetA contain variable regions (VRs), which can be separated into families of VRs exhibiting significant levels of amino acid identity and are known targets of protective immune responses (23). Clonal replacement strains may exhibit major antigenic shifts (i.e., a switch between two VR families) or minor antigenic shifts (a switch within a VR family that may involve only a small number [one to four] of amino acids). Isolates from 18 volunteers subject to clonal replacement were examined for evidence of changes in three meningococcal antigens, namely, capsule, PorA, and FetA (Table 4). For most volunteers (15/18; 83%) a major or minor shift in all three antigens was observed between the initial and replacement strains, while in 10 cases (56%) a major shift in all three antigens occurred. In both cases involving minor shifts in the PorA antigen, from P1.5,2 to P1.5-1,10-1 or P1.5-1,10-10, there were major shifts in the immunodominant VR2 domain. Although 80% (4/5 cases) of minor shifts in FetA involved a type 1 antigen, this was not statistically significantly different from involvement of this antigen in major antigenic shifts (6/12 cases).
Table 4.
Status of capsule, PorA, and FetA types during clonal replacement in individual carriers
| Volunteer | Clone (time point[s])a |
Altered antigen(s)b | |
|---|---|---|---|
| Initial | Replacement | ||
| V54 | Y:P1.21,16:F3-7:ST8510 (November, December, February) | W-135:P1.18-1,3:F4-1:ST1158 (May) | All |
| V63 | Y:P1.21,16:F3-7:ST8510 (November) | B:P1.7,30:F4-1:ST6604 (December) | All |
| V138 | Y:P1.21,16:F3-7:ST1466 (November, December, February) | 29E:P1.5,2:F1-7:ST1383 (May) | All |
| V64 | Y:P1.5-1,10-1:F1-3:ST767 (November, December, February) | NG:P1.17,9:F1-82:ST823 (May) | Minor FetA |
| V82 | Y:P1.5-1,10-1:F1-3:ST767 (November) | 29E:P1.21-7,16:F5-8:ST1157 (December, February, May) | All |
| V124 | Y:P1.5-1,10-1:F1-3:ST767 (November, December, February) | NG:P1.7,30:F4-1:ST6604 (May) | All |
| V140 | 29E:P1.5-1,10-26:F5-5:ST6798 (November, December, February) | B:P1.22,14:F5-5:ST213 (May) | Not FetA |
| V115 | 29E:P1.5,2:F1-7:ST1383 (November, December) | Y:P1.21,16:F3-7:ST1466 (February) | All |
| V120 | 29E:P1.5,2:F1-7:ST1383 (November) | Y:P1.5-1,10-1:F1-3:ST767 (May) | Minor PorA VR1 and FetA |
| V137 | 29E:P1.5,2:F1-7 (November) | B:P1.5,2:F1-5:ST8511 (February) | Not PorA and minor FetA |
| V50 | 29E:P1.5,2:F1-7:ST1383 (December, February) | Y:P1.5-1,10-10:F4-1:ST1655 (May) | Minor PorA VR1 |
| V70 | 29E:P1.21-7,16:F5-36:ST1157 (February) | NG:P1.7,30:F1-2:ST53 (May) | All |
| V226 | B:P1.22,14:F5-5:ST213 (December) | NG:P1.18,25-14:Fnull:ST823 (February, May) | All |
| V199 | B:P1.22,9:F5-5:ST283 (November, February) | W-135:P1.18-1,3:F4-1:ST2638 (May) | All |
| V192 | W-135:P1.18-1,3:F4-1:ST1617 (November) | NG:P1.18-1,3:F3-6:ST3808 (December, February, May) | Not PorA |
| V56 | NG:P1.7,30-3:F1-2:ST53 (November, December) | 29E:P1.5,2:F1-7:ST1383 (May) | Minor FetA |
| V206 | NG:P1.22,1:F1-1:ST103 (November) | 29E:P1.5-1,10-8:F3-6:ST254 (December, February) | All |
| V145 | B:P1.22,14:F5-5:ST213 (November) | 29E:P1.21-7,16:F5-36:NDc (December) | Minor FetA |
Time points indicate when each strain was isolated from a particular carrier. Note that volunteers V50, V70, and V226 were noncarriers in November, while the absence of data for other time points indicates that a volunteer was not subject to sampling.
