Abstract
Tuberculosis (TB) is rarely observed in cystic fibrosis (CF) patients. We report the first case of mediastinal TB, associated with leg pain and skin rash, in an adult patient with CF, and discuss factors suggestive of TB in the course of CF.
CASE REPORT
A 24-year-old man attending our French adult cystic fibrosis (CF) center but living in Madagascar and of Indian origin was diagnosed with CF (CFTR F508del homozygous) at the age of 1 year and with diabetes at the age of 18. His respiratory tract was colonized with Pseudomonas aeruginosa, Staphylococcus aureus, and Achromobacter xylosoxidans. He was treated daily with pancreatic extracts, vitamin E, insulin, azithromycin, and inhaled formoterol, and he received two courses of intravenous antibiotics per year.
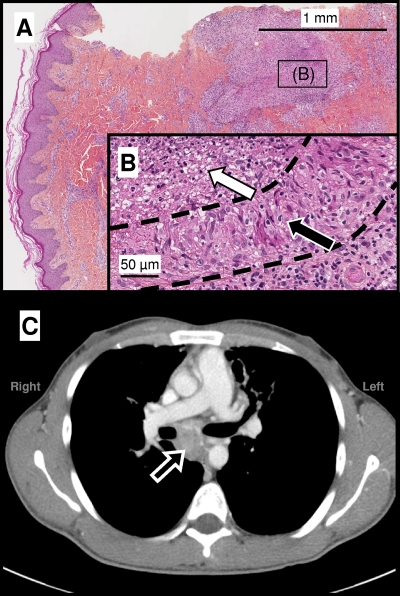
In July 2008, this patient was admitted to hospital with asthenia, pain, and edema in both legs. Clinical examination was normal apart from enlarged inguinal bilateral lymph nodes (1 cm in diameter) and an erythematous papular rash on both feet and the lower legs. Laboratory tests revealed a high C-reactive protein concentration (83 mg/liter) and anemia (hemoglobin, 86 g/liter). Serum transaminase levels were normal, but the levels of gamma glutamyl transferase (77 IU/liter) and alkaline phosphatase (331 IU/liter) activity were slightly high. Glycosylated hemoglobin (HbA1c) was increased to 9%, due to poor compliance with insulin treatment. Serological tests for HIV were negative. Skin biopsy of the left foot, on which the lesions were more typical, showed a palisading granuloma with central necrosis (Fig. 1). Ziehl-Neelsen staining and cultures for mycobacteria on solid (Coletsos and Lowenstein-Jensen; Bio-Rad, Marnes-la Coquette, France) and liquid (MGIT, BD; Franklin Lakes, NJ) media incubated at 37°C or 30°C for up to 90 days were negative. Abdominal ultrasound and computed tomography (CT) scan were normal, but a chest CT scan showed that the mediastinal lymph nodes (Fig. 1) had increased in size over the preceding 3 months. No change in bilateral diffuse bronchiectasis was observed.
Fig. 1.
(A and B) Skin biopsy specimen obtained from an erythematous papula on the foot. Hematoxylin-eosin staining shows alterations in the dermis (A), with a palisading epithelioid granuloma (B, black arrow) and a central abscess with surrounding histiocytic infiltrate (B, white arrow), as shown by the dotted lines. Histologic observations were compatible with TB, but Ziehl-Neelsen staining and mycobacterial cultures from skin samples remained negative, suggesting an immune reaction-based mechanism. (C) Mediastinal window of an axial contrast-enhanced CT scan showing an enlarged subcarinal lymph node (arrow) measuring 5 by 5 by 3 cm, located between the right and left main-stem bronchi, ascending aorta, right pulmonary artery, and esophagus.
At this point, the patient refused further medical evaluation and returned to Madagascar. He was readmitted 3 months later for weight loss (4 kg, resulting in a body mass index of 15.8 kg/m2) and fever. His forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1), expressed as percentages of normal predicted values for age, sex, and height, had decreased from 55% to 35% and from 45% to 27%, respectively, over a 6-month period. A positive gamma interferon (IFN-γ) response to Mycobacterium tuberculosis antigens was observed (QuantiFERON-TB gold in-tube; Cellestis, Chadstone, VIC, Australia). A needle aspiration biopsy of a mediastinal node was carried out by ultrasound-guided endoscopy. Auramine O staining of the aspirate for acid-fast bacilli was negative, but M. tuberculosis grew in culture. Four early-morning sputum specimens were tested for mycobacteria by culture in liquid (MGIT, 42-day incubation; BD) and solid (Coletsos, 90-day incubation; Bio-Rad) culture media. All these cultures remained negative. Antituberculosis (anti-TB) treatment was administered, with 2 months of daily four-drug therapy (isoniazid, rifampin, ethambutol, and pyrazinamide) followed by 4 months of isoniazid and rifampin. The patient's condition improved rapidly, with resolution of the leg pain and erythematous papular rash within 2 weeks. By the end of the treatment, the patient had regained 6.5 kg of body weight, his FVC and FEV1 had increased to 68% and 44% of predicted values, respectively, and the mediastinal lymph nodes had decreased in size.
