Abstract
Adenoviruses can cause a broad spectrum of clinical diseases, most of which are self-limited. However, adenovirus infection can occasionally result in severe or lethal infection. Fifty-five adenovirus serotypes are known today, and they are classified into 7 subgroups (subgroups A to G). Here we examined 282 samples derived from hospitalized patients in Israel (September 2006 to August 2008) who were diagnosed as suffering from adenovirus infections. We used a recently described PCR amplification method and subsequent sequencing to identify the adenovirus. In addition, we studied the medical charts of 106 hospitalized patients from Sheba Medical Center in Israel. The most prevalent adenovirus serotypes found were serotypes 1 (22.8%), 2 (19.2%), 7 (18%), and 3 (14%). In addition, we identified several serotypes that have not been identified previously in Israel. Overall, serotypes of subgroup B were found to be approximately 4 times more prevalent among immunocompromised children than among generally healthy children (52.6%; P < 0.007). The realization that the virus subtypes are different among healthy and immunocompromised patients may lead to more efficient treatment of adenovirus infections among immunocompromised children in the future.
Adenoviruses are responsible for approximately 7 to 8% of reported childhood viral respiratory infections worldwide (3, 20). Adenoviruses cause a broad spectrum of clinical disease, including respiratory tract infection, pharyngoconjunctival fever, conjunctivitis, hemorrhagic cystitis, and gastroenteritis. Although most infections are self-limited, adenoviruses have been associated with severe and even fatal infections in both immunocompromised and healthy individuals (6, 22, 27). In the immunocompromised host, adenovirus can cause severe localized disease or disseminated disease with multiorgan failure (2, 11). Case fatality rates for those patients can reach 50 to 80% (1, 2, 11, 20).
There are 55 known human adenoviruses serotypes, classified into 7 species (subgroups A to G) (8, 12, 27, 28).
Certain subgroups and serotypes are more commonly associated with specific disease syndromes, epidemiological settings, and demographic risk groups. For example, serotypes 1, 2, 3, 5, 6, and 7 cause mainly respiratory illness (3), while serotypes 40 and 41 cause mainly gastroenteritis (26). Some studies imply an association between specific serotypes and subgroups and more severe disease presentation (4, 14, 15).
A study conducted between 1988 and 1996 in Chile (15) reported that children infected with adenovirus serotype 7 (subgroup B) had longer hospital stays, a higher frequency of rectal temperatures over 39°C, and a greater need for oxygen support than children infected with adenovirus subgroup C. In addition, certain adenovirus serotypes that also belong to subgroup B, such as serotypes 34, 35, and 11, which are usually rare in the healthy population, are much more common in immunocompromised patients (11, 24).
Recent studies suggested that the in vitro susceptibility to some antiviral drugs such as cidofovir and ribavirin may be serotype specific (19), and therefore, a rapid diagnosis of the specific adenovirus serotype may become essential for proper treatment. Furthermore, adenovirus serotypes 4 and 7 were recognized as being the most common strains responsible for respiratory disease in soldiers. Vaccines have been developed for use against those individual adenovirus serotypes and have been successfully used by the U.S. army for about 25 years (9, 23).
To the best of our knowledge, there is little information about the epidemiology of adenovirus serotypes prevalent in Israel and in the nearby area. To date, adenovirus serotyping in Israel was done retrospectively by using the neutralization method. Only the specific serotypes known to be prevalent in Israel were tested, given that the appropriate antibodies were available. In addition, this serological method is known to be nonspecific due to the cross-reactivity of the different antibodies against the various serotypes.
Recently, an innovative and advanced molecular method for the rapid detection of different adenovirus serotypes was described. This new method is based on the nucleotide sequencing of the adenovirus hexon gene. In 2006, Lu and Erdman demonstrated that by using PCR amplification and sequencing of the areas of HVR1 to HVR6 in the adenovirus hexon gene, almost all of the 51 different serotypes could be identified, excluding serotypes 15 and 19 (16). This method has been adopted by other studies and was noted to be fast, simple, and more precise than the traditional method (10).
We used this new molecular method to characterize adenoviruses serotypes prevalent in hospitalized patients in Israel. In addition, we characterized the morbidity of generally healthy hospitalized children with a positive adenovirus test and identified the serotypes prevalent in immunocompromised versus healthy children.
MATERIALS AND METHODS
Population.