All = capsule, PorA, and FetA. See Materials and Methods for a definition of minor and major variations in PorA and FetA.
ND, not determined.
To extend this analysis to other surface proteins, PCR-based assays were utilized to test for the presence/absence of four “dispensable” meningococcal genes, opc, hpuA, hmbR, and nadA, encoding outer membrane proteins, which are missing in 30 to 70% of meningococcal strains (Table 5). The hpuA and opc genes were present in the majority of our isolates, and extensive sequence analysis will be required to detect antigenic shifts in these antigens during clonal replacement. In contrast, nadA and hmbR were present in a lower percentage of these strains. Seven clonal replacement events involved strains differing in their nadA status, with only one replacement event (V145) involving two nadA+ strains. Similarly, 10 clonal replacement events involved strains differing in hmbR status, with only five events involving two hmbR+ strains. Recently, Evans et al. (13) identified loops 2, 3, and 4 of HmbR as being hypervariable and developed a typing scheme based on the amino acid sequences of these loops. Five of the clonal replacement events involved two hmbR+ clones. Sequencing of the VR loops from these isolates detected changes in two or more loops for four of these pairs of clones (from 2,1,2 to 1,3,5 in V145; from 1,1,1 to 2,1,2 in V140; from 1,3,5 to 1,1,1 in V70; and from 1,1,1 to 2,1,2 in V137) and no change in the fifth (both 1,1,1 in V56). These results indicated that clonal replacement strains have a propensity to differ in either the antigenic regions or presence/absence of HmbR. In summary, antigenic exclusion in N. meningitidis during clonal replacement appears to cover the NadA and HmbR outer membrane proteins.
Table 5.
Presence or absence of four genes encoding outer membrane proteins in meningococcal strains involved in clonal replacement in individual carriers
| Carrier | Presence of genea in: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Initial clone |
Replacement clone |
|||||||
| nadA | hpu | hmbR | opc | nadA | hpu | hmbR | opc | |
| V54 | + | + | − | + | − | + | − | + |
| V63 | + | + | − | + | − | + | + | + |
| V138 | + | + | − | + | − | + | + | + |
| V64 | − | + | − | + | − | + | − | + |
| V82 | − | + | − | + | + | + | + | + |
| V124 | − | + | − | + | − | + | + | + |
| V140 | − | + | + | + | + | − | + | + |
| V115 | − | + | + | + | + | + | − | + |
| V120 | − | + | + | + | − | + | − | + |
| V137 | − | + | + | + | − | + | + | + |
| V50 | − | + | + | + | − | + | − | + |
| V70 | + | + | + | + | − | − | + | ND |
| V226 | + | − | + | + | − | + | − | + |
| V199 | − | + | + | + | − | + | − | + |
| V192 | − | + | − | + | − | + | − | + |
| V56 | − | − | + | + | − | + | + | + |
| V206 | − | + | + | + | − | + | − | + |
| V145 | + | − | + | − | + | + | + | + |
The typing characteristics of these strains are as presented in Table 3. +, gene detected by PCR; −, gene not detected by PCR; ND, not determined.
Examination of the frequency of simultaneous carriage of multiple meningococcal strains.
