Abstract
Intravenous cholecystokinin octapeptide (CCK-8) elicits vago-vagal reflexes that inhibit phasic gastric contractions and reduce gastric tone in urethane-anaesthetized rats. A discrete proximal subdivision of the ventral gastric vagus nerve (pVGV) innervates the proximal stomach, but the fibre populations within it have not been characterized previously. We hypothesized that i.v. CCK-8 injection would excite inhibitory efferent outflow in the pVGV, in contrast to its inhibitory effect on excitatory efferent outflow in the distal subdivision (dVGV), which supplies the distal stomach. In each VGV subdivision, a dual-recording technique was used to record afferent and efferent activity simultaneously, while also monitoring intragastric pressure (IGP). CCK-8 dose dependently (100–1000 pmol kg−1, i.v.) reduced gastric tone, gastric contractile activity and multi-unit dVGV efferent discharge, but increased pVGV efferent firing. Single-unit analysis revealed a minority of efferent fibres in each branch whose response differed in direction from the bulk response. Unexpectedly, efferent excitation in the pVGV was significantly shorter lived and had a significantly shorter decay half-time than did efferent inhibition in the dVGV, indicating that distinct pathways drive CCK-evoked outflow to the proximal vs. the distal stomach. Efferent inhibition in the dVGV began several seconds before, and persisted significantly longer than, simultaneously recorded dVGV afferent excitation. Thus, dVGV afferent excitation could not account for the pattern of dVGV efferent inhibition. However, the time course of dVGV afferent excitation paralleled that of pVGV efferent excitation. Similarly, the duration of CCK-8-evoked afferent responses recorded in the accessory celiac branch of the vagus (ACV) matched the duration of dVGV efferent responses. The observed temporal relationships suggest that postprandial effects on gastric complicance of CCK released from intestinal endocrine cells may require circulating concentrations to rise to levels capable of exciting distal gastric afferent fibres, in contrast to more immediate effects on distal gastric contractile activity mediated via vago-vagal reflexes initiated by paracrine excitation of intestinal afferents.
Non-technical summary
Gut activity is controlled by the vagus nerves. In anaesthetized rats, both sensory and motor nerve activity evoked by intravenous injection of the gut hormone cholecystokinin were recorded in separate sub-branches of the gastric vagus nerve that supply the forestomach and hindstomach, respectively. Activity in the forestomach branch has not been studied before. Motor nerve activity in response to cholecystokinin differed between the two branches, in both timing and direction. Motor output to the forestomach paralleled sensory input from the hindstomach, while motor output to the hindstomach paralleled sensory input from the intestines. The data suggest that cholecystokinin released in the intestines after a meal immediately influences churning and propulsion of food by the hindstomach, via reflexes initiated by nearby intestinal sensory nerve terminals, but may influence gastric capacity only later, once circulating levels of cholecystokinin rise to levels capable of activating sensors in the hindstomach.
Introduction
The mechanical repertoire of the stomach can be divided broadly into a reservoir function responsible for adjusting stomach capacity and tone, and a pumping function responsible for mixing ingesta and gastric secretions, trituration and propulsion of the resulting chyme. These functions are regionally segregated to the proximal and distal stomach, respectively. While anatomically distinct, the activities of these two regions of the stomach are typically regulated coordinately. Stimuli that delay gastric emptying often cause both a reduction in phasic contractile activity of the hindstomach and relaxation of the forestomach, while stimuli or conditions that increase gastric emptying generally have the opposite effects (Azpiroz & Malagelada, 1986; van der Schaar et al. 2001; Tack et al. 2006).
Central control of gastric contractile activity and of accommodation during normal digestion is mediated primarily via efferent pathways in the subdiaphragmatic vagi, and both functions are subject to modulation by vago-vagal reflexes, i.e. changes in vagal afferent input from the gut which in turn alter efferent output to the gut (Harper et al. 1959; Azpiroz & Malagelada, 1986). It has been known for over a century that a major vagal pathway controlling gastric accommodation is inhibitory, i.e. that excitation of the relevant preganglionic fibres reduces forestomach tone, while the predominant vagal pathway controlling the gastric pump is excitatory (Langley, 1898; McSwiney, 1931). However, all regions of the stomach appear to receive a combination of excitatory and inhibitory vagal efferent inputs, whose relative roles in controlling motor functions of the stomach are an area of ongoing research interest (Zheng et al. 1999; Guo et al. 2001; Hermann et al. 2006; Pearson et al. 2007; Herman et al. 2008). The clinical importance of the vagal regulation of gastric compliance is emphasized by recent findings linking impaired accommodation to functional dyspepsia (Tack et al. 1998, 2001).
