Abstract
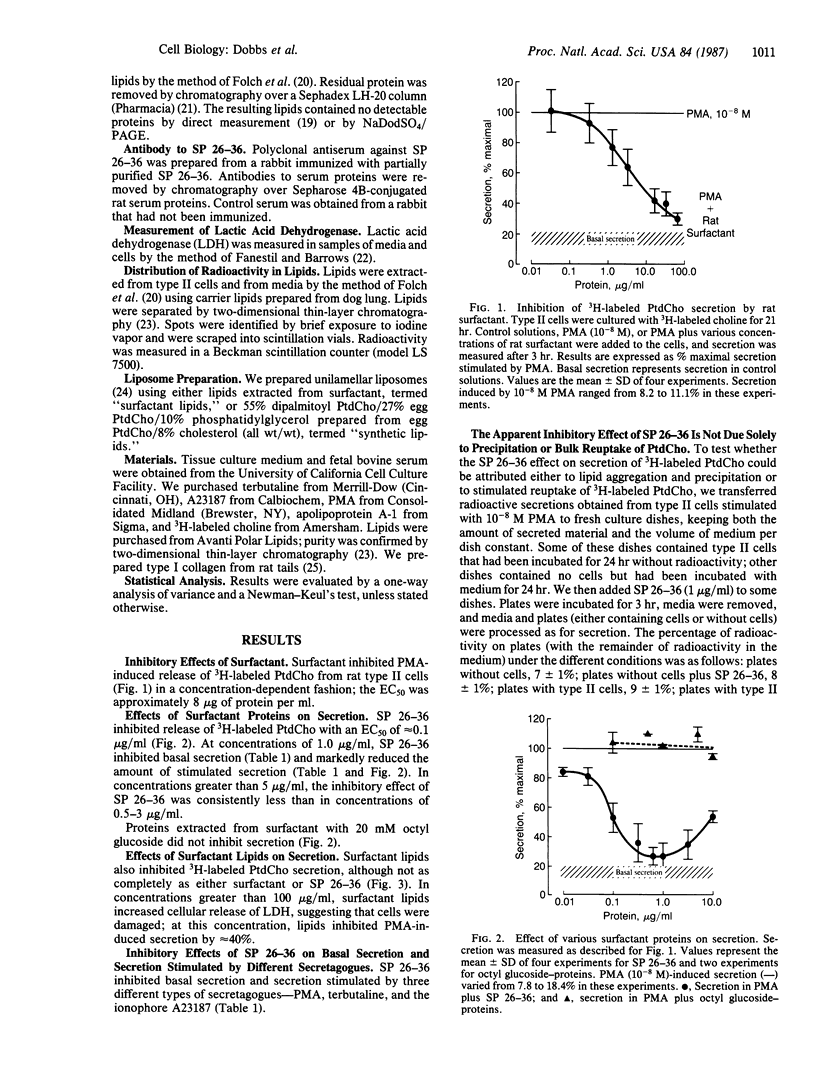
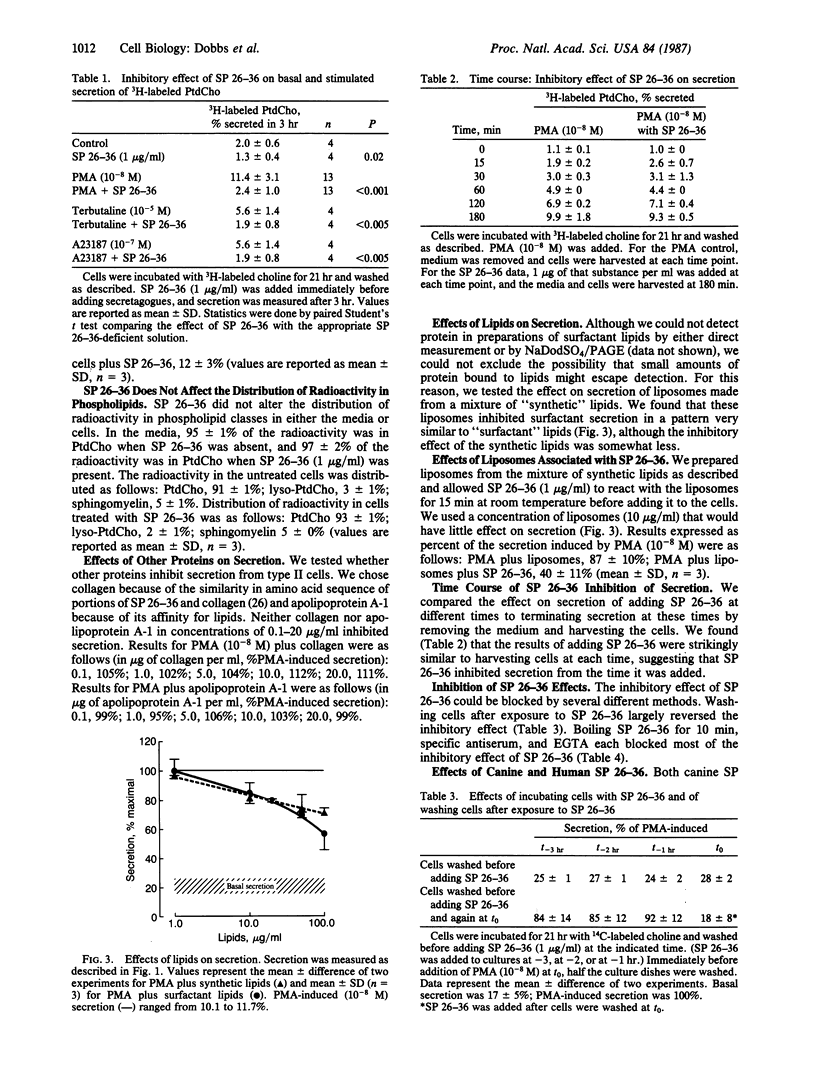
Pulmonary surfactant is synthesized and secreted by alveolar type II cells. Radioactive phosphatidylcholine has been used as a marker for surfactant secretion. We report findings that suggest that surfactant inhibits secretion of 3H-labeled phosphatidylcholine by cultured rat type II cells. The lipid components and the surfactant protein group of Mr 26,000-36,000 (SP 26-36) inhibit secretion to different extents. Surfactant lipids do not completely inhibit release; in concentrations of 100 micrograms/ml, lipids inhibit stimulated secretion by 40%. SP 26-36 inhibits release with an EC50 of 0.1 microgram/ml. At concentrations of 1.0 microgram/ml, SP 26-36 inhibits basal secretion and reduces to basal levels secretion stimulated by terbutaline, phorbol 12-myristate 13-acetate, and the ionophore A23187. The inhibitory effect of SP 26-36 can be blocked by washing type II cells after adding SP 26-36, by heating the proteins to 100 degrees C for 10 min, by adding antiserum specific to SP 26-36, or by incubating cells in the presence of 0.2 mM EGTA. SP 26-36 isolated from canine and human sources also inhibits phosphatidylcholine release from rat type II cells. Neither type I collagen nor serum apolipoprotein A-1 inhibits secretion. These findings are compatible with the hypothesis that surfactant secretion is under feedback regulatory control.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansfield M. J., Benson B. J. Identification of the immunosuppressive components of canine pulmonary surface active material. J Immunol. 1980 Sep;125(3):1093–1098. [PubMed] [Google Scholar]
- Benson B., Hawgood S., Schilling J., Clements J., Damm D., Cordell B., White R. T. Structure of canine pulmonary surfactant apoprotein: cDNA and complete amino acid sequence. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6379–6383. doi: 10.1073/pnas.82.19.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. A., Longmore W. J. Adrenergic and cholinergic regulation of lung surfactant secretion in the isolated perfused rat lung and in the alveolar type II cell in culture. J Biol Chem. 1981 Jan 10;256(1):66–72. [PubMed] [Google Scholar]
- Brown L. A., Pasquale S. M., Longmore W. J. Role of microtubules in surfactant secretion. J Appl Physiol (1985) 1985 Jun;58(6):1866–1873. doi: 10.1152/jappl.1985.58.6.1866. [DOI] [PubMed] [Google Scholar]
- Chander A., Dodia C. R., Gil J., Fisher A. B. Isolation of lamellar bodies from rat granular pneumocytes in primary culture. Biochim Biophys Acta. 1983 Aug 29;753(1):119–129. doi: 10.1016/0005-2760(83)90105-4. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Gonzalez R. F., Marinari L. A., Mescher E. J., Hawgood S. The role of calcium in the secretion of surfactant by rat alveolar type II cells. Biochim Biophys Acta. 1986 Jun 27;877(2):305–313. doi: 10.1016/0005-2760(86)90308-5. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Gonzalez R., Williams M. C. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986 Jul;134(1):141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fanestil D. D., Barrows C. H., Jr Aging in the rotifer. J Gerontol. 1965 Oct;20(4):462–469. [PubMed] [Google Scholar]