Multiple strain carriage may lead to an underestimation of the distribution of strains or the length of persistent carriage when only a single isolate per carrier is examined. Similarly, this may result in an overestimation of clonal replacement. Low rates of simultaneous carriage of multiple strains were reported in two previous studies, occurring in 2 of 33 and 2 of 19 carriers (9a). Seven carriers subject to persistent carriage with either a Y:P1.21,16:F3-7:CC-174 (V43, V54, V59, and V88) or a 29E:P1.5,2:F1-7:CC60 (V114, V134, and V185) strain were examined for multiple strain carriage using 20 meningococcal isolates collected in November and 20 isolates collected in either February or May from each carrier. All isolates were confirmed as meningococci by amplification of the meningococcal ctrA and crgA genes (data not shown). A PCR-based approach was used to determine whether the isolates had similar PorA types. All 20 isolates from both time points generated products with primers specific for one of the VRs (i.e., VR1 for P1.21,16 and VR2 for P1.5,2) in combination with a primer specific for a constant region (data not shown). These results indicated that all the isolates were of a similar PorA type, suggesting that a mixed infection with a strain with an unrelated PorA type had not occurred. Multiple isolates from two time points (February and May) for two carriers (V50 and V54) subject to clonal replacement were also examined. In these cases, the isolates were subjected to PCR with primers specific for the relevant PorA VRs and capsular genes. Carrier V50 exhibited replacement of 29E:P1.5,2:F1-7:ST1383 with Y:P1.5-1,10-10:F4-1:ST1655 between February and May (Table 4). All 20 isolates from the February time point were found to have a P1.2 VR2 region in PorA and a 29E capsular genotype, while conversely, all the May isolates had a P1.10-10 VR2 in PorA and a Y capsular genotype (data not shown). A similar result was obtained for V54, suggesting that the initial strain had been completely replaced by the incoming strain. Overall, we have been unable to detect evidence of carriage of multiple strains in nine carriers.
DISCUSSION
In this report we provide a detailed analysis of the spatial and temporal changes in the prevalence of meningococcal clones in a cohort of students from Nottingham University during the 2008-2009 academic year. The epidemiological survey encompassed six catered residential halls in which students are in close and frequent contact, providing the close-contact environments conducive to spread of meningococci. Hall-to-hall variations in carriage rate were apparent, with one hall (C) exhibiting an extremely high carriage rate of 78% at the first time point (Table 1). This and two other halls (A and B) were dominated by one or two specific clones, with these clones accounting for >50% of the isolates (Fig. 1). These results may be an underestimate of the distribution of these clones, as only one isolate per carrier was examined in the majority of cases. Multiple strain carriage is, however, likely to be low, as we were unable to detect evidence for this phenomenon by analysis of multiple colonies from nine carriers. Halls A, B, and C each contain 200 to 300 people, while halls D1, D2, and E each contain more people (300 to 400), and D1/D2 are newer in construction and have air-conditioned bars. The absence of dominant clones in these halls may reflect these differing characteristics or result from the absence of a highly transmissible clone. The latter conclusion seems unlikely, as the Y:P1.21,16:F3-7:CC174 clone was found in three of the six halls of residence.