The predominant vagal input to the stomach arrives via the gastric vagi (Berthoud et al. 1991a,b;). In a number of species including the rat, dog and human, the ventral gastric vagus emits one or more fine branches that proceed to selectively innervate the proximal portion of the stomach (Legros & Griffith, 1969; Prechtl & Powley, 1990; Berthoud et al. 1991a; Walter et al. 2009). The remainder of the VGV courses toward the lesser curvature and supplies the distal stomach, pylorus, proximal duodenum and pancreas (Berthoud et al. 1991a). This anatomical arrangement provides a previously unexploited opportunity to investigate vagal control of the distal and proximal stomach separately. The fine branch(es) of the VGV that supply the forestomach have been previously referred to as the anterior fundic branch(es), but we are unaware of any specific designation for the remaining larger trunk, other than the gastric division of the gastric branch (Legros & Griffith, 1968, 1969). This larger trunk is analogous to what is referred to in the human as the anterior nerve of Latarjet (Skandalakis et al. 1980). In the present work we have opted to refer to this larger branch as the distal branch of the VGV, or ‘distal VGV’, a term which de-emphasizes the gastric course intentionally since it also innervates the proximal duodenum and pancreas. We have further chosen to refer to what has previously been termed the fundic branch(es) of the anterior gastric vagus as the ‘proximal VGV’. We suggest the term ‘common VGV’ to refer to the short portion of the VGV rostral to the divergence of the proximal VGV. To our knowledge, no prior study has used a recording site within the proximal VGV to investigate the vagal motor output to the forestomach.
CCK is a gut hormone released endogenously by intestinal endocrine cells in response to intraluminal nutrients (Raybould & Lloyd, 1994; Raybould et al. 1994; van der Schaar et al. 2001; Reeve et al. 2003). CCK potently excites a large subpopulation of mucosal vagal afferent chemosensitive fibres in the stomach and intestines, and plays a physiological role in the regulation of postprandial gastric emptying, pancreatic exocrine activity and satiation, in large part via vagal pathways (Smith et al. 1985; Forster et al. 1990; Li & Owyang, 1994). Interest in CCK-mediated vagal signalling has grown in recent years, as a number of other GI hormones including leptin, apolipoprotein A-IV, PYY(3–36), and orexin have been shown to exert physiological effects via CCK-dependent vagal afferent mechanisms (Wang et al. 2000; Burdyga et al. 2003; Glatzle et al. 2004; Whited et al. 2007). In anaesthetized in vivo experimental models, gastric motor effects of intravenously (i.v.) administered CCK-8 include inhibition of gastric contractile activity, a reduction in gastric intraluminal pressure, and a relaxation of the lower oesophageal sphincter primarily via vago-vagal reflexes, although vago-sympathetic and humoral–vagal pathways also may play a role in these responses, depending in part upon the dose of peptide (Raybould et al. 1987; Raybould & Taché, 1988; Takahashi & Owyang, 1999; Bucinskaite et al. 2000). This pattern of gastric motor responses differs from those observed in ex vivo preparations, in which extrinsic neural reflex arcs are absent (Morgan et al. 1978; Scheurer et al. 1983). Prior electrophysiological studies have demonstrated inhibition of bulk gastric vagal efferent activity in response to i.v. CCK-8, consistent with a reduction in excitatory drive to the gastric pump (Niijima et al. 1996; Bucinskaite et al. 2000). However, these studies used a recording site in either the distal or common VGV, and thus any excitation of inhibitory drive to the forestomach would have been either absent or obscured.
The present study was undertaken to provide the first electrophysiological characterization of the proximal VGV efferent and afferent supply, and to explore the relationship between the vagal responses elicited by i.v. administration of CCK-8 in the proximal vs. distal VGV. Multi-unit afferent and efferent activity changes were recorded in each subdivision of the VGV in response to consecutive bolus i.v. injections of CCK-8 at ascending doses, using the recently developed dual-recording technique (Adelson et al. 2007). Vago-vagal responses to mild gastric distention were also recorded. Intragastric pressure (IGP) was monitored concomitantly with nerve recording. Phasic and tonic components of IGP were analysed separately to serve as indices of gastric contractile and, with a caveat, accommodative activities, and were compared with the pattern of nerve activity in each branch of the VGV. Single-unit records of vagal efferent activity were sorted offline from recorded multi-unit activity, to determine the relative contributions of so-called excitatory and inhibitory efferent fibres to the motor response in each branch of the VGV. In addition to providing a detailed electrophysiological characterization of the efferent fibre population in each branch of the VGV, the data revealed unexpected temporal differences in CCK-8-evoked efferent responses between branches, with potentially important physiological implications. Parallels observed in the time course of afferent and efferent responses between branches suggest that distinct CCK-sensitive afferent pathways drive the vago-vagal reflexes that modulate gastric emptying and gastric accommodation, respectively.
Methods
Animals
Male Sprague-Dawley rats (250–350 g, Harlan Laboratories, San Diego, CA, USA) were housed under controlled conditions of temperature (22–24°C) and illumination (12 h light cycle, starting at 06:00 h) and maintained with Purina Chow and tap water ad libitum. Rats were deprived of food overnight (>14 h) but allowed free access to tap water up to the beginning of the experiments. Rats were anaesthetized by intramuscular injection of 25% urethane (1.5 ml kg−1) into the right hindlimb, followed by additional doses of up to 0.2 ml 25% urethane injected intraperitoneally (i.p.), if needed, to render the animal areflexic to strong paw or tail pinch prior to initiating surgery. Additional booster doses of up to 0.2 ml 25% urethane were administered i.p. occasionally during the course of the experiment as necessary to maintain areflexia. At the end of the experiment, the anaesthetized rat was killed by exsanguination via section of the abdominal aorta. Protocols were approved by the Veterans Administration Greater Los Angeles Healthcare System (VAGLAHS) Institutional Animal Care and Use Committee (IACUC).
