Abstract
Seven body polishers working in the same “hot spa” presented with multiple red nodules and papules on their hands and forearms. A causative agent was successfully isolated from two of the subjects and from a swab sample collected from the underside of a bed cover in the body-polishing facility. The two cutaneous isolates and the environmental isolate were rapidly growing mycobacteria that formed nonphotochromogenic smooth or smooth/rough colonies on Ogawa egg slants. They were identified as Mycobacterium massiliense by multigenotypic analysis using the 16S rRNA, hsp65, and rpoB genes and the 16S–23S rRNA internal transcribed spacer (ITS) region. However, the use of the 16S rRNA gene sequence and/or DNA-DNA hybridization (DDH Mycobacteria Kit) alone would not distinguish M. massiliense from mycobacteria in the M. chelonae-M. abscessus group. The three isolates were significantly more susceptible to clarithromycin, doxycycline, and minocycline than the M. abscessus and M. bolletii reference strains. One cutaneous isolate and the environmental isolate were in a related cluster by randomly amplified polymorphic DNA PCR (RAPD-PCR). Of the several mycobacterial species found in the day spa, only M. massiliense was isolated from biopsy specimens of the skin lesions, suggesting that this bacterium is a human skin pathogen. This is the first known report of cutaneous M. massiliense infections that could not be attributed to a prior invasive procedure. This is also the first report of M. massiliense infection in Japan.
Mycobacterium massiliense was initially isolated from the sputum of a patient with pneumonia in France in 2004 (1). Epidemiologically, M. massiliense has been recognized as an emerging pathogen in the United States (16, 24) and Brazil, where outbreaks have been associated with postsurgical and cosmetic procedures (2, 4, 22). In Korea, an outbreak was linked to intramuscular injections of an antimicrobial agent (9). This bacterium was also the source of a lethal case of sepsis in Italy and has been found in cystic fibrosis patients in France (15, 20). Among pulmonary M. abscessus group isolates, almost half of the isolates in Korea and 30% of those in the Netherlands are M. massiliense (8, 21). It has been suggested that M. massiliense should be reclassified taxonomically as a subspecies of M. abscessus (11). The clinical significance of differentiating these two species has also been explored (7). However, M. massiliense has not been fully characterized. Although mycobacteria are a frequent source of dermal infection, M. massiliense has never been reported as an etiological agent. This report describes the first case of an M. massiliense dermal infection in Japan.
Case Reports
In November 2007, a 49-year-old female who worked as a body polisher in a hot spa developed multiple red nodules and papules on her hands and forearms. The number of lesions gradually increased over several months, precipitating a visit to a local hospital in June 2008 (case 1). A skin biopsy specimen of a nodule stained with hematoxylin and eosin (H&E) revealed that the lesion was a structured form of granuloma that contained giant cells and infiltrating lymphocytes with necrosis. Acid-fast bacilli were identified by Ziehl-Neelsen staining.
In October 2008, multiple red nodules and papules appeared on the hands and forearms of a 26-year-old female who worked in the same body-polishing facility as the individual with case 1. She visited the same local hospital in December 2008 (case 2) and received similar biopsy results: acid-fast bacilli and granuloma formation with giant cells and infiltrating lymphocytes.
In addition to cases 1 and 2, in the same spa during the same period, there were five more puzzling cases of body polishers with similar symptoms. Three of these patients (with cases 3 to 5) visited the hospital. However, the presence of acid-fast bacilli was not confirmed, even after the observation of granulomas in the skin biopsy specimen of case 3. In April 2009, environmental sampling was conducted at this hot spa in order to discover the causative agent(s).
MATERIALS AND METHODS
Identification and characterization of isolates.
