Abstract
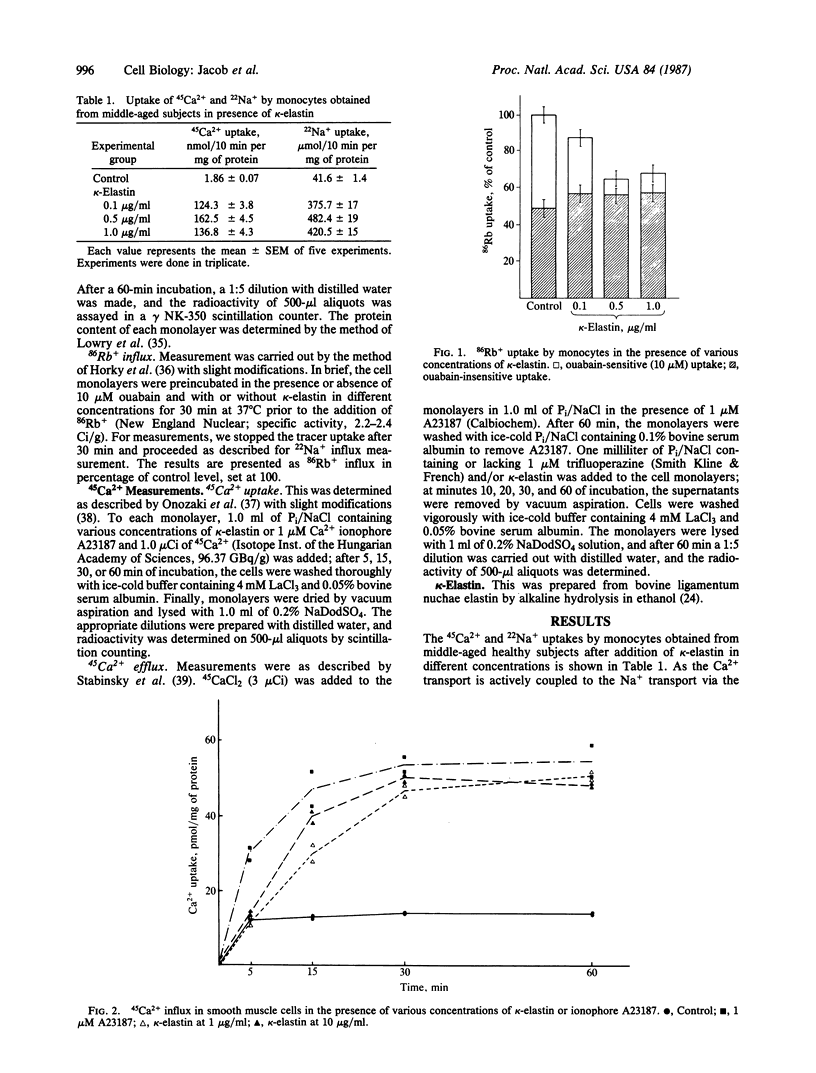
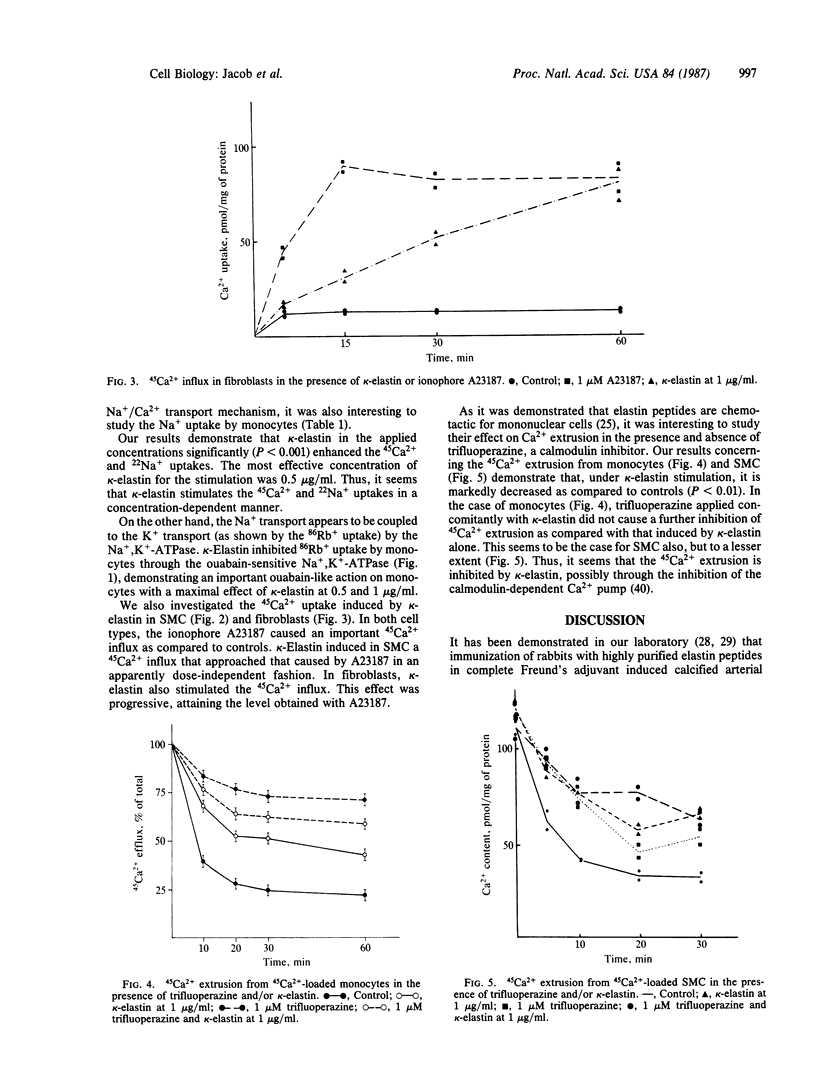
Elastin peptides prepared by alcoholic potassium hydroxide degradation of highly purified fibrous elastin from bovine ligamentum nuchae (kappa-elastin) were shown to act on the ion channels of human monocytes, aorta smooth muscle cells, and skin fibroblasts. In small amounts (between 0.1 and 1 microgram/ml), elastin peptides strongly increased calcium influx and inhibited calcium efflux by an apparently calmodulin-dependent mechanism. They also were shown to increase sodium influx and to decrease rubidium influx in monocyte preparations obtained from human blood. Only the ouabain-sensitive portion of rubidium influx was inhibited. The action of elastin peptides is strongly concentration-dependent; the maximal activity observed in the above reactions was less than 1 microgram/ml. These results suggest that elastin peptides may play a role in the regulation of the biological activity of mesenchymal cells, in the proximity of which they are released by the action of elastase-type enzymes. Such enzymes were demonstrated in aorta smooth muscle cells (membrane-bound serine protease) and in fibroblasts (metalloprotease). Monocytes and polymorphonuclear leukocytes were also shown to carry elastase-type enzymes. The release of peptides from elastin by elastase-type enzymes and the action of such peptides on the ion fluxes through the cell membrane may well be involved in mechanisms of the modulation of the phenotype of mesenchymal cells during aging as well as in the development of age-dependent pathologies such as arterioclerosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein M. P., Hamlyn J. M. Sodium transport inhibition, cell calcium, and hypertension. The natriuretic hormone/Na+-Ca2+ exchange/hypertension hypothesis. Am J Med. 1984 Oct 5;77(4A):45–59. doi: 10.1016/s0002-9343(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Codogno P., Hornebeck W., Goussault Y., Robert L., Aubery M. Elastase activity in chick embryo fibroblasts, quantitative changes induced by agents which affect cell functions and variations during embryogenesis. Cell Biol Int Rep. 1983 Jun;7(6):433–439. doi: 10.1016/0309-1651(83)90132-7. [DOI] [PubMed] [Google Scholar]
