Abstract
A human astrovirus (HAstV) strain from Kenya was characterized by nucleotide sequence analysis. Sequences from open reading frame 1a (ORF1a) clustered with genotype 6/7, those from ORF1b clustered with genotype 3, and those from ORF2 clustered with genotype 2. A recombination point in the ORF1b-ORF2 junction was identified, with a second possible recombination point within the ORF1a region.
Human astroviruses (HAstVs), in the genus Mamastrovirus of the family Astroviridae, have a single-stranded positive-sense RNA genome of approximately 6.4 kb in length comprised of three open reading frames (ORFs) (16, 19). ORF1a and ORF1b, located at the 5′ end of the genome, are conserved among astroviruses (AstVs) and encode the nonstructural proteins serine protease and RNA-dependent RNA polymerase, respectively. ORF2, located at the 3′ end of the genome, is highly variable and encodes the capsid proteins (16, 24). Eight HAstV serotypes have been defined by immunology-based assays (16, 19, 21). Based on nucleotide sequence analysis of a partial region of the 3′ or 5′ end of ORF2, eight HAstV genotypes have been described, with correlation between antigenic serotypes and genotypes (1, 18, 21, 27). An investigation to genotype HAstVs detected in diarrheal stool specimens, by commercial immunoassay and/or reverse transcriptase PCR (RT-PCR), from Kenyan pediatric patients revealed discordant genotyping results for one of the strains, NK180. The aim of this investigation was to analyze, by nucleotide sequence analysis, this HAstV strain and to compare its genetic relationships to known HAstV genotypes.
Viral nucleic acid was extracted from 120 μl of the HAstV-positive stool suspension using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) and amplified using published primers (Table 1). The RT-PCR conditions used were identical to those described for the detection of HAstVs using type-common primers (1), except that an annealing temperature of 45°C was used to improve amplification. The purified RT-PCR products were cloned using a CloneJet PCR cloning kit (Fermentas Inc., Glen Burnie, MD). At least 10 clones each from the different regions were sequenced using the BigDye Terminator kit (Applied Biosystems, Foster City, CA). The sequence data were assigned the following GenBank accession numbers (in parentheses): ORF1a (FJ842149), ORF1b-ORF2 junction (FJ842147), and ORF2 (FJ842148). Published HAstV gene sequences used in the phylogenetic analysis included the following (GenBank accession numbers are in parentheses): HAstV-1 (L23513), HAstV-2 (L13745), HAstV-3 (AF117209), HAstV-4 (DQ070852 and Z33883), HAstV-5 (DQ028633 and U15136), HAstV-6 (Z46658), HAstV-7 (AF248738), HAstV-8 (AF260508), and newly described KS106210 (AF361035) and KS106209 (AF361034) from Korea (11) and MLB1 (FJ222451) from Australia (4). Sequences from ORF1b/ORF2 for the HAstV-3 to HAstV-6 Oxford strains (AF292074-8), HAstV-7 (AF248738), and an HAstV-8 South African strain (AF292073) were used in further analysis. Nucleotide sequences were aligned and compared using Sequencher 4.7. Multiple sequence alignments were generated by using MAFFT version 6 (http://mafft.cbrc.jp/alignment/server/) and edited using BioEdit version 7.0.9.0 (27 June 2007) (9). Phylogenetic trees were constructed using MEGA software version 4 (25) by both neighbor-joining and maximum parsimony methods. A Recombination Detection Program (RDP version 3.34) and SimPlot (version 3.2) (24) were used to investigate recombination events in the aligned sequences.
Table 1.