Skin samples were decontaminated with N-acetyl-l-cysteine sodium hydroxide (NALC-NaOH) (13). Briefly, an equal volume of NALC-NaOH solution (2% NaOH, 1.45% sodium citrate, 0.5% NALC) was added to as much as 10 ml of a skin specimen homogenized in normal saline. The mixture was vortexed and allowed to stand for 15 to 20 min before neutralization with sterile 0.067 M phosphate buffer (pH 6.8), to a final volume of 50 ml, and centrifugation at 3,000 rpm for 20 min. The supernatant was discarded, and the sediment was resuspended in 2 ml of phosphate-buffered saline. Half of the sediment was stored at −80°C, while the other half was used for acid-fast staining and inoculation into a 2% Ogawa egg slant (case 1) or Middlebrook 7H9 broth enriched with 10% oleic acid-albumin-dextrose-catalase (OADC; Nippon Becton Dickinson, Fukushima, Japan) (7H9 broth) (case 2). Mycobacterial isolates were subcultured on Middlebrook 7H11 agar plates enriched with 10% OADC (Nippon Becton Dickinson) for more than 3 days at 36.5°C.
A total of 15 environmental samples were collected from the body-polishing facility in sterile containers or bags. There were four water samples from different bathtubs, eight swab samples, and three scurf scrub equipment samples (two gloves and one brush). All samples were centrifuged at 3,000 rpm for 20 min to concentrate any organisms; the swab and equipment samples were stirred in sterile normal saline before centrifugation. Following centrifugation, precipitated samples were resuspended in normal saline and were added to 1.5 volume of 1 N hydrogen chloride. After incubation for 20 min, the samples were neutralized with 1 N NaOH. The mixture was centrifuged at 3,000 rpm for 20 min, and the sediment was resuspended in 1 ml of phosphate-buffered saline (5). Suspensions were inoculated onto 2% Ogawa egg slants or into 7H9 broth and were incubated at 36.5°C. Mycobacterial isolates were subcultured on Middlebrook 7H11 agar for more than 3 days at 36.5°C. The characteristics of the cultured isolates were determined as described previously (3).
DNA-DNA hybridization.
DNA-DNA hybridization was performed with a DDH Mycobacteria Kit (Kyokuto Pharmaceutical Industrial Co., Tokyo, Japan) to identify mycobacterial species (10). In brief, one-half loopful of a mycobacterial colony was used for the test. Biotin-labeled denatured DNA was extracted from a colony and was distributed into the wells of a microdilution plate where the single stranded DNA from 18 reference strains had been immobilized. After a 2-h hybridization at 55°C, hybridized DNA was detected with peroxidase-conjugated streptavidin and the substrate tetramethylbenzidine. The optical density at 630 nm was measured for each well within 30 min. The labeled strain was identified as one of the 18 species when the maximum color intensity was 1.9 times higher than the intensity of the negative control and the second strongest color intensity was lower than 70% of the maximum color intensity.
DNA extraction.
One loopful of a mycobacterial colony on solid medium was suspended in 400 μl sterilized phosphate-buffered saline supplemented with 0.05% Tween 80 and was stored at −80°C until DNA was extracted. A frozen mycobacterial sample was crushed in a bead-beating instrument (Magnalizer; Roche Diagnostics) at 3,000 rpm for 90 s with zirconia beads (diameter, 2 mm). Total genomic DNA was purified from the crushed suspension using the High Pure PCR template preparation kit according to the manufacturer's instructions (Roche Diagnostics) and was stored at −20°C.
Sequence and phylogenetic analysis.
Sequences of clinical and environmental isolates, which had been preliminarily identified as M. abscessus by the DDH Mycobacteria Kit, were compared to those of the reference strains M. massiliense JCM 15300T, M. chelonae JCM 6388T, M. abscessus JCM 13569T, and M. bolletii JCM 15297T, obtained from the Japan Collection of Microorganisms of the Riken BioResource Center (BRC-JCM; Saitama, Japan). The majority of the 16S rRNA gene, the partial hsp65 and rpoB genes, and the internal transcribed spacer (ITS) region between the 16S and 23S rRNA genes were amplified by PCR using AmpliTaq Gold polymerase (Applied Biosystems, Foster City, CA) with the primers listed in Table 1. Both strands were sequenced with the BigDye Terminator cycle sequencing kit, version 3.1 (Applied Biosystems), and were run on the ABI Prism 310 genetic analyzer (Applied Biosystems) (13). Analyses were performed after removal of the primers from the sequences.
Table 1.