- Davis P. W., Vincenzi F. F. Ca-ATPase activation and NaK-ATPase inhibition as a function of calcium concentration in human red cell membranes. Life Sci II. 1971 Apr 8;10(7):401–406. doi: 10.1016/0024-3205(71)90051-8. [DOI] [PubMed] [Google Scholar]
- De Cremoux H., Hornebeck W., Jaurand M. C., Bignon J., Robert L. Partial characterisation of an elastase-like enzyme secreted by human and monkey alveolar macrophages. J Pathol. 1978 Aug;125(4):171–177. doi: 10.1002/path.1711250402. [DOI] [PubMed] [Google Scholar]
- Frances C., Robert L. Elastin and elastic fibers in normal and pathologic skin. Int J Dermatol. 1984 Apr;23(3):166–179. doi: 10.1111/j.1365-4362.1984.tb04506.x. [DOI] [PubMed] [Google Scholar]
- Fóris G., Medgyesi G. A., Hauck M. Bidirectional effect of met-enkephalin on macrophage effector functions. Mol Cell Biochem. 1986 Feb;69(2):127–137. doi: 10.1007/BF00224759. [DOI] [PubMed] [Google Scholar]
- Fülöp T., Fóris G., Wórum I., Leövey A. Age-dependent changes of the Fc gamma-receptor-mediated functions of human monocytes. Int Arch Allergy Appl Immunol. 1984;74(1):76–79. doi: 10.1159/000233520. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Godeau G., Frances C., Hornebeck W., Brechemier D., Robert L. Isolation and partial characterization of an elastase-type protease in human vulva fibroblasts: its possible involvement in vulvar elastic tissue destruction of patients with lichen sclerosus et atrophicus. J Invest Dermatol. 1982 Apr;78(4):270–275. doi: 10.1111/1523-1747.ep12506899. [DOI] [PubMed] [Google Scholar]
- Holian A., Daniele R. P. The role of calcium in the initiation of superoxide release from alveolar macrophages. J Cell Physiol. 1982 Oct;113(1):87–93. doi: 10.1002/jcp.1041130115. [DOI] [PubMed] [Google Scholar]
- Horký K., Schreiber V., Pribyl T. Radioactive 86rubidium influx into red blood cells in essential hypertension in relation to the plasma renin activity. Horm Metab Res. 1984 Jan;16(1):41–45. doi: 10.1055/s-2007-1014689. [DOI] [PubMed] [Google Scholar]
- Hornebeck W., Pignaud G., Legrand Y. J., Robert L. Differentiation of the elastase-type protease of platelets from other elastases. Clin Physiol Biochem. 1984;2(4):166–175. [PubMed] [Google Scholar]
