Abstract
To determine the prevalence of serotypes of Streptococcus pneumoniae responsible for pneumonia with pleural effusion, we determined the capsular polysaccharide (PS) type directly on 49 pleural fluid specimens collected from pediatric patients during 2007 to 2009 with laboratory-confirmed pneumococcal pneumonia by using monoclonal antibodies and a multiplex, bead array immunoassay. Because the fluids had to be heated to remove nonspecific reactivity before being tested in the immunoassay and type 19A PS is heat labile, the pleural fluid samples were also tested for serotype 19A capsule gene locus by PCR. Use of the multiplex immunoassay combined with type-specific 19A PCR allowed for serotype determination on 40 of 49 pleural fluids. Pneumococcal pneumonia with pleural effusion was associated with a limited number of serotypes, with types 1, 3, 7F/A, and 19A accounting for 75% of the typeable cases. The concentration of capsular PS in the pleural fluids was often greater than 1 μg/ml and sufficient to inhibit the opsonic capacity of sera from individuals who had received the 23-valent pneumococcal PS vaccine. Based on the serotypes observed before and after introduction of the 7-valent pneumococcal conjugate vaccine, the recently licensed 13-valent pneumococcal conjugate vaccine may reduce the incidence of pneumonia with pleural effusions.
Parapneumonic pleural effusions in children are most commonly associated with pneumococcal infections, and they may lead to the more serious complication of empyema (10). With the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in 2000, there has been a dramatic decrease in the number of cases of invasive pneumococcal disease (IPD) in the United States; however, the incidence of empyema has actually increased by as much as 50% (6). To investigate the apparent failure of PCV7 in reducing the incidence of pneumonia with empyema, it is important to investigate pneumococcal serotypes associated with parapneumonic pleural effusions obtained after PCV7 became widely used.
While pneumococcal serotypes associated with pleural effusions have been studied in the past (6), most studies used pneumococcal isolates obtained from blood or pleural fluid cultures of patients; however, this approach is insensitive and may preferentially recover antibiotic-resistant serotypes because most patients are treated with antibiotics and only a small percentage of cases yield positive cultures. To avoid these limitations, two reports investigated pleural fluids directly for the presence of pneumococcal capsular polysaccharide (PS) with monoclonal antibodies (10, 12); however, these studies were performed in England before PCV7 was widely used and they tested the fluids for only 13 capsular PS serotypes.
Recently, we developed a multiplex immunoassay to detect capsular PS of 36 pneumococcal serotypes on cultured isolates (29). The method was modified to detect and quantify the concentration of capsular PS directly in clinical samples such as pleural fluids. We used this modified method to identify pneumococcal serotypes in pleural fluids recently collected from a pediatric population in central Ohio, a geographic region where PCV7 is widely used to immunize children.
MATERIALS AND METHODS
Pleural fluid specimens.
Pleural fluid specimens examined in this study were obtained from children admitted to Nationwide Children's Hospital, Columbus, OH, from 2007 to 2009 with suspected bacterial pneumonia and from whom pleural fluid was obtained as per standard of care by thoracentesis with or without chest tube placement. A laboratory diagnosis of pneumococcal pneumonia was made based on a positive blood or pleural fluid culture or a positive PCR on pleural fluid for both the pneumococcal autolysin and pneumolysin genes (17a). Residual pleural fluid samples were stored at −70°C. A total of 49 positive samples from 49 children aged 0.4 to 15 years (median, 4 years) were removed from storage, coded so as to remove patient identifiers, and sent to the University of Alabama at Birmingham for serotype analysis. This study was approved by the Nationwide Children's Hospital Institutional Review Board.
Multiplex immunoassay for capsular polysaccharide.