Primers used in this study
| Primer | Sequence (positions) | Target and/or purpose (amplified fragment size) | Reference |
|---|---|---|---|
| 8F16S | 5′-AGAGTTTGATCCTGGCTCAG-3′ (8–27)a | 16S rRNA gene, PCR (ca. 1,500 bp), sequencing | 17 |
| 1047R16S | 5′-TGCACACAGGCCACAAGGGA-3′ (1047–1028)a | ||
| 830F16S | 5′-GTGTGGGTTTCCTTCCTTGG-3′ (830–849)a | ||
| 1542R16S | 5′-AAGGAGGTGATCCAGCCGCA-3′ (1542–1523)a | ||
| TB11 | 5′-ACCAACGATGGTGTGTCCAT-3′ | hsp65, PCR (441 bp), sequencing | 19 |
| TB12 | 5′-CTTGTCGAACCGCATACCCT-3′ | ||
| MabrpoF | 5′-GAGGGTCAGACCACGATGAC-3′ (2112–2131)b | rpoB, PCR (449 bp), sequencing | This study |
| MabrpoR | 5′-AGCCGATCAGACCGATGTT-3′ (2559–2541)b | ||
| ITSF | 5′-TTGTACACACCGCCCGTC-3′ | 16S–23S ITS region, PCR (ca. 340 bp), sequencing | 14 |
| ITSR | 5′-TCTCGATGCCAAGGCATCCACC-3′ | ||
| OPA2 | 5′-TGCCGAGCTG-3′ | RAPD-PCR | 25 |
| OPA18 | 5′-AGGTGACCGT-3′ | ||
| INS-2 | 5′-GCGTAGTGCGTCGGTGACAAA-3′ |
Nucleotide positions were assigned using the Escherichia coli 16S rRNA gene sequence as a reference.
Primer design and nucleotide positions were based on the M. tuberculosis rpoB gene sequence (GenBank/EMBL/DDBJ accession no. L27989).
Similarity searches were performed in the DNA Data Bank of Japan (DDBJ) (6). Phylogenetic analyses were performed using the MEGA software package, version 4.0.2 (Build no. 4028) (18). The tree was constructed using the neighbor-joining method with Kimura's two-parameter distance correction model with 1,000 bootstrap replications.
RAPD-PCR.
Randomly amplified polymorphic DNA PCR (RAPD-PCR) (25) was performed with three random primers in order to compare clinical and environmental isolates with the M. massiliense JCM 15300T reference strain (Table 1). In brief, 50 μl of a mixture containing 60 mM Tris-HCl (pH 9.0), 2.5 mM MgCl2, 15 mM (NH4)2SO4, 250 μM each deoxynucleoside triphosphate (dNTP), 50 pmol of the primer, 1 U of Taq DNA polymerase (Takara Bio Inc., Japan), and 100 ng of total genomic DNA, which was freshly extracted or stored for as long as 30 days at −20°C, was used for the PCR. Amplification was performed in the Takara PCR thermal cycler SP using 40 cycles of 94°C for 1 min, 36°C for 1 min, and 72°C for 2 min. The PCR products were separated in the same run by 2% agarose gel electrophoresis and ethidium bromide staining. Strains were assigned to the same cluster when the same band patterns were observed with the three primers or one major band difference was observed in only one of the three primers.
Drug susceptibility assays.
Drug susceptibility assays were performed with 7H9 broth microdilutions according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (23), with a modification in drug choice for rapidly growing mycobacteria. Amikacin (AMK), azithromycin (AZM), ciprofloxacin (CIP), clofazimine (CLF), clarithromycin (CLR), doxycycline (DOX), meropenem (MEM), minocycline (MIN), and panipenem (PAPM) were tested against the clinical and environmental isolates and the M. abscessus, M. massiliense, and M. bolletii reference strains. AZM was provided by Pfizer Japan Inc.; MEM and PAPM were provided by Dainippon Sumitomo Pharma Co. Ltd. and Daiichi Sankyo Co. Ltd., respectively; and the other drugs were purchased from Sigma-Aldrich Co. MIC testing was carried out in triplicate on different days, with two of three matching MICs used as the criteria for MIC determination. Susceptibility was evaluated according to the CLSI breakpoint recommendations.
Nucleotide sequence accession numbers.