Surgery
Surgical procedures were similar to those previously described (Adelson et al. 2007). Animals were tracheally cannulated, and maintained at 35.5–37.5°C on a feedback-controlled heating block, and rectal temperature was monitored continuously. The left jugular vein was cannulated to allow continuous i.v. infusion of 0.57 ml h−1 sterile, pyrogen-free 0.9% saline throughout the experiment to maintain hydration, and for i.v. injection of test solutions. EKG electrodes were attached to fore and hind paws and connected to a differential amplifier (Model 1700, A-M Systems, Carlsborg, WA, USA) to allow recording of heart rate. Laparotomy was performed, and the skin flaps were secured to a stainless-steel ring, exposing the abdominal viscera. All exposed surfaces were kept moist by covering with cotton soaked in warm 0.1 m phosphate-buffered (pH 7.4) saline. A plastic holder was positioned to reflect the liver away from the stomach and against the diaphragm, and the omentum was cut near the lower esophageal sphincter (LES) to allow unrestricted access to the VGV.
The duodenum was ligated 1–2 cm distal to the pylorus, a small incision was made in the non-glandular forestomach, and the stomach lumen was rinsed several times with saline. To permit gastric distention, the stomach was cannulated, via the forestomach incision, with Tygon (Saint-Gobain SA, Paris) B-44-3 tubing (i.d. 3/16 in, o.d. 5/16 in (∼4.8 and 7.9 mm, respectively)) shaped into a sigmoidal form with several small holes cut in the tubing wall near the tip (to avoid clogging). The cannula tip was positioned in the stomach immediately distal to the gastric dividing line, in the proximal portion of the corpus, and the incision was sutured closed around the cannula.
Intragastric manometry
Intragastric pressure (IGP) was monitored via a catheter pressure transducer (SPR-524, Millar Instruments) led into the stomach via the mouth and oesophagus, and connected to a transducer amplifier (TBM-4A, World Precision Instruments, Boca Raton, FL, USA). The pressure sensor at the tip of the catheter was positioned in the mid-corpus region (∼1 cm from the greater curvature). Saline (1–1.5 ml) was injected into the stomach via the intragastric cannula to establish an initial IGP of 3–6 cmH2O, and the cannula outlet valve was closed.
Nerve dissection and recording
Approximately 1 cm rostral to the gastroesophageal junction, connective tissue anchoring the VGV to the oesophagus was dissected away, and a segment of either the dVGV or the pVGV was placed on a dissecting platform immediately above the oesophagus. In animals in which two pVGV branches were apparent, we referred to the branch closer to the dVGV as the medial pVGV and the more lateral branch as the lateral pVGV. The nerve sheaths were opened using fine forceps, the abdominal cavity was filled with warm mineral oil to provide electrical isolation for the recording, and a quadripolar platinum wire electrode (wire diameter 25 μm, A-M Systems) comprising two separate bipolar electrodes was positioned above the picking window. Two separate twigs of the selected VGV branch, each typically 15–30 μm in diameter, were dissected free of the nerve trunk, and each was placed on one pole of one of the two pairs of bipolar electrodes. One was picked (severed) at the caudal margin of the picking window and teased free toward the rostral margin, and thus only centrifugal (efferent) impulse activity was recorded from this ‘efferent’ filament. The other was picked from the rostral margin of the picking window and teased toward the caudal margin, and thus only centripetal (afferent) activity was recorded from this ‘afferent’ filament. In picking these filaments, care was taken to maintain intact as much of the source trunk as possible. Epineurial connective tissue taken from as close to the recording site as possible was attached to the indifferent pole of each bipolar electrode. This arrangement allowed simultaneous recording of vagal efferent (VE) and vagal afferent (VA) activity, in separate filaments of either the dVGV or pVGV. Once both an ‘efferent’ and an ‘afferent’ filament with stable activity and waveforms much larger than the surrounding noise were isolated, the preparation was left undisturbed for at least half an hour, to allow stabilization of recording conditions and activity levels. In experiments comparing activity in the accessory celiac branch of the vagus (ACV) with that in the dVGV, filaments from each were teased from the respective trunk and placed on the quadripolar electrode as above. ACV filaments were teased at a site immediately distal to the point of ACV divergence from the ventral vagal trunk. Each bipolar electrode pair was connected to a separate channel of a multi-channel differential amplifier (Model 1700, A-M Systems) to allow separate recording from each of the two nerve twigs. Nerve activity was amplified 10,000-fold and the signal was filtered with a passband of 100 Hz to 1 kHz. Impulse activity, along with all physiological data (rectal temperature, EKG and IGP) were acquired using a Micro1401 A/D interface (Cambridge Electronic Design, Ltd, Cambridge, UK) connected to a PC running Spike 2 (Cambridge Electronic Design) data acquisition software under Microsoft Windows XP. The times of stimulus application were marked on a separate stimulus mark channel using a foot pedal controller.