Primers used to genotype HAstV strains in this study
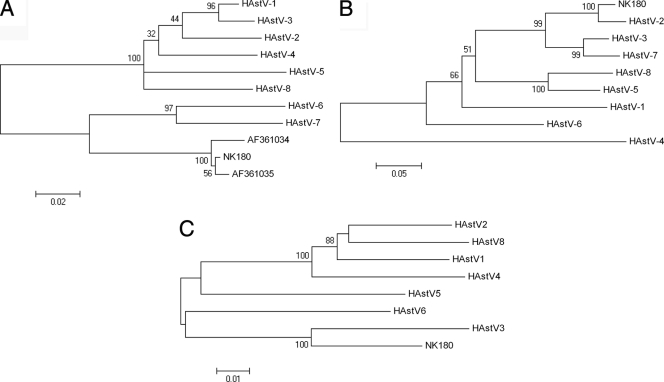
The presence of HAstV in the diarrheal stool specimen was confirmed by RT-PCR. Based on a pairwise comparison of the 266-bp nucleotide sequence from ORF1a, strain NK180 could be assigned to genogroup B (Fig. 1A), while analysis of the 3′ end of ORF2 assigned the same strain to genotype 2 (Fig. 1B), which resorts within genogroup A. Pairwise comparison of the ORF1b region assigned NK180 to genotype 3 (Fig. 1C). The ORF1a region of strain NK180 showed high sequence identity to the HAstV-7 Oxford reference strain (86% of nucleotides and 97.5% of amino acids), but the highest sequence identity was to two as-yet-untyped strains, KS106210 (99% of nucleotides and 100% of amino acids) and KS106209 (98% of nucleotides and 100% of amino acids) (Fig. 1A). Within genogroup B, the Kenyan strain and the two Korean strains clustered separately to the Oxford HAstV-6 and HAstV-7 reference strains (Fig. 1A). The phylogenetic relationship of strain NK180 to HAstV-6 and HAstV-7 was similar to that of Korean strains KA613 and KA-SUN to HAstV-6 and HAstV-7 (11) and is suggestive of a new distinct lineage in genogroup B. Through comparison of the nucleotide sequences from the conserved region of ORF1a, the 3′ end of ORF2, and the ORF1b-ORF2 transition region of strain NK180 with those of AstVs AstV-MLB1, AstV-MLB2, VA1-3, HMO-A, and HMO-B, it was evident that these viruses were unrelated (results not shown).
Fig. 1.
Phylogenetic analysis of different genetic regions of HAstV strain NK180. Neighbor-joining phylogenetic trees of the ORF1a protease region (nt 1182 to 1448) (A), ORF2 capsid region (nt 6459 to 6777) (B), and ORF1b (nt 3676 to 4278) (C) were determined using MEGA version 4. Bars indicate the number of changes per site, and bootstrap percentages are indicated.
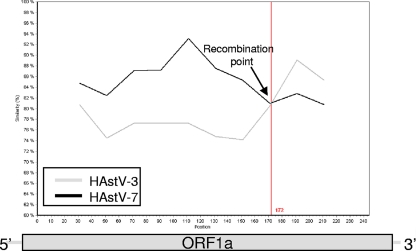
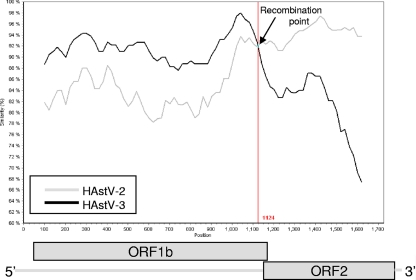
SimPlot analysis (24) of ORF1a showed a possible crossover site at nucleotide (nt) position 1377 (Fig. 2). Nucleotide sequences after the crossover point were highly similar to those of HAstV-3 (83%) and HAstV-7 (81%). However, before the putative crossover point, the homology was notably different, and the SimPlot analysis showed high nucleotide identity to HAstV-7 (86%) but not to HAstV-3 (81%). Nucleotide sequence and similarity plot analysis also identified a putative recombination site within ORF1b-ORF2. Pairwise sequence comparison of the first 1,074 nt at the 5′ end of the ORF1b-ORF2 amplicon (ORF1b) with the HAstV reference strains showed the highest sequence identity to HAstV-3 (92% of nucleotides and 98% of amino acids), while the 593 nucleotides at the 3′ end of the fragment (ORF2) showed the highest sequence identity to HAstV-2 (94% of nucleotides and 99% of amino acids) (results not shown). The recombination site was mapped to the highly conserved 52-nt junction region (bases 4274 to 4328) between ORF1b and ORF2 (Fig. 3). The possibility of a mixed infection was excluded, as all the clones from each genomic region produced identical sequence data. There is only one previously documented record of recombination in HAstVs in which intragenogroup recombination was identified in HAstV strains from Houston, TX, and Mexico City, Mexico (28). In these strains, the recombination point, also in the ORF1b-ORF2 transition region, was between HAstV-3 in ORF1b and HAstV-5 in ORF2. Coinfection with different HAstV antigenic types, as described in epidemiological studies in Japan (15), provides an opportunity for recombination to occur. Although coinfections were not identified in the specimen analyzed in this study, the patient was from a closed setting, i.e., an urban hospice (13), where different HAstV types were cocirculating (data not shown), thus providing the ideal setting for recombination to occur.
Fig. 2.