The research was conducted at the National Influenza and Respiratory Virus Center at the Central Virology Laboratory, Sheba Medical Center (MC), Tel Hashomer, Israel. This laboratory serves patients hospitalized at the Sheba MC but also receives samples from other medical centers located in Israel for the diagnosis of respiratory viral infections. Our research included all samples that tested positive for adenoviruses by real-time PCR during the period between September 2006 and August 2008, including samples from patients who were hospitalized at Sheba MC and other medical centers. The research was approved by the Helsinki committee of Sheba Hospital.
DNA extraction and sequencing.
Routinely, the process of adenovirus detection in clinical samples includes DNA extraction using a QIAamp blood minikit (Qiagen, Germany) and real-time PCR. In our study, we further sequenced all PCR-amplified DNA extracts of the adenoviruses using an ABI Prism 3100 Avant genetic analyzer (Applied Biosystems, CA) according to the method described previously by Lu and Erdman (16).
Review of medical records.
In order to characterize the clinical symptoms associated with adenovirus infections in hospitalized children, we studied all computerized medical charts of patients under 18 years of age who were hospitalized at Sheba MC between September 2006 and August 2008 and who had been diagnosed with adenovirus infection.
For each patient the following data were obtained: demographic data (including age upon admission, sex, and city of residence), duration of hospitalization, hospital ward, source of the sample (e.g., throat culture, nasal culture, or stool sample), comorbidities with an emphasis on immunosuppressive diseases or states, symptoms compatible with adenovirus infection (e.g., cough, conjunctivitis, sore throat, diarrhea, vomiting, and dysuria), vital signs at admission (e.g., fever and oxygen saturation in room air), physical findings (e.g., dyspnea, lung crepitations, and obstructive lung findings), laboratory tests (e.g., complete blood count and liver enzymes), imaging tests (e.g., chest X-ray), and documented complications (e.g., apnea, oxygen support, and artificial mechanical ventilation).
Based on the data from the medical charts, the children were divided into two groups: children with and those without substantial underlying diseases which altered their immunological system (immunocompromised versus generally healthy children, respectively). The immunocompromised group included children with the following underlying diseases: solid and hematological malignant tumors, immunosuppressive treatment, genetic syndromes (such as Down's syndrome and fragile X), complex neurological diseases, congenital cardiac defects, chronic lung diseases, prematurity, and metabolic disorders.
Statistical analysis.
We used descriptive statistics to describe the adenoviruses serotypes prevalent in Israel and to characterize the illness caused by them.
A chi-square test and a t test were used for comparisons between categorical and continuous variables, respectively. A P value of <0.05 was considered significant.
RESULTS
Study population.
Between September 2006 and August 2008, our laboratory diagnosed 282 out of 1,542 samples (18.3%) as being positive for adenovirus infections, accounting for 206 patients (76 samples were either repeated samples or from different sites in the same patient). All of these patients were hospitalized at various general hospitals in central Israel.
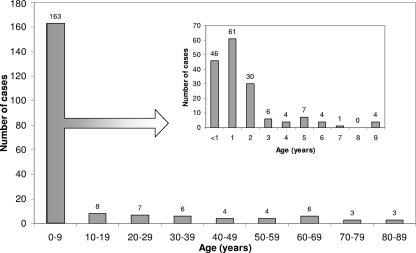
Adenovirus infections were prevalent throughout the year. The disease predominated in children and in males: 137 (66.5%) patients were under the age of 3 years (Fig. 1), and 123 (59.7%) were males (male-to-female ratio, 1.48).
Fig. 1.
Age distribution of 206 hospitalized patients diagnosed with adenovirus infection, September 2006 to August 2008.
Adenovirus subgroups and serotypes.
To gain precise information on the types of adenoviruses present in Israel during the study period, we used the sequencing method outlined in Materials and Methods to type 115 of the 282 (40.8%) adenovirus-positive samples, corresponding to 83 (40.3%) patients.
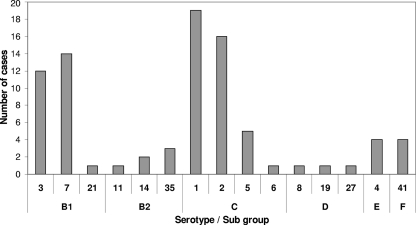
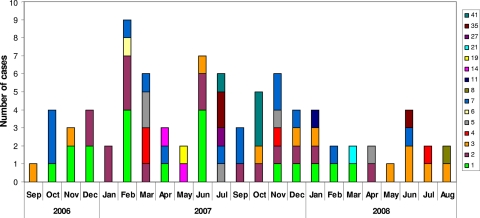
We identified 15 out of the 35 known different serotypes (data not shown). The most common were serotypes 1 (n = 19; 22.9%) and 2 (n = 16; 19.3%) from subgroup C and serotypes 7 (n = 14; 16.9%) and 3 (n = 12; 14%) from subgroup B (Fig. 2). No association was observed between the different serotypes and seasonality, apart from serotype 1, which seemed to be predominant in the fall and winter (Fig. 3).