Dominance of particular clones in certain halls in November 2008 is indicative of rapid clonal expansion since the start of the term 5 weeks prior to this initial sampling point. In the absence of sampling at the beginning of the term, inferences about the expansion of clones were based on the distribution of clones in the other residential halls and on the analysis of carriage rates in incoming students for the next academic year. The 29E:P1.5,2:F1-7:ST1383 clone was found only in hall C in November 2008, being present in 8 of 32 volunteers, which was a significantly higher prevalence than for the other halls (assuming 0.5 carriers in the other halls, P < 0.002 by Fisher's exact test; odds ratios ranged from 23.2 to 33.7, with a 95% CI of 1.28 to 607.9 [InStat 2.0]). This strongly suggests that rapid clonal expansion initiated by a single carrier was responsible for the site-specific prevalence of this clone. Three different serogroup Y clones were observed in multiple carriers in halls A, B, and C and in single carriers in other halls. The Y:P21,16:F3-7:CC174 clones were significantly overrepresented in hall A compared to other halls (P < 0.006; odds ratios ranged from 13.5 to 43.4, with a 95% CI of 1.6 to 772), while the other serogroup Y clones did not show significant variations in distribution. Epidemiology data for incoming students for Nottingham University in the first week of the following year (September 2009) indicate a prevalence of 0.029 for serogroup Y strains in first-year university students (Ala'Aldeen et al., submitted for publication), which would mean an average of nine serogroup Y carriers in a hall of 300 individuals. Assuming an even distribution between the six major serogroup Y clones (P1.21,16:ST1466, P1.12-1,16-8:ST1466, P1.5-1,10-1:ST767, P1.5-1,10-1:ST1655, P1.5-1,2-2:ST23, and P1.5-1,2-2:ST3651) observed in November 2008 and a similar initial carriage rate in incoming students, rapid clonal expansion initiated by one or two individuals may also be responsible for the higher prevalence of the CC174 clones in hall A and for the slightly higher levels of other serogroup Y clones in halls B and C. This rapid spread may have occurred due to a lack of cross-protective immunity and/or limited competition from other meningococcal clones. As three of these major clones had a serogroup Y capsule and other serogroup Y clones also occurred in significant numbers, a lack of immunity may be a significant factor, as high levels of carriage of serogroup Y strains have not previously been observed in the United Kingdom and hence immunity to the serogroup Y capsular antigen may be low. It is notable that serogroup Y spread was most prevalent among naïve individuals as opposed to carriers, as measured by an involvement in clonal replacement, suggesting that carriage of another meningococcal clone protected against acquisition of these serogroup Y clones. An alternative explanation for rapid clonal expansion is that these serogroup Y and 29E clones have genetic characteristics permitting high levels of transmission.
Persistent carriage of the same meningococcal clone for at least 6 months has been observed in a number of longitudinal studies of meningococcal carriage (1, 11). In this study, we observed persistent carriage of the same clone for at least 5 to 6 months in 45% of individuals. Persistence of these organisms may be aided by antigenic or phase variation of surface antigens. Antigenic variation in the variable regions of the PorA protein was detected in three persistent carriers, but only one of these carriers exhibited carriage for >5 months. In two of these carriers, variation was due to alterations in repetitive DNA tracts (Table 3), emphasizing the importance of these highly mutable DNA sequences not only as a mechanism of phase variation but as a significant source of antigenic variation. The PorA antigen is a major surface protein and target of adaptive immune responses. The low level of antigenic variation in this protein during long-term persistence (i.e., in only 1 of 15 individuals who exhibited 5 to 6 months of carriage) is surprising and suggests that other mechanisms such as phase-variable changes in the level of expression may contribute to immune evasion (2, 28). An alternative explanation is that meningococcal strains might escape detection by the host immune system or prevent activation of host immune responses (22).