Chemicals
Sulfated CCK-8 (Research Plus, Bayonne, NJ, USA) was dissolved in 0.9% sterile, pyrogen-free saline (Sigma-Aldrich, St Louis, MO, USA) to reach a stock solution (300–700 pmol μl−1) whose concentration was measured spectrophotometrically, using a conversion factor of 186 pmol μl−1 × absorbance at 280 nm (A280). Bovine serum albumin (BSA, Sigma-Aldrich) was added to a concentration of 0.1% and aliquots were stored at −20°C. Samples were tested after 6 months by the CURE: Digestive Diseases Center Peptidomic Core using reverse-phase HPLC to confirm that the peptide had not degraded during storage. Stock solutions were diluted to the appropriate concentration in 0.9% saline containing 0.1% BSA immediately before injection. All chemicals other than CCK-8 were purchased from Sigma-Aldrich.
Experimental protocol
Following the initial 30 min stabilization period, consecutive bolus (100 μl + flush) i.v. injections of vehicle and CCK-8 (100, 300 and 1000 pmol kg−1) were performed at 25 min intervals. After recording the response to the highest dose of CCK-8, one to three i.v. injections of other peptide hormones were performed. The results of these latter trials are not reported here. Following these injections, gastric distention was performed by infusing 1 ml of normal saline into the stomach via the intragastric cannula at a rate of 2 ml min−1 using a syringe pump (WPI SP260p, World Precision Instruments). The infused saline remained in the stomach for 2 min, after which 1 ml was withdrawn from the stomach at the same rate at which it was infused. Finally, the innervation territories of the recorded afferent bundles were explored using fine art brushes (0-4, various manufacturers) to locate the areas in which robust afferent responses to mechanical probing could be evoked. No attempt was made to identify every area from which a response could be discriminated, but particular attention was paid to whether responses could be evoked from either side of the dividing line.
Analysis of intragastric pressure
Intragastric pressure (IGP) traces comprise several distinct components, including a respiratory component resulting from the motion of the diaphragm against the stomach, a phasic component resulting from contractile activity of the stomach at the pacemaker frequency, and a tonic component. The respiratory component present in the IGP trace was removed by downsampling 5:1 and applying a low-pass finite impulse response digital filter with the passband set at 0.4 Hz and a gap width of 0.2 Hz. The period of phasic gastric contractions was obtained by performing an autocorrelation of the resulting trace, during an interval in which there was minimal variation in tonic IGP, and measuring the interpeak interval on the autocorrelogram. The phasic component of the IGP trace (phIGP) was extracted from the IGP trace after filtering out the respiratory component, by applying the ‘DC remove’ channel process internal to the Spike 2 software, with a time constant of 20 s. The magnitude of phasic activity was extracted from this trace by applying the ‘RMS amplitude’ channel process. The tonic component of IGP (tIGP) was extracted from the IGP trace, after the respiratory component was removed as described above, by applying a median filter with a 60 s window size. A 60 s window was chosen empirically by observing the correspondence between the extracted and original IGP traces during the response to CCK-8 for a variety of different window durations. The use of a wider window duration than we have used previously (Adelson et al. 2004) reduced jagged artifacts in the ‘tonic’ trace during the recovery of phasic activity and initial tone following CCK-8 injection.
Sorting of unit waveforms
Efferent and afferent activities were recorded on separate channels and efferent multi-unit activity was sorted, off-line, into single unit activity using Spike 2 software (Cambridge Electronic Design). Initial template formation was done by setting initial template widths at 12–15% of spike amplitude, requiring matching of a minimum of 60–65% of points within the template envelop. After the assignment of marker codes to spikes using this initial template set, spike waveforms corresponding to each marker were reviewed, and similar waveforms were combined into common marker designations, after which additional subsorting was conducted as needed to remove false positives. Units whose waveforms could be reliably distinguished from all other waveforms in the record were registered as single units (SUs).
Data analysis and statistics
Data were quantified using custom software scripts developed for the purpose in our laboratory, written in the Spike 2 scripting language. All error values reported are standard errors of the mean (s.e.m.). All statistical tests and exponential curve fits were performed using Prism version 5.0 software (GraphPad Software, San Diego, CA, USA).
Impulse activity levels were normalized by dividing the post-stimulus discharge rate by the pre-stimulus (control) mean, and were expressed as percent of the pre-stimulus mean value. The control period duration for gastric distention stimuli was equal to the duration of the distention period. Control period durations for i.v. injections were 2.5 min. All illustrations of impulse activity in individual experiments or for individual units show mean discharge frequency (10 s smoothing).
Response durations for each stimulus trial and each trace were measured by smoothing the raw data (30 s window) and determining the time required for the mean value to return from its maximal excursion to within 10% (i.e. 90% ‘repolarization’) of the threshold level of activity used to define the onset of the response, as previously described for analysis of ultrasonometric motion traces (Adelson et al. 2004). The response threshold was defined as the mean control period value for each trace plus (for excitation) or minus (for inhibition) two times the standard deviation of activity over the ten 15 s bins spanning the control period. On occasion the algorithm used produced spurious duration measurements, particularly for pVGV afferent and phasic IGP responses at the lower doses of CCK-8 tested, where the magnitude of response was relatively small compared to the prevailing variability. Spurious measurements were excluded from the group duration analysis, resulting in smaller n values for some duration measurements than for associated aggregate data plots (in which all responses were reflected). See Supplemental material for further detail regarding duration measurements and exclusion of spurious measurements. In each graph illustrating response durations, the number indicated at the bottom of each column indicates the total number of included duration measurements. Statistical comparisons between pairs of simultaneously recorded parameters (i.e. IGP vs. neural activity, and efferent vs. afferent responses recorded in the same branch) were made using paired t tests on the subset of trials for which both values were available. Statistical tests including comparisons between dVGV and pVGV efferent and/or afferent activity were performed using unpaired statistical tests (unpaired t test or one-way ANOVA for multiple comparisons, as indicated in the text) on all included measurements.