The DNA sequences of the 16S rRNA (1,468 bp), hsp65 (401 bp), rpoB (409 bp), and ITS (298 bp) fragments from the reference strains (M. massiliense JCM 15300T, M. chelonae JCM 6388T, M. abscessus JCM 13569T, and M. bolletii JCM 15297T) and the clinical and environmental isolates have been deposited in the International Nucleotide Sequence Databases (INSD) through the DDBJ under accession numbers AB548592 to AB548611.
RESULTS
Isolation from skin and environmental samples.
Bacteria isolated from the skin biopsy specimens of cases 1 and 2 were provisionally identified as M. abscessus by the DDH Mycobacteria Kit. None of the four environmental samples from the bathtubs yielded mycobacteria. However, mycobacteria grew from four swabs and two gloves used for the scurf scrub. The swab isolate from the underside of the bed cover in the body-polishing room was tentatively identified as M. abscessus by the DDH Mycobacteria Kit. The five remaining mycobacterial isolates included M. nonchromogenicum, from the stone wall of the body-polishing room; M. terrae, from a glove; and three M. fortuitum isolates (one from the spring spout, one from the wood wall of the body-polishing room, and one from a glove).
The clinical and environmental (bed cover) isolates were rapidly growing mycobacteria that formed nonphotochromogenic colonies at 25 to 37°C on 2% Ogawa egg slants and 7H11 agar plates but did not grow at 42°C. The isolates were negative for niacin, nitrate reduction, and Tween 80 hydrolysis and were positive for 5% NaCl tolerance, arylsulfatase (3 days), catalase, and urease. However, differences in colony morphology were observed: isolate 1 and the environmental isolate formed smooth colonies, while isolate 2 produced rough colonies.
Genotypic analysis.
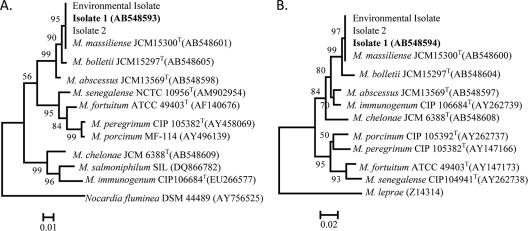
Nucleotide sequence analysis was performed with the three isolates and four reference strains (M. abscessus, M. massiliense, M. bolletii, and M. chelonae). The sequences of the 1,468-bp fragment of the 16S rRNA gene from the three isolates were identical. Only single or triple mismatches with M. abscessus, M. massiliense, and M. bolletii, or with M. chelonae, respectively, were found at nucleotide positions 1008 or 999, 1039, and 1265. The sequences of hsp65, rpoB, and the ITS region were also identical among the three isolates, showed complete identity with those of M. massiliense, and were 89.9 to 99.3% similar to those of M. abscessus, M. bolletii, and M. chelonae (Table 2). Phylogenetic trees, developed using sequences from the hsp65 and rpoB genes, clustered the isolates with M. massiliense (Fig. 1), although the clustering was not as clear with trees developed using sequences from the 16S rRNA gene and the 16S-23S rRNA ITS region (data not shown). Confirmation of these three isolates as M. massiliense led to the supposition that M. massiliense might be the underlying cause of the cutaneous lesions and that the environment of the day spa led to the acquisition of the infections.
Table 2.
Similarities of nucleotide sequences between case isolates and reference strains of closely related mycobacterial species
| Isolate | Species for comparisona | % Identity |
|||
|---|---|---|---|---|---|
| 16S rRNA (1,468 bp) | hsp65 (401 bp) | rpoB (409 bp) | ITS (298 bp) | ||
| Isolate 1 | M. abscessus | 99.9 | 98.8 | 97.6 | 99.0 |
| M. massiliense | 99.9 | 100 | 100 | 100 | |
| M. bolletii | 99.9 | 99.3 | 98.3 | 99.0 | |
| M. chelonae | 99.8 | 92.5 | 96.1 | 89.9 | |
| Isolate 2 | M. abscessus | 99.9 | 98.8 | 97.6 | 99.0 |
| M. massiliense | 99.9 | 100 | 100 | 100 | |
| M. bolletii | 99.9 | 99.3 | 98.3 | 99.0 | |
| M. chelonae | 99.8 | 92.5 | 96.1 | 89.9 | |
| Environmental isolate | M. abscessus | 99.9 | 98.8 | 97.6 | 99.0 |
| M. massiliense | 99.9 | 100 | 100 | 100 | |
| M. bolletii | 99.9 | 99.3 | 98.3 | 99.0 | |
| M. chelonae | 99.8 | 92.5 | 96.1 | 89.9 | |
Reference strains used for comparison were M. abscessus JCM 13569T, M. massiliense JCM 15300T, M. bolletii JCM 15297T, and M. chelonae JCM 6388T.