Fig. 2.
Adenovirus serotype and subgroup distribution of 83 hospitalized patients (September 2006 to August 2008), as identified by PCR followed by sequencing.
Fig. 3.
Adenovirus serotypes of 83 hospitalized patients between September 2006 and August 2008, distributed by month.
Clinical characteristics of hospitalized children.
Medical charts of 106 hospitalized children (under 18 years of age) at Sheba MC during our study period with laboratory-confirmed adenovirus infection were studied, which included 52 (49%) children who were considered to be generally healthy and 54 (51%) children who were immunocompromised.
The characteristics of the generally healthy children are detailed in Table 1. Among these children, the average age of the children upon adenovirus infection was 1.4 ± 2.2 years. A total of 52.8% of the infected children were under the age of 1 year, and 88.6% were under the age of 2 years. The male-to-female ratio was 1.68, compared to 1.05 in the general child population in Israel in those years (7). The majority had a fever above 38°C (57.7%). Respiratory symptoms were found for 38 (73.1%) children, gastrointestinal symptoms were found for 21 (40.4%) children, and conjunctivitis was found for 8 (15.4%) children. Ninety-six percent had complete blood count results. Leukocytosis of over 15,000 cells/mm3 was found for 44% of children. No leukopenia (leukocyte count under 4,000 cells/mm3) was found. Six generally healthy children were admitted due to apnea. Two children needed intensive care unit admission and mechanical ventilation, and one died due to septic shock, disseminated intravascular coagulation, and intracerebral hemorrhage. All six were aged between 2 weeks and 4 months.
Table 1.
Characteristics of generally healthy children diagnosed with adenovirus infection, Sheba Medical Center, September 2006 to August 2008
| Patient characteristic | Value for generally healthy children (n = 52) |
|---|---|
| Avg age (yr) ± SD | 1.4 ± 2.2 |
| Male/female ratio | 1.68 |
| No. (%) of children with fever above 38°C | 30 (57.7) |
| Avg reported body temp (°C) ± SD | 39.1 ± 1 |
| Avg measured body temp at admission (°C) ± SD | 38.2 ± 1.2 |
| No. (%) of children with oxygen saturation: | |
| Below 95% | 18 (34.6) |
| Below 90% | 8 (15.4) |
| No. (%) of children with symptom of: | |
| Cough | 35 (67.3) |
| Rhinorrhea | 26 (50) |
| Diarrhea | 16 (30.8) |
| Vomiting | 13 (25) |
| Conjunctivitis | 8 (15.4) |
| Avg white blood cell count/mm3 ± SD | 15,705 ± 7,630 |
| No. (%) of children with chest X-ray | 28 (54) |
| No. (%) of children with radiological evidence of infiltrates | 13 (25) |
| Avg duration of hospitalization (days) ± SD | 4 ± 1.7 |
Among the immunocompromised children the average age was significantly higher than that among the generally healthy group, 2.7 ± 3.2 years (P < 0.017), and 61.1% were under the age of 2 years. The male-to-female ratio was 1.34 (not significant).
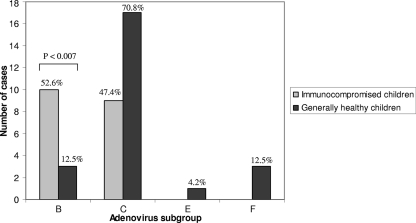
In the generally healthy children, 15 serotypes were identified, and the majority (70.8%) belonged to subgroup C. Serotypes from subgroups B, E, and F were also identified. Among the immunocompromised children, 19 serotypes were identified, and all belonged to both subgroups B (52.6%) and C (47.3%) (Fig. 4). Overall, subgroup B, which was not common among generally healthy children (13%), was very common among immunocompromised children (52.6%) (P < 0.007).
Fig. 4.
Number of hospitalized children at Sheba Medical Center with diagnosed adenovirus infection, by immunological status and adenovirus subgroup, September 2006 to August 2008.