Pleural fluid samples were brought to 0.05 M EDTA, and the mixtures were boiled for 10 min (19, 22), diluted 1:10 and 1:30, and subjected to a latex bead-based, multiplexed immunoassay for pneumococcal PS types by using a modification of a previously described procedure for typing of culture isolates (29). Briefly, latex bead sets treated with various red dyes recognizable by their differential fluorescence signals were coated with type-specific pneumococcal capsular PS and mixed with PS-specific monoclonal antibodies and pleural fluid samples. Free PS in the pleural fluid sample binds to free PS-specific monoclonal antibody and thus competitively inhibits specific binding of the free monoclonal antibody to the latex beads coated with the specific PS. The presence of type-specific PS in the pleural fluid was determined by flow cytometry by watching for a reduction in fluorescence signal after washing of the beads and addition of phycoerythrin-conjugated anti-mouse immunoglobulin antibody. The assay has been designed to identify pneumococcal capsular PS of 36 serotypes, including all those in the 23-valent pneumococcal PS vaccine.
To determine capsular PS concentrations in 15 pleural fluid samples, eight 3-fold serial dilutions of the 15 samples and purified PS controls containing 1 μg/ml of purified capsular PS were prepared. The PS controls were purchased from ATCC (Manassas, VA) and included serotypes 1, 3, 6B, 7F, 8, 19A, and 33F. The diluted samples and controls were then subjected to the multiplexed immunoassay, and the capsular PS concentrations in the pleural fluids were determined by comparing the fluorescence signals of the samples at different dilutions to that of the standard curves constructed with the PS controls.
Multiplex opsonophagocytosis assay.
To obtain an antibiotic-free pleural fluid, 100 μl of a pleural fluid sample containing serotype 3 PS (∼20 μg/ml) was loaded into a 250-μl G-25-80 column. The column was eluted with normal saline, and the eluate was collected in 35-μl fractions. The fractions were tested for antibiotic activity and the presence of serotype 3 PS. Fractions that were free of antibiotics, but with type 3 PS levels equivalent to the level in the original pleural fluid, were pooled and used in opsonophagocytosis assays.
The antibiotic-free, serotype 3-containing pleural fluid and a serotype 3 purified PS solution (10 μg/ml) were then separately mixed with an equal volume of a post-pneumococcal vaccination serum pool. The serum pool was prepared by mixing serum from 2 adults previously immunized with a 23-valent PS vaccine. The serum pool and pleural fluid alone, as well as serum pool/type 3 pleural fluid or serum pool/type 3 purified PS mixtures, were analyzed for their capacity to opsonize pneumococci in a multiplexed opsonophagocytosis assay using the HL60 human cell line as phagocytic cells, baby rabbit serum as a source of complement, and OREP3 and STREP8 as target pneumococcal strains (5, 21). OREP3 is an optochin-resistant pneumococcal strain expressing serotype 3 PS, and STREP8 is a streptomycin-resistant strain expressing serotype 8 PS. The opsonic index was defined as the serum dilution that killed 50% of target bacteria.
PCR for pneumococcal serotype 19A.
To identify the 19A-specific capsular gene, bacterial lysates or pleural fluid samples were diluted 10-fold in water and subjected to 2 PCRs—one targeting the pneumococcal wzy gene, the other targeting the cpsA gene—under previously described assay conditions (18). The primers targeting the pneumococcal wzy gene were specific for the 19A serotype, and the primers targeting the cpsA gene were specific for all pneumococcal capsular PS genes and used as a positive control. The reaction products were separated by agarose electrophoresis, stained with ethidium bromide, and visualized under UV light.
RESULTS
Determination of pneumococcal serotypes in pleural fluids.
When the 49 pleural samples were directly analyzed for capsular PS with a multiplexed immunoassay, serotypes could be identified for 36 samples (Table 1). The most common serotype was serotype 3, accounting for 13 samples, and the next most common serotypes were 1, 7F, and 19A, accounting for six, seven, and seven samples, respectively. Pneumococci were isolated from pleural fluids of 5 patients, and the serotypes of the isolates matched the serotypes identified by testing the pleural fluids directly (Table 1). The immunoassay could not identify the serotypes of 13 pleural fluid samples. Since our immunoassay tests for 36 serotypes, the 13 samples may express one of the remaining 57 serotypes that were not included in our multiplex immunoassay. Alternatively, they may contain PS below the limits of detection of our immunoassay.