Fig. 1.
Phylogenetic analysis based on the hsp65 (A) and rpoB (B) genes of isolate 1 (boldface) and other rapidly growing mycobacteria. The numbers at the nodes are the percentages of bootstrap levels supported by 1,000 resampled data sets. Bootstrap values of <50% are not shown. Nocardia fluminea (A) and M. leprae (B) were used as outgroups.
Randomly amplified polymorphic DNA PCR.
Strain typing was performed by RAPD-PCR with three random primers to clarify the relatedness of the clinical and environmental M. massiliense isolates. A comparison of the OPA2 band patterns (Fig. 2, lanes 1 to 4) revealed distinct differences in the amplification patterns of the clinical isolates versus the M. massiliense reference strain. The patterns of isolate 1 and the environmental isolate differed by a minor band. The OPA18 and INS-2 band patterns of isolate 1 and the environmental isolate were identical or differed by only one minor band, though these band patterns were clearly different between the clinical isolates and the reference strain (Fig. 2, lanes 5 to 8 and 9 to 12). Therefore, isolate 1 and the environmental isolate were assigned to the same cluster by RAPD-PCR analysis but were different from isolate 2.
Fig. 2.
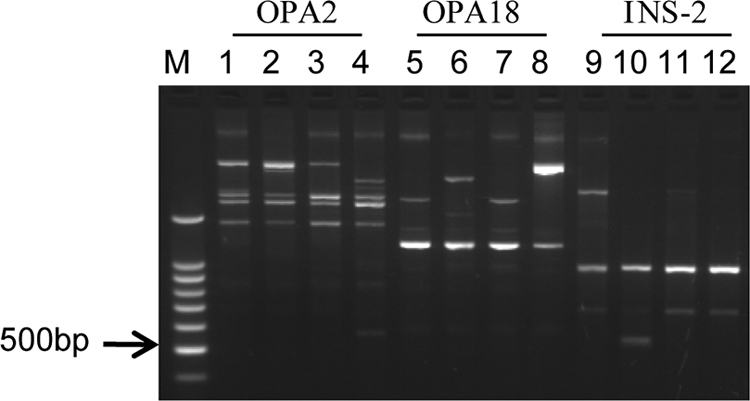
Comparison of the RAPD-PCR patterns of the clinical isolates (isolates 1 and 2), the environmental isolate, and a reference strain (M. massiliense JCM 15300T) with three different primers. Lanes 1, 5, and 9, DNA from isolate 1; lanes 2, 6, and 10, DNA from isolate 2; lanes 3, 7, and 11, DNA from the environmental isolate; lanes 4, 8, and 12, DNA from the M. massiliense reference strain; lane M, DNA size marker (100-bp ladder). RAPD-PCR patterns produced with primers OPA2 (lanes 1 to 4), OPA18 (lanes 5 to 8), and INS-2 (lanes 9 to 12) are shown.
Assays for susceptibility to antimicrobial agents.
The results of tests of the susceptibilities of the clinical and environmental isolates to antimicrobial agents are shown in Table 3. All three isolates exhibited susceptibility patterns similar to that of the M. massiliense reference strain (1, 11, 16), such as susceptibility to clarithromycin, minocycline, doxycycline, and amikacin and resistance to ciprofloxacin. The strains were also tested against azithromycin, clofazimine, meropenem, and panipenem, though these were not on the list of CLSI-recommended drugs (23). Notably, the MICs of azithromycin for the three isolates and the M. massiliense reference strain were lower than those for the M. abscessus and M. bolletii reference strains. No differences in the MIC were observed with clofazimine, meropenem, and panipenem.
Table 3.