DISCUSSION
The main goal of this study was to characterize the adenovirus serotypes and subgroups prevalent in hospitalized patients in Israel during the study period. We used a new method of PCR amplification followed by sequencing, which allowed us to identify serotypes that have not previously been detected in Israel. For example, an analysis done in Israel in 2005 by our laboratory using the serological method identified serotypes 3 (32.4%) and 7 (13.5%) of subgroup B; serotypes 2 (29.7%), 1 (19%), and 5 (2.7%) of subgroup C; and serotype 4 (2.7%) of subgroup E. In our current research, the 4 most common serotypes remained the same (serotypes 1, 2, 7, and 3) although with a different distribution. In addition, we identified rare serotypes that were not routinely reported in Israel previously, including serotypes 8, 19, and 27 of subgroup D and serotypes 11, 14, 21, and 35 of subgroup B.
A similar study was conducted between 1999 and 2002 in Egypt (18), using serotype-specific primer sets for PCR amplification rather than common primers followed by sequencing of the PCR product. Therefore, rare serotypes were probably not identified. Nevertheless, the most common serotypes were serotypes 7 (58%) and 3 (16%) of subgroup B and serotype 1 of subgroup C (12%), similar to the most common serotypes in Israel but with a different distribution.
The characterization of adenovirus infections among healthy children was consistent with what has been described in the literature. Adenoviruses are known to typically infect very young children, and indeed, in our study, the average age of the hospitalized healthy children was 1.4 ± 2.2 years, similar to the average age described in a study conducted in 2004 at Schneider Children's Medical Center of Israel (1.4 ± 0.8 years) (21). The male-to-female ratio in our study was 1.68. The tendency toward male predominance is known from other previously reported studies of adenoviruses infection (10) as well as from studies of other infectious diseases, such as influenza virus, parainfluenza virus, and respiratory syncytial virus (RSV) (25).
According to our study, the clinical manifestation of adenovirus infection in hospitalized but generally healthy children was cough, fever, rhinorrhea, diarrhea, vomiting, and conjunctivitis, which is consistent with the known literature. Over one-third of the children had an oxygen saturation level below 95%, which is similar to what was reported by other studies.
Six generally healthy infants (11.5%) were admitted to Sheba Medical Center as a result of an apnea event (or events) related to upper respiratory infection. All were also checked for RSV in addition to adenovirus, but only one tested positive for both viruses. One of the infants aged 2 weeks was admitted to the intensive care unit after a severe apnea event and was mechanically ventilated. His respiratory and general condition deteriorated due to septic shock, disseminated intravascular coagulation, and intracerebral hemorrhage, and he eventually died. This infant tested positive for adenovirus serotype 14, a rare serotype that is known to cause severe and sometimes fatal respiratory illness in patients of all ages, including healthy young adults (5). Infections as causes for apnea events among newborns or an “apparent life-threatening event” (ALTE) have been widely studied but mainly in the context of RSV, pertussis, or Mycoplasma infections (13, 17). The possible link between adenoviruses and ALTE has not been sufficiently explored to date, and our findings highlight the need for further evaluations of the role of adenovirus infections in ALTE.
The difference observed between the adenovirus subgroups that were identified among generally healthy children and the subgroups found among immunocompromised has not been previously described. There have been studies that implied that certain serotypes are more common among children with immunological impairment (and are rare in the general population), such as serotypes 11, 34, and 35 of subgroup B (11, 24). Our study demonstrated that the distribution of subgroup B among generally healthy children (13%) is significantly lower than that among immunocompromised children (52.6%; P < 0.007). Thus, the chance of being hospitalized due to adenovirus subgroup B infection is approximately 4 times higher among immunocompromised children. There are not enough data to date to determine whether serotypes of subgroup B are more virulent, as implied by some studies (15), or perhaps, some of these serotypes act as opportunistic infections, which do not cause illness among immunocompetent people but emerge when the immune system is compromised.
In summary, this study enabled us to draw a general picture of the common adenovirus serotypes in hospitalized patients in central Israel from September 2006 to August 2008 and their relative prevalence. The observation that different subtypes are present in healthy versus immunocompromised children may contribute in the future to the development of treatment protocols and may assist in the future when considering possible vaccination of the population.
ACKNOWLEDGMENTS
This work was performed in partial fulfillment of the requirements for an M.D. thesis of Pnina Dror at the Medical School of the Hebrew University of Jerusalem.