Table 1.
Pneumococcal serotypes of 49 cases of pneumonia with pleural effusion as determined by multiplex immunoassay directly on pleural fluids
| Serotype | No. of positive samples | No. of positive samples confirmed by isolation of S. pneumoniae from pleural fluidb |
|---|---|---|
| 3 | 13 | 2 |
| 19A | 11 (7 + 4)a | |
| 7F/A | 7 | 1 |
| 1 | 6 | 1 |
| 6B | 1 | 1 |
| 8 | 1 | |
| 33F/A | 1 | |
| Nontypeable | 9 (13 − 4)a | |
| Total | 49 |
Four samples contained nondetectable amounts of capsular PS but were found to contain DNA of serotype 19A capsule synthesis gene locus by PCR.
Serotyping results of all 5 available isolates agreed with direct typing results of pleural fluids.
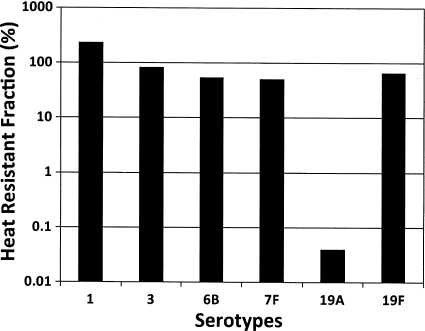
While investigating the limits of detection of the immunoassay, we found that, unlike other pneumococcal PS types tested, type 19A PS is heat labile. A 10-min boiling reduced the immunoreactive concentration of purified 19A PS to less than 0.1% of the original concentration (Fig. 1). Following this discovery, the pleural fluid samples were reexamined for the presence of capsule gene locus of 19A serotype by PCR (18). The PCR test confirmed that all 7 samples previously identified to be 19A and 4 previously nontypeable samples had DNA for 19A capsule gene locus (data not shown).
Fig. 1.
Residual pneumococcal capsular PS (y axis) activity of various PS types (x axis) as determined by a multiplex immunoassay after heat treatment. Heat treatment involved boiling purified PS samples for 10 min with 0.05 M EDTA. PSs were obtained from the ATCC.
Quantification of pneumococcal capsular PS in the pleural fluids.
To determine the PS concentrations in pleural fluids, we selected 15 samples and reanalyzed them without boiling using standard curves constituted with purified pneumococcal PS. Two samples had only about 0.1 μg/ml of capsular PS; however, 7 samples had capsular PS concentrations between 1 and 10 μg/ml, 6 samples had >10 μg/ml, and one serotype 3 sample had 800 μg/ml of PS (Table 2).
Table 2.
Concentrations of pneumococcal PS in 15 pleural fluid samples from patients with pneumococcal pneumonia with effusion
| Sample | Serotype | PS concn (μg/ml) |
|---|---|---|
| 1 | 3 | 0.1 |
| 2 | 3 | 0.1 |
| 3 | 3 | 1.0 |
| 4 | 1 | 1.0 |
| 5 | 7F | 1.0 |
| 6 | 7F | 1.0 |
| 7 | 19Aa | 1.0 |
| 8 | 1 | 8 |
| 9 | 7F | 8 |
| 10 | 19Aa | 19 |
| 11 | 3 | 20 |
| 12 | 7F | 33 |
| 13 | 19Aa | 79 |
| 14 | 1 | 90 |
| 15 | 3 | 800 |
Concentrations of 19A PS were determined with samples that were not heat pretreated.
Effect of capsular PS in pleural fluids on opsonophagocytosis.