- Jacob M. P., Hornebeck W., Lafuma C., Bernaudin J. F., Robert L., Godeau G. Ultrastructural and biochemical modifications of rabbit arteries induced by immunization with soluble elastin peptides. Exp Mol Pathol. 1984 Oct;41(2):171–190. doi: 10.1016/0014-4800(84)90034-0. [DOI] [PubMed] [Google Scholar]
- Janoff A. Human granulocyte elastase. Further delineation of its role in connective tissue damage. Am J Pathol. 1972 Sep;68(3):579–592. [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Purification of human granulocyte elastase by affinity chromatography. Lab Invest. 1973 Oct;29(4):458–464. [PubMed] [Google Scholar]
- Kornfeld-Poullain N., Robert L. Effet de différents solvants organiques sur la dégradation alcaline de l'élastine. Bull Soc Chim Biol (Paris) 1968;50(4):759–771. [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leake D. S., Hornebeck W., Bréchemier D., Robert L., Peters T. J. Properties and subcellular localization of elastase-like activities of arterial smooth muscle cells in culture. Biochim Biophys Acta. 1983 Nov 22;761(1):41–47. doi: 10.1016/0304-4165(83)90360-4. [DOI] [PubMed] [Google Scholar]
- Legrand Y., Caen J., Booyse F. M., Rafelson M. E., Robert B., Robert L. Studies on a human blood platelet protease with elastolytic activity. Biochim Biophys Acta. 1973 Jun 6;309(2):406–413. doi: 10.1016/0005-2744(73)90039-9. [DOI] [PubMed] [Google Scholar]
- Negendank W., Shaller C. The effect of metabolic inhibition on ion contents and sodium exchange in human lymphocytes. J Cell Physiol. 1982 Mar;110(3):291–299. doi: 10.1002/jcp.1041100312. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE S. M., DAVIS H. F., ADAIR G. S. The chemistry of connective tissues. 2. Soluble proteins derived from partial hydrolysis of elastin. Biochem J. 1955 Sep;61(1):11–21. doi: 10.1042/bj0610011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERT L., POULLAIN N. ETUDES SUR LA STRUCTURE DE L''ELASTINE ET LE MODE D'ACTION DE L''ELASTASE. I. NOUVELLE M'ETHODE DE PR'EPARATION DE D'ERIV'ES SOLUBLES DE L''ELASTINE. Bull Soc Chim Biol (Paris) 1963;45:1317–1326. [PubMed] [Google Scholar]
- Robert A. M., Grosgogeat Y., Reverdy V., Robert B., Robert L. Lésions artérielles produites chez le lapin par immunisation avec l'élastine et les glycoprotéines de structure de l'aorte. Atherosclerosis. 1971 May-Jun;13(3):427–449. doi: 10.1016/0021-9150(71)90084-0. [DOI] [PubMed] [Google Scholar]