Clonal replacement was observed at a significant rate (36%) in long-term meningococcal carriers; this is a higher rate than observed in longitudinal studies of teenagers but similar to those in other student or military cohorts (1, 11, 15). Nonoverlapping repertoires of antigenic variants in meningococcal populations are thought to result from immune selection (6, 16), suggesting that clonal replacement should involve strains with diverse antigenic profiles. Replacement was associated with a strong likelihood of alterations between replacement and initial strains either in the presence/absence of the antigen (e.g., capsule, NadA, or HmbR) or in the sequence of known or putative immunodominant antigenic variable regions (e.g., PorA and FetA). One interpretation of these results is that antigenic differences were due to chance and solely reflected the prevalence of the clones circulating in this community. The alternative is that carriage induces epitope-specific immune responses providing protection against homologous but not heterologous antigens or antigenic variants of specific surface determinants and hence against strains with an overlapping antigenic repertoire. A significant difference was observed between the prevalence of serogroup Y strains in carriers (56/125; 45%) and as the incoming strains involved in clonal replacement (3/18; 17%) (P = 0.04 by Fishers exact test; odds ratio of 0.25, with a 95% CI of 0.07 to 0,89). Similarly, only 10% (1/10) of clonal replacement strains at the May time point contained a FetA type 3 or 5 antigen, although these types were present in 56% (20/36) of carriage isolates at this time point (P = 0.01; odds ratio of 0.09, with a 95% CI 0.01 to 0.77). These findings support the view that there is selection against these antigens during clonal replacement. Some trends were also observed (e.g., 10% of the May clonal replacement strains were nadA+ while 43% of carried isolates were nadA+, and major circulating clones ST1466, ST8510, ST767, ST1655, and ST1383 were only involved in 12.5% [1/8] of replacement events in December and February despite being present in 51% of carriers) but were not significant, possibly due to the low number of observed replacement events. The lack of significance for other comparisons (e.g., PorA antigenic variants) may reflect the diversity of circulating strains rather than selection. The observation of major and minor outer membrane structures/proteins being influenced by clonal replacement is novel and provides further evidence that immune responses during carriage could be responsible for antigenic structuring of multiple surface structures/proteins in meningococcal populations (10). Examination of the sequences of other surface antigens in these and other similar strain collections will indicate the size of the repertoire recognized by the host immune system and whether the protective effects of carriage are restricted only to major surface antigens.
This report has described the significant potential for clonal expansion in residential halls within universities, which has implications for the implementation of prophylactic measures following a case of meningococcal disease in this type of setting, such as whether or not to target all individuals within the hall. This report also has implications for vaccine development. The low level of antigenic variation in FetA and PorA during persistent carriage suggests that escape of vaccine-induced immune responses due to antigenic variation may not be frequent even if carriage occurs in vaccinated individuals. The observation of high levels of alteration in antigenic variants of both major and minor surface structures/proteins during clonal replacement is more difficult to interpret but provides an indication that inclusion of major and minor antigens in a vaccine will prevent carriage of, and hence disease caused by, other strains carrying these antigens. This is particularly important as NadA is a component of a novel vaccine undergoing human trials (24), and others have proposed including both major and minor antigens in a meningococcal vaccine in order to provide a broader protective response (14).
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by research grants from Sanofi Pasteur and the Healthcare and Bioscience iNET.
We thank colleagues at Pfizer for generation and provision of STs for many of these isolates. We are especially grateful to all the volunteers who participated in this study. We also thank Nader Ahmed, Isfahan Tauseef, Shaun Morroll, and Karl Wooldridge for help with the collection of isolates.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 1 December 2010.
REFERENCES