Analysis of a possible recombination event occurring in the ORF1a region (244 bp) of HAstV strain NK180. The sequence was most similar to that of the HAstV-7 strain (GenBank accession number AF290508) before the recombination site at the 5′ end and that of the HAstV-3 strain (GenBank accession number AF141381.1) after the recombination site at the 3′ end, thereby depicting intergenogroup recombination. The window size was 50 bp, with a step size of 20 bp. The recombination breakpoint (indicated by an arrow) is located at nucleotide position 1377 with respect to the published sequence of HAstV-1 (GenBank accession number L23513).
Fig. 3.
Analysis of the recombination event occurring in the ORF1b-ORF2 junction (1,721 bp) of HAstV strain NK180. The sequence was most similar to that of the HAstV-3 strain (GenBank accession number AF141381.1) before the recombination site at the 5′ end and that of the HAstV-2 strain (GenBank accession number EU327561.1) after the recombination site at the 3′ end, thereby depicting intragenogroup recombination. The window size was 200 bp, with a step size of 20 bp. The recombination breakpoint (indicated by an arrow) is located at nucleotide position 4328 with respect to the published sequence of HAstV-1 (GenBank accession number L23513).
RNA viruses have a tendency toward recombination due to the nature of their polymerase, which naturally shifts frame at the ORF1a-ORF1b junction (15), and various immunological and intracellular constraints can allow a recombinant virus to adapt and rapidly emerge as the predominant population (31). Recombinant viruses have been demonstrated among other single-stranded positive-sense RNA viruses, including those in the families Picornaviridae and Caliciviridae (2, 3, 6, 10, 14, 23, 26). In the family Caliciviridae, novel recombination events in the norovirus (NoV) ORF1-ORF2 overlap (2, 22) and polymerase gene (30) have been described, and a double recombination event within the NoV polymerase region has been described (2).
As HAstVs are important pediatric diarrheal pathogens (7, 17, 20, 29), the application of reliable diagnostic assays and characterization methods are essential for burden of disease investigations. An alternate typing scheme was proposed to address viral virulence studies (8), and a new classification scheme was proposed (12) to accommodate highly divergent HAstVs (AstV-MLB1, AstV-MLB2, VA1-3, HMO-A, and HMO-B) (4, 5, 12). The recent identification of novel HAstVs (4) and human, mink, and ovine-like AstVs (12) and the occurrence of HAstV recombinants may have a significant impact on epidemiological studies. By ignoring the presence of recombination and newly identified HAstVs, phylogenetic data may be skewed and misinterpreted.
Nucleotide sequence accession numbers.
Nucleotide sequence data for ORF1a, the ORF1b-ORF2 junction, and ORF2 have been added to GenBank under accession numbers FJ842149, FJ842147, and FJ842148, respectively.
Acknowledgments
This work was supported by a grant from the South African Co-operation Fund for Scientific Research and Technological Developments of the National Research Foundation in a collaborative project between the Department of Medical Virology, University of Pretoria, Pretoria, South Africa, and the Institute of Primate Research (IPR) and the Kenya Medical Research Institute (KEMRI) (Kenya).
Footnotes
Published ahead of print on 24 November 2010.
REFERENCES
- 1. Belliot G., Laveran H., Monroe S. S. 1997. Detection and genetic differentiation of human astrovirus: phylogenetic grouping varies by coding region. Arch. Virol. 142:1323–1334 [DOI] [PubMed] [Google Scholar]
- 2. Bull R. A., Tanaka M. M., White P. A. 2007. Norovirus recombination. J. Gen. Virol. 88:3347–3359 [DOI] [PubMed] [Google Scholar]
- 3. Costa-Mattioli M., et al. 2003. Evidence of recombination in natural populations of hepatitis A virus. Virology 311:51–59 [DOI] [PubMed] [Google Scholar]
- 4. Finkbeiner S. R., Kirkwood C. D., Wang D. 2008. Complete genome sequence of a highly divergent astrovirus isolated from a child with acute diarrhea. Virol. J. 5:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finkbeiner S. R., et al. 2009. Identification of a novel astrovirus (astrovirus VA1) associated with an outbreak of acute gastroenteritis. J. Virol. 83:10836–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furione M., et al. 1993. Polioviruses with natural recombinant genomes isolated from vaccine-associated paralytic poliomyelitis. Virology 196:199–208 [DOI] [PubMed] [Google Scholar]
- 7. Glass R. I., et al. 1996. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch. Virol. 12(Suppl.):287–300 [DOI] [PubMed] [Google Scholar]