- Robert B., Legrand Y., Pignaud G., Caen J., Robert L. Activité élastinolytique associée aux plaquettes sanguines. Pathol Biol (Paris) 1969 Jun-Jul;17(11):615–622. [PubMed] [Google Scholar]
- Robert L., Robert B., Robert A. M. Molecular biology of elastin as related to aging and atherosclerosis. Exp Gerontol. 1970 Dec;5(4):339–356. doi: 10.1016/0531-5565(70)90017-3. [DOI] [PubMed] [Google Scholar]
- Robert L., Stein F., Pezess M. P., Poullain N. Propriétés immunochimiques de l'élastine. Leur importance dans l'athéromatose. Rev Atheroscler (Paris) 1967;9(1):233–241. [PubMed] [Google Scholar]
- Scully S. P., Segel G. B., Lichtman M. A. Calcium exchange and ionized cytoplasmic calcium in resting and activated human monocytes. J Clin Invest. 1984 Aug;74(2):589–599. doi: 10.1172/JCI111456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully S. P., Segel G. B., Lichtman M. A. Plasma membrane vesicles prepared from unadhered monocytes: characterization of calcium transport and the calcium ATPase. Cell Calcium. 1982 Dec;3(6):515–530. doi: 10.1016/0143-4160(82)90042-2. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger K. B. Alteration of cellular calcium metabolism as primary cause of hypertension. Clin Physiol Biochem. 1985;3(4):208–220. [PubMed] [Google Scholar]
- Stabinsky Y., Bar-Shavit Z., Fridkin M., Goldman R. On the mechanism of action of the phagocytosis-stimulating peptide tuftsin. Mol Cell Biochem. 1980 Apr 18;30(2):71–77. doi: 10.1007/BF00227920. [DOI] [PubMed] [Google Scholar]
- Stickle D. F., Daniele R. P., Holian A. Cytosolic calcium, calcium fluxes, and regulation of alveolar macrophage superoxide anion production. J Cell Physiol. 1984 Dec;121(3):458–466. doi: 10.1002/jcp.1041210303. [DOI] [PubMed] [Google Scholar]
- Szendroi M., Meimon G., Bakala H., Frances C., Robert L., Godeau G., Hornebeck W. On the presence of a metalloprotease in human skin fibroblasts that degrades the human skin elastic fiber system. J Invest Dermatol. 1984 Sep;83(3):224–229. doi: 10.1111/1523-1747.ep12263609. [DOI] [PubMed] [Google Scholar]
- Takamori K., Ikeda S., Naito K., Ogawa H. Proteases are responsible for blister formation in recessive dystrophic epidermolysis bullosa and epidermolysis bullosa simplex. Br J Dermatol. 1985 May;112(5):533–538. doi: 10.1111/j.1365-2133.1985.tb15260.x. [DOI] [PubMed] [Google Scholar]