Unlike nontuberculous mycobacteria (NTM), which are isolated in 7 to 13% of CF patients (5, 8), TB is rare in CF patients and only pulmonary forms have been reported (1, 4, 9). Thus, this is the first reported case of mediastinal TB in a CF patient. Several risk factors, although not specific to CF, appeared to be associated with the occurrence of TB in this patient. First, the prevalence of TB in Madagascar (478/100,000 in 2009) is significantly higher than that in France (7.3/100,000) (10). Geographic origin has previously been identified as a risk factor for epidemiologically linked cases of TB in CF patients in an area of high TB prevalence and multiple drug resistance (1). Second, ethnic origin may be an additional risk factor for TB development in this patient, as mediastinal TB was first described in West Indian and Asian immigrants (2). Third, poorly controlled diabetes is an additional risk factor, because the odds ratio for TB development in diabetic as opposed to nondiabetic subjects ranges from 2.44 to 8.3 (3).
The clinical presentation, associating leg pain and skin rash with visceral TB, was suggestive of Poncet's disease, but neither arthralgia nor erythema nodosum was present. The resolution of clinical symptoms on anti-TB treatment, together with negative cultures for mycobacteria from skin lesions, is consistent with the hypothesis of an immunological reactive mechanism (7).
Despite the positive results obtained for our patient in the QuantiFERON-TB gold in-tube assay, the diagnosis of TB rather than NTM infection was based on the isolation of M. tuberculosis from a mediastinal node. As for the Mantoux test, for which false-positive results have been reported for CF patients due to cross-reactivity between mycobacterial species (5), IFN-γ release assays, such as the QuantiFERON TB-2G (Cellestis), have also been reported to give positive results in some cases of NTM infection (6). Therefore, diagnosis can be confirmed only by direct detection of the causal mycobacterium, and TB can be no more than suspected on the basis of IFN-γ release assays.
In conclusion, our report demonstrates that mediastinal TB can occur in CF patients. With the extension of life span and the diverse geographic origins of patients with CF, TB should be more systematically investigated in these patients, with a combination of direct and indirect diagnostic methods.
Acknowledgments
We thank Brigitte Radenen (Université Paris Descartes) for her assistance with microscopy.
This work required no specific financial support.
None of the authors has any conflict of interest to declare.
Footnotes
Published ahead of print on 24 November 2010.
REFERENCES
- 1. Asherova I. K., Feigelson J., Vasilyeva L. A., Gabitov V. J. 2006. Cystic fibrosis complicated by multiresistant tuberculosis. Acta Paediatr. 95:1513–1514 [DOI] [PubMed] [Google Scholar]
- 2. Bloomberg T. J., Dow C. J. 1980. Contemporary mediastinal tuberculosis. Thorax 35:392–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dooley K. E., Chaisson R. E. 2009. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 9:737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feigelson J., et al. 1997. Tuberculous pneumopathy in the course of cystic fibrosis. Arch. Pediatr. 4:1209–1212 [DOI] [PubMed] [Google Scholar]
- 5. Giron R. M., et al. 2008. Nontuberculous mycobacterial infection in patients with cystic fibrosis: a multicenter prevalence study. Arch. Bronconeumol. 44:679–684 [PubMed] [Google Scholar]
- 6. Kobashi Y., et al. 2009. Clinical evaluation of the QuantiFERON-TB Gold test in patients with non-tuberculous mycobacterial disease. Int. J. Tuberc. Lung Dis. 13:1422–1426 [PubMed] [Google Scholar]
- 7. Kroot E. J., Hazes J. M., Colin E. M., Dolhain R. J. 2007. Poncet's disease: reactive arthritis accompanying tuberculosis. Two case reports and a review of the literature. Rheumatology 46:484–489 [DOI] [PubMed] [Google Scholar]
- 8. Pierre-Audigier C., et al. 2005. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J. Clin. Microbiol. 43:3467–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith M. J., Efthimiou J., Hodson M. E., Batten J. C. 1984. Mycobacterial isolations in young adults with cystic fibrosis. Thorax 39:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization 2009. Global Health Observatory, health-related millennium development goals. MDG6:HIV/AIDS, malaria and other diseases, prevalence of tuberculosis. World Health Organization, Geneva, Switzerland: http://apps.who.int/ghodata [Google Scholar]