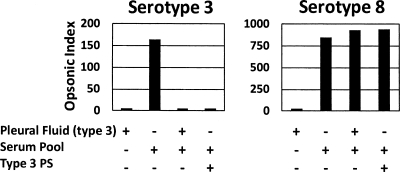
About 0.1 to 1 μg/ml of capsular PS was shown to inhibit the opsonophagocytic capacity of postimmune sera from many vaccine recipients (15; unpublished data). Since most pleural fluids had more than 1 μg/ml of capsular PS (Table 2), we hypothesized that pleural fluids could inhibit the opsonic capacity of immune sera. To directly test this possibility, we selected a serotype 3 pleural fluid sample containing 20 μg/ml PS and of sufficient volume to allow for additional testing. Because this sample, similarly to other pleural fluids, contained antibiotics and was bactericidal (data not shown), it was subjected to column chromatography as described above to remove the antibiotics. When the antibiotic-free pleural fluid containing type 3 pneumococcal PS was mixed with an immune serum pool with a high opsonic index against both serotypes 3 and 8, the opsonic index to serotype 3 was almost completely neutralized while that of serotype 8 was not (Fig. 2). The opsonic index of the serum pool to serotype 3 could be completely neutralized with 5 μg/ml of capsular PS of serotype 3 (left panel). Thus, the pleural fluids of patients with pleural effusions have sufficient capsular PS to neutralize type-specific anticapsular antibodies in vitro.
Fig. 2.
Opsonic index (y axis) of a post-pneumococcal vaccine serum pool tested against a serotype 3 (left panel) and a serotype 8 (right panel) pneumococcal isolate in different sample mixtures. The serum pool had high opsonic activity with respect to both serotypes 3 and 8. Pleural fluid containing serotype 3 PS and purified type 3 PS (5 μg/ml) was used in all experiments.
DISCUSSION
Previous studies have shown that pneumococcal pneumonia with pleural effusion is associated with a limited number of serotypes. Table 3 summarizes 9 published studies of pneumococcal pneumonia with pleural effusion and lists the five most common pneumococcal serotypes isolated in culture. It is possible that these serotype prevalence studies based on typing of culture isolates may be biased toward serotypes that express antibiotic resistance and thus are more likely to be recovered in culture. This is because many patients with suspected community-acquired pneumonia are treated as outpatients with oral antibiotics. Amoxicillin alone or in combination with azithromycin is commonly used to manage pediatric patients without severe pneumonia and without underlying disease. Of those patients who fail oral outpatient therapy and go on to develop pleural effusion and require hospitalization, only a small percentage, typically 10 to 20% in most studies, yield culture isolates. There are reported differences in antibiotic resistance among serotypes.
Table 3.
Summary of 11 published reports showing the five most prevalent pneumococcal serotypes isolated from pleural effusions
| No. of samples | Collection date (yr) | Serotype (% of total) by ranking: |
Sum (%) | Location of study | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||
| 10 | 1975–1978 | 1 (50) | 3 (37.5) | 7F (12.5) | Xa | X | 100 | Pennsylvania | 24 |
| 26 | 1993–1999 | 1 (50.0) | 14 (15.4) | 9V (15.4) | 19F (3.8) | 18C (3.8) | 88.5 | Utah | 6 |
| 133 | 1993–2000 | 14 (29.1) | 1 (24.4) | 19 (9.0) | 3 (8.4) | 6 (8.4) | 79.3 | U.S., multiple centers | 26 |
| 24 | 1996–2000 | 1 (45.8) | 14 (12.5) | 6B (8.3) | 19F (8.3) | 6A (4.2) | 79.2 | Utah | 7 |
| 27 | 1997–2001 | 1 (53.1) | 14 (15.6) | 3 (9.4) | X | X | 78.1b | UK | 10 |
| 11 | 1990–2002 | 1 (62.5) | 4 (25.0) | 5 (12.5) | X | X | 100.0 | Israel | 13 |
| 35 | 2000–2003 | 14 (26.3) | 3 (23.7) | 1 (21.1) | 6B (7.9) | 9V (5.3) | 84.2 | Canada | 17 |
| 30 | 2002–2004 | 19A (26.7) | 1 (23.3) | 14 (13.3) | 3 (10) | 23F (6.7) | 100 | France | 3 |
| 27 | 2003–2004 | 1 (66.7) | 4 (11.1) | 3 (7.4) | 7F (3.7) | 9V (3.7) | 92.6 | UK | 12 |
| 50 | 2001–2005 | 1 (34.0) | 3 (20.0) | 19A (14.0) | 19F (6.0) | 7 (4.0) | 78.0 | Utah | 7 |
| 51 | 2001–2007 | 1 (33.3) | 3 (27.5) | 19A (25.5) | 7F (3.9) | 17 (2.0) | 92.2 | Utah | 6 |
“X” indicates the absence of reported serotypes.