- 1. Ala'Aldeen D. A., et al. 2000. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J. Clin. Microbiol. 38:2311–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bayliss C. D., et al. 2008. Neisseria meningitidis escape from the bactericidal activity of a monoclonal antibody is mediated by phase variation of lgtG and enhanced by a mutator phenotype. Infect. Immun. 76:5038–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett D. E., Mulhall R. M., Cafferkey M. T. 2004. PCR-based assay for detection of Neisseria meningitidis capsular serogroups 29E, X, and Z. J. Clin. Microbiol. 42:1764–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broome C. V. 1986. The carrier state: Neisseria meningitidis. J. Antimicrob. Chemother. 18(Suppl. A):25–34 [DOI] [PubMed] [Google Scholar]
- 5. Buckee C. O., Gupta S., Kriz P., Maiden M. C., Jolley K. A. 2010. Long-term evolution of antigen repertoires among carried meningococci. Proc. Biol. Sci. 277:1635–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callaghan M. J., et al. 2008. Opa protein repertoires of disease-causing and carried meningococci. J. Clin. Microbiol. 46:3033–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cartwright K. A., Stuart J. M., Jones D. M., Noah N. D. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caugant D. A. 2008. Genetics and evolution of Neisseria meningitidis: importance for the epidemiology of meningococcal disease. Infect. Genet. Evol. 8:558–565 [DOI] [PubMed] [Google Scholar]
- 9. Caugant D. A., Hoiby E. A., Rosenqvist E., Froholm L. O., Selander R. K. 1992. Transmission of Neisseria meningitidis among asymptomatic military recruits and antibody analysis. Epidemiol. Infect. 109:241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a. Caugant D. A., Tzanakaki G., Kriz P. 2007. Lessons from meningococcal carriage studies. FEMS Microbiol. Rev. 31:52–63 [DOI] [PubMed] [Google Scholar]
- 10. Caugant D. A., Maiden M. C. 2009. Meningococcal carriage and disease—population biology and evolution. Vaccine 27:B64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caugant D. A., Tzanakaki G., Kriz P. 2007. Lessons from meningococcal carriage studies. FEMS Microbiol. Rev. 31:52–63 [DOI] [PubMed] [Google Scholar]
- 12. De Wals P., et al. 1983. Longitudinal study of asymptomatic meningococcal carriage in two Belgian populations of schoolchildren. J. Infect. 6:147–156 [DOI] [PubMed] [Google Scholar]
- 13. Evans N. J., et al. 2010. Variation and molecular evolution of HmbR, the Neisseria meningitidis haemoglobin receptor. Microbiology 156:1384–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feavers I. M., Pizza M. 2009. Meningococcal protein antigens and vaccines. Vaccine 27:B42–B50 [DOI] [PubMed] [Google Scholar]
- 15. Glitza I. C., et al. 2008. Longitudinal study of meningococcal carrier rates in teenagers. Int. J. Hyg. Environ. Health 211:263–272 [DOI] [PubMed] [Google Scholar]
- 16. Gupta S., et al. 1996. The maintenance of strain structure in populations of recombining infectious agents. Nat. Med. 2:437–442 [DOI] [PubMed] [Google Scholar]
- 17. Jolley K. A., Brehony C., Maiden M. C. 2007. Molecular typing of meningococci: recommendations for target choice and nomenclature. FEMS Microbiol. Rev. 31:89–96 [DOI] [PubMed] [Google Scholar]
- 18. Jordens J. Z., Williams J. N., Jones G. R., Christodoulides M., Heckels J. E. 2004. Development of immunity to serogroup B meningococci during carriage of Neisseria meningitidis in a cohort of university students. Infect. Immun. 72:6503–6510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. MacLennan J., et al. 2006. Social behavior and meningococcal carriage in British teenagers. Emerg. Infect. Dis. 12:950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maiden M. C., et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neal K. R., et al. 2000. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional study. BMJ 320:846–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roussel-Jazede V., Jongerius I., Bos M. P., Tommassen J., van Ulsen P. 2010. NalP-mediated proteolytic release of lactoferrin-binding protein B from the meningococcal cell surface. Infect. Immun. 78:3083–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Russell J. E., Jolley K. A., Feavers I. M., Maiden M. C., Suker J. 2004. PorA variable regions of Neisseria meningitidis. Emerg. Infect. Dis. 10:674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seib K. L., et al. Influence of serogroup B meningococcal vaccine antigens on growth and survival of the meningococcus in vitro and in ex vivo and in vivo models of infection. Vaccine 28:2416–2427 [DOI] [PubMed] [Google Scholar]
- 25. Taha M. K., et al. 2005. Interlaboratory comparison of PCR-based identification and genogrouping of Neisseria meningitidis. J. Clin. Microbiol. 43:144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson E. A., Feavers I. M., Maiden M. C. 2003. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 149:1849–1858 [DOI] [PubMed] [Google Scholar]
- 27. Trotter C. L., Gay N. J. 2003. Analysis of longitudinal bacterial carriage studies accounting for sensitivity of swabbing: an application to Neisseria meningitidis. Epidemiol. Infect. 130:201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Ende A., Hopman C. T., Dankert J. 2000. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect. Immun. 68:6685–6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.