Michal Mandelboim and Pnina Dror contributed equally to this study.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1. Abzug M. J., Levin M. J. 1991. Neonatal adenovirus infection: four patients and review of the literature. Pediatrics 87:890–896 [PubMed] [Google Scholar]
- 2. Blanke C., et al. 1995. Evolving pathogens in allogeneic bone marrow transplantation: increased fatal adenoviral infections. Am. J. Med. 99:326–328 [DOI] [PubMed] [Google Scholar]
- 3. Brandt C. D., et al. 1969. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am. J. Epidemiol. 90:484–500 [DOI] [PubMed] [Google Scholar]
- 4. Carballal G., Videla C., Misirlian A., Requeijo P. V., del Carmen Aguilar M. 2002. Adenovirus type 7 associated with severe and fatal acute lower respiratory infections in Argentine children. BMC Pediatr. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 2007. Acute respiratory disease associated with adenovirus serotype 14—four states, 2006-2007. MMWR Morb. Mortal. Wkly. Rep. 56:1181–1184 [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 1998. Civilian outbreak of adenovirus acute respiratory disease—South Dakota, 1997. MMWR Morb. Mortal. Wkly. Rep. 47:567–570 [PubMed] [Google Scholar]
- 7. Central Bureau of Statistics 2008. Statistical abstract of Israel, no. 59. Central Bureau of Statistics, Jerusalem, Israel [Google Scholar]
- 8. Davison A. J., Benko M., Harrach B. 2003. Genetic content and evolution of adenoviruses. J. Gen. Virol. 84:2895–2908 [DOI] [PubMed] [Google Scholar]
- 9. Gaydos C. A., Gaydos J. C. 1995. Adenovirus vaccines in the U.S. military. Mil. Med. 160:300–304 [PubMed] [Google Scholar]
- 10. Gray G. C., et al. 2007. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004-2006. Clin. Infect. Dis. 45:1120–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hierholzer J. C. 1992. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 5:262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones M. S., II, et al. 2007. New adenovirus species found in a patient presenting with gastroenteritis. J. Virol. 81:5978–5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahn A. 2004. Recommended clinical evaluation of infants with an apparent life-threatening event. Consensus document of the European Society for the Study and Prevention of Infant Death, 2003. Eur. J. Pediatr. 163:108–115 [DOI] [PubMed] [Google Scholar]
- 14. Kajon A., Wadell G. 1994. Genome analysis of South American adenovirus strains of serotype 7 collected over a 7-year period. J. Clin. Microbiol. 32:2321–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larranaga C., Kajon A., Villagra E., Avendano L. F. 2000. Adenovirus surveillance on children hospitalized for acute lower respiratory infections in Chile (1988-1996). J. Med. Virol. 60:342–346 [PubMed] [Google Scholar]
- 16. Lu X., Erdman D. D. 2006. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch. Virol. 151:1587–1602 [DOI] [PubMed] [Google Scholar]
- 17. McGovern M. C., Smith M. B. 2004. Causes of apparent life threatening events in infants: a systematic review. Arch. Dis. Child. 89:1043–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metzgar D., et al. 2005. PCR analysis of Egyptian respiratory adenovirus isolates, including identification of species, serotypes, and coinfections. J. Clin. Microbiol. 43:5743–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morfin F., et al. 2005. In vitro susceptibility of adenovirus to antiviral drugs is species-dependent. Antivir. Ther. 10:225–229 [PubMed] [Google Scholar]
- 20. Munoz F. M., Piedra P. A., Demmler G. J. 1998. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin. Infect. Dis. 27:1194–1200 [DOI] [PubMed] [Google Scholar]
- 21. Peled N., et al. 2004. Adenovirus infection in hospitalized immunocompetent children. Clin. Pediatr. (Phila.) 43:223–229 [DOI] [PubMed] [Google Scholar]
- 22. Pichler M. N., Reichenbach J., Schmidt H., Herrmann G., Zielen S. 2000. Severe adenovirus bronchiolitis in children. Acta Paediatr. 89:1387–1389 [DOI] [PubMed] [Google Scholar]
- 23. Russell K. L., et al. 2006. Vaccine-preventable adenoviral respiratory illness in US military recruits, 1999-2004. Vaccine 24:2835–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shields A. F., Hackman R. C., Fife K. H., Corey L., Meyers J. D. 1985. Adenovirus infections in patients undergoing bone-marrow transplantation. N. Engl. J. Med. 312:529–533 [DOI] [PubMed] [Google Scholar]
- 25. Tsai H., Kuo P., Liu C., Wang J. 2001. Respiratory viral infections among pediatric inpatients and outpatients in Taiwan from 1997 to 1999. J. Clin. Microbiol. 39:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uhnoo I., Wadell G., Svensson L., Johansson M. E. 1984. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J. Clin. Microbiol. 20:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walls T., Shankar A. G., Shingadia D. 2003. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect. Dis. 3:79–86 [DOI] [PubMed] [Google Scholar]
- 28. Walsh M. P., et al. 2010. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J. Clin. Microbiol. 48:991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]