The kinetics of efferent responses to CCK-8 were quantified using recovery half-times. Single-phase exponential curves were fitted, using the least-squares method with automatic outlier elimination, to the portion of the activity profile following the maximum post-stimulation excursion of the recorded trace (i.e. the ‘decay’ portion of the trace). The adequacy of the single-phase exponential fit was verified by comparing it to a two-phase decay, which, in the case of the traces evaluated, did not provide a significant improvement in the quality of the fit, as determined by the F test. Differences between decay half-times of exponential curves fitted to multi-unit response profiles were tested using the F test. Differences in the half-times of decay determined for responses to CCK-8 of each population of efferent single-units were analysed using a non-parametric ANOVA with Dunn's post hoc test between groups.
The strength of linear correlations between pairs of neural response profiles were evaluated using the coefficient of determination (r2) obtained from Deming regression of the mean ± s.e.m. of the normalized curves (15 s bins).
Results
VGV recordings were performed in 17 urethane-anaesthetized rats, eight using a recording site in the dVGV, and nine in the pVGV. In all dVGV recordings the trunk from which filaments were dissected remained at least 40% intact, and in five of these, it appeared >80% intact. Due to the much smaller size of pVGV, the trunk from which recordings were made was severed in four of nine experiments, and in the others it was difficult to make a credible estimate of the extent to which the trunk remained intact. Further details concerning the number of complete afferent and efferent records obtained are contained in Supplemental material. Figure 1 illustrates raw traces of IGP, multi-unit efferent and multi-unit afferent activity recorded simultaneously during typical experiments in each branch in urethane-anaesthetized rats.
Figure 1. Representative traces of afferent(upper traces) and efferent (lower traces) nerve activity (mean discharge rate) and intragastric pressure (IGP) (centre traces) in dual-recording experiments performed in the distal and proximal VGV.
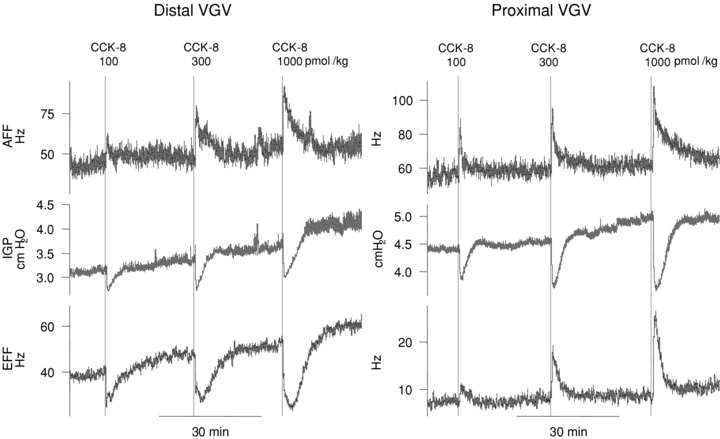
CCK-8 i.v. (100–1000 pmol kg−1) causes a dose-related decline in IGP and excites both distal and proximal VGV afferent activity (upper traces). CCK-8 i.v. inhibits distal VGV efferent multi-unit activity (bottom trace, left panel) but excites efferent activity in the proximal VGV (bottom trace, right panel).
Receptive fields of mechanosensitive afferent fibres present in pVGV recordings were distributed throughout the non-glandular forestomach, but none were encountered in the hindstomach. Mechanoreceptive fields of dVGV afferent fibres were located in the glandular hindstomach, including the cardia region, the corpus, antrum and pylorus, as well as in the proximal duodenum. A figure indicating the locations of the identified mechanosensitive zones in each experiment is included in Supplemental Fig. S1.
Below, the quantitative aspects of individual IGP and nerve discharge responses in each experiment are described, after which the temporal relationships between traces are evaluated.
CCK-8-evoked changes in IGP
Prior to any i.v. injections, basal tonic intragastric pressure (tIGP) in was 4.7 ± 0.3 cmH2O (range: 2.2 to 6.8, n = 17). Phasic activity at the pacemaker frequency was present in 16 of 17 preparations, with a basal amplitude of 0.07 ± 0.01 cmH2O (range: 0.03 to 2.0) and a period of 13.6 ± 0.51 s (n = 16). There was no significant difference in basal tIGP or phIGP between dVGV vs. pVGV recording experiments.
Bolus i.v. injection of vehicle did not significantly alter tIGP or phIGP. Successive i.v. bolus injections of CCK-8 (100, 300 and 1000 pmol kg−1) dose-dependently decreased tIGP and inhibited phIGP (Fig. 2) There was no significant difference in the CCK-8-evoked pressure changes observed in dVGV vs. pVGV recording experiments. It should be noted that while the aggregate plots of the normalized traces show a progressively greater drop in tonic IGP with increasing CCK-8 dose (Fig. 2B), the absolute nadirs in IGP were similar at each dose tested (Fig. 2A). The increase in the per cent inhibition despite similar absolute values of IGP at the nadirs of the responses resulted from a rise in prevailing gastric tone with time (Fig. 2A). There was a statistically significant difference in the tonic pressure prior to each i.v. injection (4.7 ± 0.3, 4.9 ± 0.2, 5.3 ± 0.2 and 5.6 ± 0.2 cmH2O prior to vehicle, 100, 300 and 1000 pmol kg−1 CCK-8, respectively, n = 17). Interestingly, the duration of tIGP responses was significantly less than that of phIGP responses (Fig. 2E, and see also Supplemental Fig. S2).