Three samples (11.9%) were negative for the 13 serotypes tested.
With our combination of multiplex immunoassay and 19A PCR performed directly on pleural fluid, we were able to determine the serotype in 40 of 49 (82%) cases of pneumococcal pneumonia with pleural effusion. The 4 most common serotypes in our study, accounting for 75% of typeable cases, were serotypes 1, 3, 7F, and 19A. We cannot draw conclusions on how the 9 nontypeable pleural fluids might have impacted the serotype distribution in our study. Notwithstanding, these same 4 serotypes were also found to be common in another recent study of pneumococcal pneumonia (Table 3) (6), but not all were commonly represented in studies where samples were collected before 2001 (Table 3). These observations suggest that serotype prevalence data based on testing of culture isolates may not be biased toward isolates that express antibiotic resistance.
Furthermore, all 5 of the culture-positive isolates in our study were very susceptible to the beta-lactam agents tested, including penicillin, amoxicillin, and ceftriaxone, with MICs in the 0.01- to 0.04-μg/ml range. Four of five isolates were also susceptible to erythromycin, with MICs of 0.12 μg/ml. Clearly, other factors such as dose and length of oral antibiotic therapy, host immune response, and virulence of the serotype are important in determining which patients develop effusion and which have positive cultures.
The multiplexed immunoassay for typing of pneumococcal PS in pleural fluids required heat pretreatment to remove interfering substances; however, during this processing, we discovered that 19A PS is quite heat labile whereas other PS types, including 19F, are heat stable. This selective heat lability is interesting since 19A and 19F PSs are structurally very similar and a previous study noted that both 19A and 19F PSs were heat labile (25). Of note is that 6A and 6B PSs are very similar in structure but differ in hydrolytic stability (4, 30). It is possible that only the epitope recognized with the 19A-specific monoclonal antibody used in our assay is heat labile and that the heat lability may not be observed with a different 19A-specific monoclonal antibody. Additional studies are being performed to investigate these possibilities. Heat pretreatment is widely used to reduce interfering substances in serum or urine samples before subjecting them to immunoassay (9, 19, 22, 23), and one should be aware that heating may reduce the apparent concentration of certain pneumococcal capsule types.
We also found that the level of capsular PS in pleural fluid is generally greater than 1 μg/ml, which is sufficient to neutralize the opsonic capacity of anticapsular antibodies in vitro. It is possible that free pneumococcal capsular PS in pleural effusions in vivo can neutralize type-specific anticapsular antibodies and thus promote the replication of pneumococci. Neutralization of opsonic capacity by free capsular PS may be an important mechanism of pathogenesis of pleural effusion and progression to empyema. This mechanism may also apply to infections involving limited tissue spaces such as otitis media, and it should be interesting to determine the concentration of capsular PS found in ear fluids from patients with otitis media.
Pleural effusion is a complication of pneumococcal pneumonia, and serotype prevalence among cases of pleural effusions may reflect serotype prevalence in all cases of pneumococcal pneumonia. We believe that serotypes 7F and 19A may be such serotypes. These two serotypes have become more common among cases of IPD following the use of PCV7 as a part of a general shift of serotypes (1, 14, 16). Also, these serotypes have become noticeable among studies of pneumococcal pneumonia with empyema performed in the post-PCV7 era but not among studies performed before introduction of PCV7 (Table 3). Interestingly, serotype 14 was very common, accounting for 12 to 29% of cases in 6 separate studies before introduction of PCV7 (Table 3), but it has not been observed in three studies performed after introduction of PCV7 (6). Similarly, none of the pleural fluid samples collected in the 2007-2009 time period of our study were serotype 14. These data suggest that PCV7 has been effective in preventing pneumonia with pleural effusions due to serotype 14 pneumococci.