- 8. Guix S., Caballero S., Fuentes C., Bosch A., Pinto R. M. 2008. Genetic analysis of the hypervariable region of the human astrovirus nsP1a coding region: design of a new RFLP typing method. J. Med. Virol. 80:306–315 [DOI] [PubMed] [Google Scholar]
- 9. Hall T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 10. Hansman G. S., Oka T., Katayama K., Takeda N. 2007. Human sapoviruses: genetic diversity, recombination, and classification. Rev. Med. Virol. 17:133–141 [DOI] [PubMed] [Google Scholar]
- 11. Kang Y.-H., Park Y.-K., Ahn J.-B., Yeun J.-B., Jee Y.-M. 2002. Identification of human astrovirus infections from stool samples with diarrhea in Korea. Arch. Virol. 147:1821–1827 [DOI] [PubMed] [Google Scholar]
- 12. Kapoor A., et al. 2009. Multiple novel astrovirus species in human stool. J. Gen. Virol. 90:2965–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiulia N. M., et al. 2007. Astrovirus infection in young Kenyan children with diarrhoea. J. Trop. Pediatr. 53:206–208 [DOI] [PubMed] [Google Scholar]
- 14. Lukashev A. N. 2005. Role of recombination in evolution of enteroviruses. Rev. Med. Virol. 15:157–167 [DOI] [PubMed] [Google Scholar]
- 15. Matsui M., et al. 1998. Determination of serotypes of astroviruses by reverse transcription—polymerase chain reaction and homologies of the types by the sequencing of Japanese isolates. Microbiol. Immunol. 42:539–547 [DOI] [PubMed] [Google Scholar]
- 16. Mendéz E., Arias C. F. 2007. Astroviruses, p. 982–984 In Knipe D. M., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E. (ed.), Fields virology, 5th ed., vol. 1 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 17. Mitchell D. K., et al. 1995. Virologic features of an astrovirus diarrhea outbreak in a day care center revealed by reverse transcriptase-polymerase chain reaction. J. Infect. Dis. 172:1437–1444 [DOI] [PubMed] [Google Scholar]
- 18. Monroe S. S. 2003. Molecular epidemiology of human astroviruses, p. 607–616 In Desselberger U., Gray J. (ed.), Viral gastroenteritis. Elsevier Science B.V., Amsterdam, Netherlands [Google Scholar]
- 19. Monroe S. S., Carter M. J., Herrmann J., Mitchell D. K., Sanchez-Fauquier A. 2005. Astroviridae, p. 859–864 In Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A. (ed.), Virus taxonomy: classification and nomenclature of viruses. Elsevier Academic Press, San Diego, CA [Google Scholar]
- 20. Nadan S., Walter J. E., Grabow W. O. K., Mitchell D. K., Taylor M. B. 2003. Molecular characterization of astroviruses by reverse transcriptase PCR and sequence analysis: comparison of clinical and environmental isolates from South Africa. Appl. Environ. Microbiol. 69:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noel J. S., Lee T. W., Kurtz J. B., Glass R. I., Monroe S. S. 1995. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J. Clin. Microbiol. 33:797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phan T. G., et al. 2006. Viral gastroenteritis and genetic characterisation of recombinant norovirus circulating in eastern Russia. Clin. Lab. 52:247–253 [PubMed] [Google Scholar]
- 23. Phan T. G., Okitsu S., Müller W. E. G., Kohno H., Ushijima H. 2006. Novel recombinant sapovirus, Japan. Emerg. Infect. Dis. 12:865–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ray S. C. 1998. SimPlot for Windows (version 1.6). Baltimore, MD: Distributed by author (http://www.welch.jhu.edu/∼sray/download) [Google Scholar]
- 25. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 26. Tapparel C., et al. 2009. New respiratory enterovirus and recombinant rhinoviruses among circulating picornaviruses. Emerg. Infect. Dis. 15:719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor M. B., et al. 2001. Characterisation of a South African human astrovirus as type 8 by antigenic and genetic analysis. J. Med. Virol. 64:256–261 [DOI] [PubMed] [Google Scholar]
- 28. Walter J. E., et al. 2001. Molecular characterization of a novel recombinant strain of human astrovirus associated with gastroenteritis in children. Arch. Virol. 146:2357–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walter J. E., Mitchell D. K. 2003. Astrovirus infection in children. Curr. Opin. Infect. Dis. 16:247–253 [DOI] [PubMed] [Google Scholar]
- 30. Waters A., Coughlan S., Hall W. W. 2007. Characterisation of a novel recombination event in the norovirus polymerase gene. Virology 363:11–14 [DOI] [PubMed] [Google Scholar]
- 31. Worobey M., Holmes E. C. 1999. Evolutionary aspects of recombination in RNA viruses. J. Gen. Virol. 80:2535–2543 [DOI] [PubMed] [Google Scholar]