- FitzGerald G. A. Peripheral presynaptic adrenoreceptor regulation of norepinephrine release in humans. Fed Proc. 1984 Apr;43(5):1379–1381. [PubMed] [Google Scholar]
- Floros J., Phelps D. S., Taeusch H. W. Biosynthesis and in vitro translation of the major surfactant-associated protein from human lung. J Biol Chem. 1985 Jan 10;260(1):495–500. [PubMed] [Google Scholar]
- Hamilton R. L., Jr, Goerke J., Guo L. S., Williams M. C., Havel R. J. Unilamellar liposomes made with the French pressure cell: a simple preparative and semiquantitative technique. J Lipid Res. 1980 Nov;21(8):981–992. [PubMed] [Google Scholar]
- Harford J., Wolkoff A. W., Ashwell G., Klausner R. D. Monensin inhibits intracellular dissociation of asialoglycoproteins from their receptor. J Cell Biol. 1983 Jun;96(6):1824–1828. doi: 10.1083/jcb.96.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Hamilton R. L., Jr Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry. 1985 Jan 1;24(1):184–190. doi: 10.1021/bi00322a026. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Efrati H., Schilling J., Benson B. J. Chemical characterization of lung surfactant apoproteins: amino acid composition, N-terminal sequence and enzymic digestion. Biochem Soc Trans. 1985 Dec;13(6):1092–1096. doi: 10.1042/bst0131092. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Limitations of presynaptic theory: no support for feedback control of autonomic effectors. Fed Proc. 1984 Apr;43(5):1358–1364. [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972 Sep;223(3):715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- King R. J. Pulmonary surfactant. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jul;53(1):1–8. doi: 10.1152/jappl.1982.53.1.1. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Tonge D. A., Baker P. F. Inhibition of exocytosis in bovine adrenal medullary cells by botulinum toxin type D. Nature. 1985 Oct 24;317(6039):719–721. doi: 10.1038/317719a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Clubb K. W., Barrow D. A., Meissner G. Ordered and disordered phospholipid domains coexist in membranes containing the calcium pump protein of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1983 May;80(10):2917–2921. doi: 10.1073/pnas.80.10.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoon M. W., Wright J. R., Baritussio A., Williams M. C., Goerke J., Benson B. J., Hamilton R. L., Clements J. A. Subfractionation of lung surfactant. Implications for metabolism and surface activity. Biochim Biophys Acta. 1983 Jan 7;750(1):18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- Marino P. A., Rooney S. A. Surfactant secretion in a newborn rabbit lung slice model. Biochim Biophys Acta. 1980 Dec 5;620(3):509–519. doi: 10.1016/0005-2760(80)90143-5. [DOI] [PubMed] [Google Scholar]