Results of drug susceptibility tests
| Antimycobacterial druga | MIC (μg/ml) for: |
|||||
|---|---|---|---|---|---|---|
| Isolate 1 | Isolate 2 | Environmental isolate | M. massiliense JCM 15300T | M. abscessus JCM 13569T | M. bolletii JCM 15297T | |
| AMK | 16 | 16 | 16 | 16 | 16 | 16 |
| AZM | 16 | 32 | 16 | 16 | 64 | 128 |
| CIP | 8 | 16 | 8 | 8 | 8 | 8 |
| CLF | 1 | 2 | 1 | 2 | 1 | 2 |
| CLR | 0.25 | 0.25 | 0.25 | 0.25 | 4 | 4 |
| DOX | 2 | 8 | 1 | 1 | 64 | 64 |
| MEM | 8 | 8 | 16 | 8 | 16 | 8 |
| MIN | 1 | 2 | 0.5 | 0.5 | 16 | 8 |
| PAPM | 64 | 32 | 64 | 64 | 64 | 64 |
AMK, amikacin; AZM, azithromycin; CIP, ciprofloxacin; CLF, clofazimine; CLR, clarithromycin; DOX, doxycycline; MEM, meropenem; MIN, minocycline; PAPM, panipenem.
DISCUSSION
In 2004, M. massiliense was proposed as a new species in the M. chelonae-M. abscessus group (1). Its 16S rRNA gene had complete identity with that of M. abscessus and more than 99.6% similarity with the M. chelonae and M. immunogenum genes. Therefore, genotypic analysis using single-target sequencing of the 16S rRNA gene would not distinguish M. massiliense from other mycobacteria in the M. chelonae-M. abscessus group. Two independent groups have reported on the inaccuracy of single-target sequencing for the diagnosis of M. massiliense (11, 12). Similarly, the DDH Mycobacteria Kit could not distinguish M. massiliense from M. abscessus, because the objective species of this kit were limited to 18 mycobacterial species: M. tuberculosis, M. kansasii, M. marinum, M. simiae, M. scrofulaceum, M. gordonae, M. szulgai, M. avium, M. intracellulare, M. gastri, M. xenopi, M. nonchromogenicum, M. terrae, M. triviale, M. fortuitum, M. chelonae, M. abscessus, and M. peregrinum. However, with this kit, the one isolate provisionally identified as M. abscessus was easily distinguished from several environmental surveillance mycobacterial isolates.
The appearance of skin lesions among day spa workers led to the collection and analysis of workplace environmental samples. Although environmental surveillance was performed several months after case 1 first presented with symptoms, RAPD-PCR showed that isolate 1 and the environmental isolate were part of the same cluster (Fig. 2). In contrast, the same analysis revealed that isolate 2 belonged to a different cluster. The relationship between isolate 1 and the environmental isolate suggests that the unhygienic conditions in the day spa led to the acquisition of the infections, but the cause and effect could not be resolved, because the origin of isolate 2 was not specified. RAPD-PCR typing also showed that the M. massiliense reference strain isolated in France had a different amplification pattern, which was indicative of the geographical distinction between the Japanese and French isolates.
Based on published reports, this is the first presentation of cutaneous M. massiliense infections that were not preceded by an invasive procedure. M. massiliense may be more pathogenic to human skin than other species, since only M. massiliense was isolated from the skin biopsy specimens, though several species of mycobacteria were isolated from the day spa facility. The antimicrobial susceptibility profile of M. massiliense is shown in Table 3. Further studies are required to determine if the profile differs from those of other members of the M. chelonae-M. abscessus group and if any differences can be used as a typing tool. Interestingly, the MICs of azithromycin, clarithromycin, doxycycline, and minocycline for both clinical isolates, the environmental isolate, and the M. massiliense reference strain were much lower than those for the M. abscessus and M. bolletii reference strains. Reinvestigation of the genotypic and drug susceptibility characteristics of the M. chelonae-M. abscessus group is needed. However, some differences in drug susceptibilities have been described that may allow clinicians to differentiate M. massiliense from other mycobacteria in the M. chelonae-M. abscessus group and to design specific therapies targeting the organism (1, 11, 16). Further study is needed to document the clinical features of, and treatment options for, cutaneous M. massiliense infection.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor, and Welfare of Japan (for Y.H., M.M., and N.I.) and by a Grant-in-Aid for Scientific Research (C) from The Ministry of Education, Culture, Sport, Science, and Technology of Japan (for Y.H.).