PCV7 does not contain serotypes 1 and 3, and it would not have affected their prevalence; however, the incidence of pneumonia with pleural effusions associated with serotypes 1 and 3 has been disproportionately high compared to their pneumonia incidence in many studies (Table 3) (11, 24). Serotype 3 has been known to produce high levels of capsule, and the level of capsular PS may be important in pathogenesis of pneumonia with pleural effusions, as discussed above. Perhaps serotype 3 pneumococci are prone to causing pleural effusions because they can produce high and protective concentrations of capsular PS, overcome host immunity, and proliferate better than other pneumococcal serotypes. Interestingly, the prevalence of serotype 3 is disproportionately higher in otitis media than in IPD in many different geographic regions (20). Perhaps the pleural cavity and the middle ear are body cavities conducive for the high-capsule-producing serotypes.
Serotype 1 pneumococcus has been an uncommon (only 1 to 2%) cause of IPD since the 1960s (11). Yet, it is the primary serotype associated with empyema, accounting for 21 to 67% for 9 studies done in several countries from 1993 until 2007 (Table 3). One may be able to understand this remarkable clinical observation with recent discoveries of immunological properties of zwitterionic PS (2, 8). Zwitterionic PS has both positive and negative charges in its repeating unit, whereas most bacterial capsular PSs have only either neutral or negative charges. Serotype 1 PS is zwitterionic, since its repeating unit has one positively charged amino group and two negatively charged groups, and it has been shown to stimulate T cells (28) and induce intra-abdominal abscess in animal models (27). Induction of abdominal abscess was not unique to serotype 1 PS because zwitterionic PSs from other bacteria can induce abscess formation (27). These biological properties would permit serotype 1 pneumococci to form abscesses and to enhance development of empyema. In fact, serotype 1 has been clinically associated with complicated clinical presentations among empyema cases (26).
While PCV7 appears to have been effective in reducing empyema by serotype 14, our study confirms that PCV7 would be inadequate against current cases of pneumococcal empyema since the vaccine does not cover the serotypes responsible for the majority of cases. Since the recently approved PCV13 includes the four main detected serotypes causing pleural effusions, 1, 3, 7F, and 19A, it may be useful in reducing the incidence of pneumococcal empyema. Future studies should be done to monitor the effect of the new vaccine on empyema among children.
ACKNOWLEDGMENT
The work was supported by Public Health Service grant AI-31473 from the National Institutes of Health to M.H.N.
The University of Alabama at Birmingham owns the intellectual property rights to the monoclonal antibodies described in this work. M.H.N. and J.Y. are employees of the University of Alabama.
Footnotes
Published ahead of print on 1 December 2010.
REFERENCES
- 1. Aguiar S. I., Brito M. J., Goncalo-Marques J., Melo-Cristino J., Ramirez M. 2010. Serotypes 1, 7F and 19A became the leading causes of pediatric invasive pneumococcal infections in Portugal after 7 years of heptavalent conjugate vaccine use. Vaccine 28:5167–5173 [DOI] [PubMed] [Google Scholar]
- 2. Avci F. Y., Kasper D. L. 2010. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 28:107–130 [DOI] [PubMed] [Google Scholar]
- 3. Bekri H., et al. 2007. Streptococcus pneumoniae serotypes involved in children with pleural empyemas in France. Arch. Pediatr. 14:239–243 [DOI] [PubMed] [Google Scholar]
- 4. Bratcher P. E., Kim K. H., Kang J. H., Hong J. Y., Nahm M. H. 2010. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical, and serological characterization. Microbiology 156:555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burton R. L., Nahm M. H. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 13:1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byington C. L., et al. 2010. Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah. J. Clin. Microbiol. 48:520–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byington C. L., et al. 2006. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr. Infect. Dis. J. 25:250–254 [DOI] [PubMed] [Google Scholar]