- Mescher E. J., Dobbs L. G., Mason R. J. Cholera toxin stimulates secretion of saturated phosphatidylcholine and increases cellular cyclic AMP in isolated rat alveolar type II cells. Exp Lung Res. 1983 Nov;5(3):173–182. doi: 10.3109/01902148309061512. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Miles P. R., Wright J. R., Bowman L., Castranova V. Incorporation of [3H]palmitate into disaturated phosphatidylcholines in alveolar type II cells isolated by centrifugal elutriation. Biochim Biophys Acta. 1983 Aug 29;753(1):107–118. doi: 10.1016/0005-2760(83)90104-2. [DOI] [PubMed] [Google Scholar]
- Oyarzún M. J., Clements J. A. Ventilatory and cholinergic control of pulmonary surfactant in the rabbit. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jul;43(1):39–45. doi: 10.1152/jappl.1977.43.1.39. [DOI] [PubMed] [Google Scholar]
- Poorthuis B. J., Yazaki P. J., Hostetler K. Y. An improved two dimensional thin-layer chromatography system for the separation of phosphatidylglycerol and its derivatives. J Lipid Res. 1976 Jul;17(4):433–437. [PubMed] [Google Scholar]
- Redman C. M. Proteolipid involvement in human erythrocyte membrane function. Biochim Biophys Acta. 1972 Sep 1;282(1):123–134. doi: 10.1016/0005-2736(72)90316-1. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Osterhoudt K. C., Whitsett J. A. Effect of cytochalasins on surfactant release from alveolar type II cells. Biochim Biophys Acta. 1984 Sep 14;805(1):12–18. doi: 10.1016/0167-4889(84)90030-2. [DOI] [PubMed] [Google Scholar]
- Sano K., Voelker D. R., Mason R. J. Involvement of protein kinase C in pulmonary surfactant secretion from alveolar type II cells. J Biol Chem. 1985 Oct 15;260(23):12725–12729. [PubMed] [Google Scholar]
- Sueishi K., Benson B. J. Isolation of a major apolipoprotein of canine and murine pulmonary surfactant. Biochemical and immunochemical characteristics. Biochim Biophys Acta. 1981 Sep 24;665(3):442–453. doi: 10.1016/0005-2760(81)90257-5. [DOI] [PubMed] [Google Scholar]
- Tsilibary E. C., Williams M. C. Actin and secretion of surfactant. J Histochem Cytochem. 1983 Nov;31(11):1298–1304. doi: 10.1177/31.11.6688627. [DOI] [PubMed] [Google Scholar]
- Westfall T. C. Evidence that noradrenergic transmitter release is regulated by presynaptic receptors. Fed Proc. 1984 Apr;43(5):1352–1357. [PubMed] [Google Scholar]
- White R. T., Damm D., Miller J., Spratt K., Schilling J., Hawgood S., Benson B., Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. 1985 Sep 26-Oct 2Nature. 317(6035):361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- Williams M. C., Benson B. J. Immunocytochemical localization and identification of the major surfactant protein in adult rat lung. J Histochem Cytochem. 1981 Feb;29(2):291–305. doi: 10.1177/29.2.7019304. [DOI] [PubMed] [Google Scholar]
- Williams M. C. Conversion of lamellar body membranes into tubular myelin in alveoli of fetal rat lungs. J Cell Biol. 1977 Feb;72(2):260–277. doi: 10.1083/jcb.72.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. C. Endocytosis in alveolar type II cells: effect of charge and size of tracers. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6054–6058. doi: 10.1073/pnas.81.19.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]