We thank Pfizer Japan Inc., Dainippon Sumitomo Pharma Co. Ltd., and Daiichi Sankyo Co. Ltd. for the kind gifts of AZM, MEM, and PAPM, respectively.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1. Adékambi T., et al. 2004. Amoebal coculture of “Mycobacterium massiliense” sp. nov. from the sputum of a patient with hemoptoic pneumonia. J. Clin. Microbiol. 42:5493–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cardoso A. M., et al. 2008. Emergence of nosocomial Mycobacterium massiliense infection in Goiás, Brazil. Microbes Infect. 10:1552–1557 [DOI] [PubMed] [Google Scholar]
- 3. Della-Latta P., Weitzman I. 1998. Mycobacteriology, p. 169–203 In Isenberg H. D. (ed.), Essential procedures for clinical microbiology, 1st ed ASM Press, Washington, DC [Google Scholar]
- 4. Duarte R. S., et al. 2009. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J. Clin. Microbiol. 47:2149–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eddyani M., et al. 2008. Primary culture of Mycobacterium ulcerans from human tissue specimens after storage in semisolid transport medium. J. Clin. Microbiol. 46:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaminuma E., et al. 2010. DDBJ launches a new archive database with analytical tools for next-generation sequence data. Nucleic Acids Res. 38(Database issue):D33–D38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim H. Y., et al. 2010. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol. Immunol. 54:347–353 [DOI] [PubMed] [Google Scholar]
- 8. Kim H. Y., et al. 2008. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J. Clin. Microbiol. 46:3384–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim H. Y., et al. 2007. Outbreak of Mycobacterium massiliense infection associated with intramuscular injections. J. Clin. Microbiol. 45:3127–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kusunoki S., et al. 1991. Application of colorimetric microdilution plate hybridization for rapid genetic identification of 22 Mycobacterium species. J. Clin. Microbiol. 29:1596–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leao S. C., et al. 2009. Characterization of mycobacteria from a major Brazilian outbreak suggests that revision of the taxonomic status of members of the Mycobacterium chelonae-M. abscessus group is needed. J. Clin. Microbiol. 47:2691–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Macheras E., et al. 2009. Inaccuracy of single-target sequencing for discriminating species of the Mycobacterium abscessus group. J. Clin. Microbiol. 47:2596–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakanaga K., et al. 2007. “Mycobacterium ulcerans subsp. shinshuense” isolated from a skin ulcer lesion: identification based on 16S rRNA gene sequencing. J. Clin. Microbiol. 45:3840–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roth A., et al. 1998. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S–23S rRNA gene internal transcribed spacer sequences. J. Clin. Microbiol. 36:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roux A. L., et al. 2009. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J. Clin. Microbiol. 47:4124–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simmon K. E., et al. 2007. Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J. Clin. Microbiol. 45:1978–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Springer B., et al. 1996. Isolation and characterization of a unique group of slowly growing mycobacteria: description of Mycobacterium lentiflavum sp. nov. J. Clin. Microbiol. 34:1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 19. Telenti A., et al. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tortoli E., Gabini R., Galanti I., Mariottini A. 2008. Lethal Mycobacterium massiliense sepsis, Italy. Emerg. Infect. Dis. 14:984–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Ingen J., de Zwaan R., Dekhuijzen R. P., Boeree M. J., van Soolingen D. 2009. Clinical relevance of Mycobacterium chelonae-abscessus group isolation in 95 patients. J. Infect. 59:324–331 [DOI] [PubMed] [Google Scholar]
- 22. Viana-Niero C., et al. 2008. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. J. Clin. Microbiol. 46:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woods G. L., et al. 2003. Susceptibility testing of mycobacteria, nocardia and other aerobic actinomycetes; approved standard. Document M24-A. Clinical and Laboratory Standards Institute, Wayne, PA: [PubMed] [Google Scholar]
- 24. Zelazny A. M., et al. 2009. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J. Clin. Microbiol. 47:1985–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y., Rajagopalan M., Brown B. A., Wallace R. J., Jr 1997. Randomly amplified polymorphic DNA PCR for comparison of Mycobacterium abscessus strains from nosocomial outbreaks. J. Clin. Microbiol. 35:3132–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]