- 8. Cobb B. A., Kasper D. L. 2005. Zwitterionic capsular polysaccharides: the new MHCII-dependent antigens. Cell. Microbiol. 7:1398–1403 [DOI] [PubMed] [Google Scholar]
- 9. Doskeland S. O., Berdal B. P. 1980. Bacterial antigen detection in body fluids: methods for rapid antigen concentration and reduction of nonspecific reactions. J. Clin. Microbiol. 11:380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eastham K. M., et al. 2004. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax 59:522–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finland M., Barnes M. W. 1977. Changes in occurrence of capsular serotypes of Streptococcus pneumoniae at Boston City Hospital during selected years between 1935 and 1974. J. Clin. Microbiol. 5:154–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fletcher M., Leeming J., Cartwright K., Finn A. 2006. Childhood empyema: limited potential impact of 7-valent pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 25:559–560 [DOI] [PubMed] [Google Scholar]
- 13. Goldbart A. D., et al. 2009. Complicated community acquired pneumonia in children prior to the introduction of the pneumococcal conjugated vaccine. Scand. J. Infect. Dis. 41:182–187 [DOI] [PubMed] [Google Scholar]
- 14. Hicks L. A., et al. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J. Infect. Dis. 196:1346–1354 [DOI] [PubMed] [Google Scholar]
- 15. Hu B. T., et al. 2005. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 12:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang S. S., et al. 2009. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 124:e1–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langley J. M., et al. 2008. Empyema associated with community-acquired pneumonia: a Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect. Dis. 8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a. Marcon M. J., Salamon D., Cuartas J. 2009. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., abstr. D-780 [Google Scholar]
- 18. Pai R., Gertz R. E., Beall B. 2006. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 44:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Requejo H. I. 2007. Community-acquired pneumonia in the childhood: analysis of the diagnostic methods. Braz. J. Infect. Dis. 11:246–248 [DOI] [PubMed] [Google Scholar]
- 20. Rodgers G. L., Arguedas A., Cohen R., Dagan R. 2009. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine 27:3802–3810 [DOI] [PubMed] [Google Scholar]
- 21. Romero-Steiner S., et al. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schaffner A., Michel-Harder C., Yeginsoy S. 1991. Detection of capsular polysaccharide in serum for the diagnosis of pneumococcal pneumonia: clinical and experimental evaluation. J. Infect. Dis. 163:1094–1102 [DOI] [PubMed] [Google Scholar]
- 23. Scott J. A., et al. 1996. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin. Infect. Dis. 22:973–981 [DOI] [PubMed] [Google Scholar]
- 24. Siegel J. D., Gartner J. C., Michaels R. H. 1978. Pneumococcal empyema in childhood. Am. J. Dis. Child. 132:1094–1096 [DOI] [PubMed] [Google Scholar]
- 25. Sweeney J. A., Sumner J. S., Hennessey J. P., Jr 2000. Simultaneous evaluation of molecular size and antigenic stability of PNEUMOVAX 23, a multivalent pneumococcal polysaccharide vaccine. Dev. Biol. (Basel) 103:11–26 [PubMed] [Google Scholar]
- 26. Tan T. Q., et al. 2002. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics 110:1–6 [DOI] [PubMed] [Google Scholar]
- 27. Tzianabos A. O., Onderdonk A. B., Rosner B., Cisneros R. L., Kasper D. L. 1993. Structural features of polysaccharides that induce intra-abdominal abscesses. Science 262:416–419 [DOI] [PubMed] [Google Scholar]
- 28. Velez C. D., Lewis C. J., Kasper D. L., Cobb B. A. 2009. Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation. Immunology 127:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu J., Carvalho M. D. G., Beall B., Nahm M. H. 2008. A rapid pneumococcal serotyping system based on monoclonal antibodies and PCR. J. Med. Microbiol. 57:171–178 [DOI] [PubMed] [Google Scholar]
- 30. Zon G., Szu S. C., Egan W., Robbins J. D., Robbins J. B. 1982. Hydrolytic stability of pneumococcal group 6 (type 6A and 6B) capsular polysaccharides. Infect. Immun. 37:89–103 [DOI] [PMC free article] [PubMed] [Google Scholar]