Figure 2. Tonic and phasic intragastric pressure responses to i.v. CCK-8 (0–1000 pmol kg−1, N = 17).
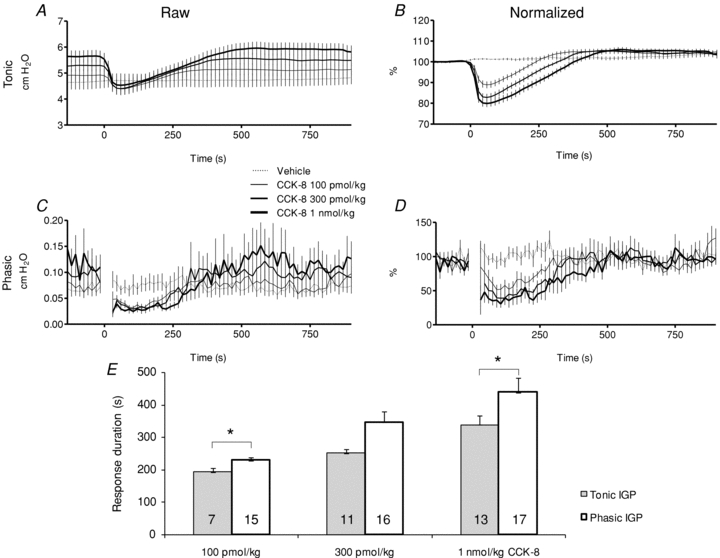
A and C, aggregate plots of raw tonic and phasic IGP records. CCK-8 was injected beginning at t = 0 in each plot. B and D, normalized counterparts of A and C. Points immediately following an injection of CCK-8 injection are excluded from phasic IGP plots (C and D) to omit the transient resulting from the abrupt change in tonic IGP evoked by the injection. E, phasic IGP inhibition persisted longer than did the drop in tonic IGP, and this difference was significant at the 100 pmol kg−1 and 1 nmol kg−1 doses (*P < 0.05, paired t test). The number at the base of each column indicates the number of records for which a reliable measurement of duration was obtained (see Methods for details).
Proximal and distal VGV efferent responses to i.v. CCK-8
Bolus i.v. injection of vehicle did not alter vagal afferent or efferent activity. CCK-8 i.v. elicited dose-dependent changes in vagal afferent and vagal efferent multi-unit activity in both dVGV and pVGV, but the changes in activity observed in the two branches differed markedly.
Prior to any i.v. injections, basal multi-unit efferent discharge was 29.6 ± 8.7 impulses per second (imp s−1, or Hz; n = 7, range: 2.7 to 73.4) in dVGV filaments, and 14.3 ± 3.7 Hz (n = 9, range: 2.3–34.4) in pVGV filaments. In 8/9 (89%) pVGV preparations, CCK-8 increased multi-unit efferent activity. In contrast, in 6/7 (86%) dVGV preparations, CCK-8 inhibited multi-unit efferent activity. In one dVGV preparation CCK-8 evoked efferent excitation rather than inhibition, and in one pVGV preparation CCK-8 evoked efferent inhibition rather than excitation. In each group, the divergent response occurred in the preparation with the lowest initial discharge rate (2.7 imp s−1 in the dVGV preparation, 2.3 imp s−1 in the pVGV preparation) and in each case this rate was less than half that in the next least active bundle. Aggregate response plots are shown in Fig. 3. Data for all experiments in each branch are included in the plots of absolute activity (imp s−1), while the divergent experiments in each branch are excluded from the plots showing the per cent change in activity because, owing to the very low discharge rates of the divergent experiments, their inclusion would disproportionately affect the profile of the aggregate normalized responses. Supplemental Fig. S3 illustrates representative raw multi-unit responses of each type encountered. Of interest, multi-unit efferent responses to CCK-8 in dVGV recordings lasted significantly longer than pVGV efferent responses (Fig. 3E), and also differed in temporal profile (Fig. 4A). At the 1 nmol kg−1 dose, the decay half-time obtained from an exponential curve fit to the recovery (‘decay’) portion of the aggregate dVGV efferent response (inhibition) was 293 s (95% confidence interval: 208 to 495 s, r2 = 0.74, n = 6). This was significantly greater (P < 0.0001, F test) than the 73 s (95% confidence interval: 63 to 87 s, r2 = 0.73, n = 8) decay half-time obtained for the curve fit to the aggregate pVGV efferent response (excitation). As shown in Fig. 4B, the duration of dVGV efferent and phasic IGP reponses were similar and did not differ significantly from each other (paired t test), while both differed significantly from the duration of pVGV efferent responses (one-way ANOVA, P < 0.05). The duration of tonic IGP responses did not differ significantly from the duration of pVGV efferent responses (paired t test). The differences in the temporal profiles of efferent activity between the two branches were similar to the differences observed between the temporal profiles of tonic and phasic IGP described above, and comparison of the response profiles of pVGV efferent activity with tonic IGP and of dVGV efferent activity with phasic IGP revealed a distinct parallelism (Fig. 4C and D). These temporal relationships were similar at all doses of CCK-8 tested (Supplemental Fig. S4).
Figure 3. Dose-related multi-unit efferent activity changes evoked by CCK-8 i.v.
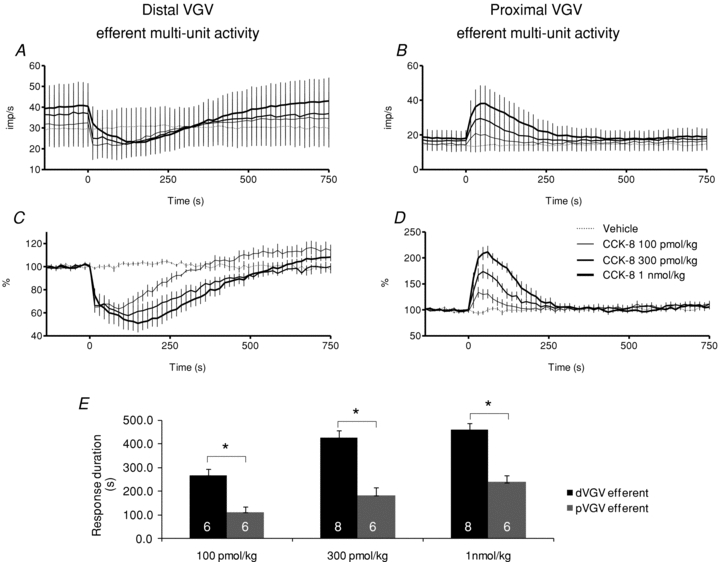
A, distal VGV efferent impulse activity (imp s−1) for all bundles (N = 7). B, proximal VGV efferent impulse activity (imp s−1) for all bundles (N = 9). C, distal VGV normalized activity (%) in bundles with inhibitory responses to CCK-8 (N = 6, excludes the single excitatory response, see text). D, proximal VGV normalized activity (%) in bundles with excitatory responses to CCK-8 (N = 8, excludes the single inhibitory response). CCK-8 injections were initiated at t = 0. E, the duration of distal VGV multi-unit efferent inhibition significantly exceeded the duration of proximal VGV efferent excitation, at each dose tested (*P < 0.05, unpaired t test).
Figure 4. The temporal profiles of efferent responses to i.v. CCK-8 in proximal VGV and distal VGV differ from each other, but are similar to the profiles of tonic and phasic IGP, respectively.
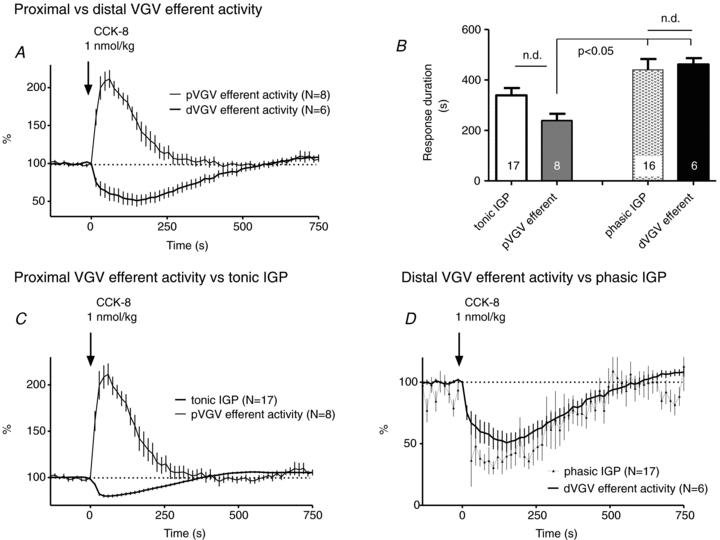
A, a comparison of normalized (%) aggregate multi-unit efferent responses to i.v. CCK-8 (1 nmol kg−1) in the proximal VGV (pVGV) and distal VGV (dVGV). B, durations of efferent and IGP responses to 1 nmol kg−1 CCK-8. The duration of dVGV efferent inhibition did not differ (n.d.) significantly from the duration of phasic IGP inhibition (paired t test), but both were significantly longer than the duration of pVGV efferent excitation (P < 0.05, one-way ANOVA with Bonferroni post hoc test). The duration of pVGV efferent excitation did not differ (n.d.) significantly from that of tonic IGP (paired t test). C and D, plotting tonic IGP vs. pVGV efferent activity (C) and phasic IGP vs. dVGV efferent activity (D) emphasizes the correspondence in time courses. The relationships shown were consistent at all doses tested (100 pmol kg−1–1 nmol kg−1; see Supplemental Material for full dose–response illustration). See text for further details.
Single-unit analysis of CCK-8-evoked efferent activity in the proximal and distal VGV
The aggregate multi-unit activity records reflect the responses of the dominant fibre population in each branch. However, the occurrence of responses in one preparation in each group that differed in direction from those in the majority of recordings in that group indicated the existence of a minority of fibres in each branch whose responses differed from that of the dominant population. To investigate the diversity of efferent unit types present in each branch, multi-unit efferent activity records were sorted to yield single-unit records.
A total of 28 single units (SUs) were discriminated from multi-unit records from 7 of the 9 preparations in which pVGV efferent activity was recorded. Of these 19 (68%) SUs were excited by i.v. CCK-8, 5 (18%) were inhibited and 4 (14%) showed no response. Ongoing discharge rates, measured in the 15 min prior to testing with 1 nmol kg−1 CCK-8, were not significantly different between pVGV efferent SUs excited (1.7 ± 0.4 imp s−1, range 0.1–5.6 imp s−1), those inhibited (2.2 ± 0.5 imp s−1, range 0.5–3.7 imp s−1) and those unaffected (2.0 ± 0.5 imp s−1, range 1.0–3.5) by CCK-8 (one-way ANOVA with Bonferroni post hoc test). The variation in the temporal profile of response among pVGV efferent SUs inhibited by CCK-8 suggested that these comprise a heterogeneous population of unit types (Fig. 5A–C).
Figure 5. Examples of single-unit efferent response to 1 nmol kg−1 in three proximal VGV recordings (upper panels, A–C) and three distal VGV recordings (lower panels, D–F).
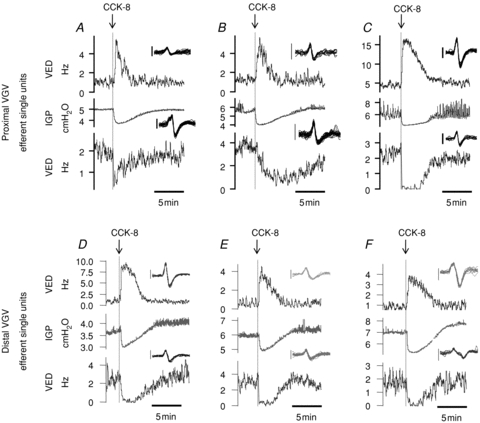
In each panel, a single unit excited by i.v. CCK-8 is shown in the upper trace, a single unit inhibited by i.v. CCK-8 present in the same bundle is shown in the lower trace, and simultaneously recorded IGP is shown in the centre trace. The inset in each frame shows superimposed waveforms of consecutive spikes fired over a 10 s interval prior to CCK-8 injection, with the exception of the excited units in panels E and F which show 20 s of activity – vertical scale bars are 50 μV in each inset, and the duration of each waveform trace is 7.5 ms.
A total of 22 SUs were sorted from multi-unit records obtained from 5 of the 6 preparations in which dVGV efferent activity was recorded. Of these, 12/22 (55%) SUs were inhibited and 6/22 (27%) were excited in a dose-related manner by i.v. CCK-8. Examples of CCK-evoked activity in dVGV SUs are illustrated in Fig. 5D–F. An additional 2/22 units (9%) were only weakly inhibited at the highest dose tested, and their responses were qualitatively different than those of the other 14 units inhibited in a dose-related fashion, while two units showed no response to CCK-8 at any dose tested. Ongoing discharge rates, measured in the 15 min prior to testing with 1 nmol kg−1 CCK-8, were significantly different between dVGV efferent SUs excited (1.1 ± 0.3 imp s−1, range 0.2–2.2 imp s−1, n = 6) and those inhibited (2.0 ± 0.3 imp s−1, range 0.5–4.6 imp s−1, n = 12) by CCK-8 in a dose-related fashion (t test, P < 0.05).
In the pVGV, the profile of the aggregate of all sorted efferent SUs matched that of the multi-unit activity as a whole (not shown). This was not the case in the dVGV. A comparison of the aggregated activity of all sorted dVGV efferent SUs with that of all dVGV efferent multi-units indicated a greater preponderance of excited dVGV efferent units in the sorted population compared to the population as a whole. The greater proportion of dVGV efferent units excited by CCK-8 in the group of sorted SUs compared to that in the total multi-unit activity is probably a result of factors related to the sorting process itself. The sorting criteria demanded that spike waveforms of a given unit be similar to each other, and recognizably different from those of all other units in the record. In dVGV efferent recordings, it was often the case that many units had waveforms of similar amplitude and often similar shape, and therefore, only a minority of units in the record could be separated into reliable single-unit records. Several of the dVGV efferent SUs that were excited by CCK-8 had much higher amplitude spikes than other spikes in the same recording, and each multi-unit record typically included only a small number of units with these large waveforms. Thus, the sorting process may have introduced a bias toward the excited dVGV units, though this did not appear to be the case in the pVGV.
The aggregated response profiles of those SUs excited by CCK-8 were similar between the two branches, as were those for units inhibited by CCK-8 (Fig. 6). The distribution of curve fits for single units is shown in Fig. 6C. No statistically significant difference existed between the populations of calculated t1/2 values for inhibited units in the two branches (dVGV: 269 ± 66 s, n = 12; pVGV: 503 ± 307 s, n = 5) or for excited units in the two branches (dVGV: 62 ± 9 s, n = 6; pVGV: 89 ± 9 s, n = 19), but the t1/2 of inhibited and excited units differed significantly (P < 0.05, one-way non-parametric ANOVA with Dunn's post hoc test).
Figure 6. Comparisons of the temporal profile of efferent single-unit responses to 1 nmol kg−1i.v. CCK-8 in proximal vs. distal VGV.
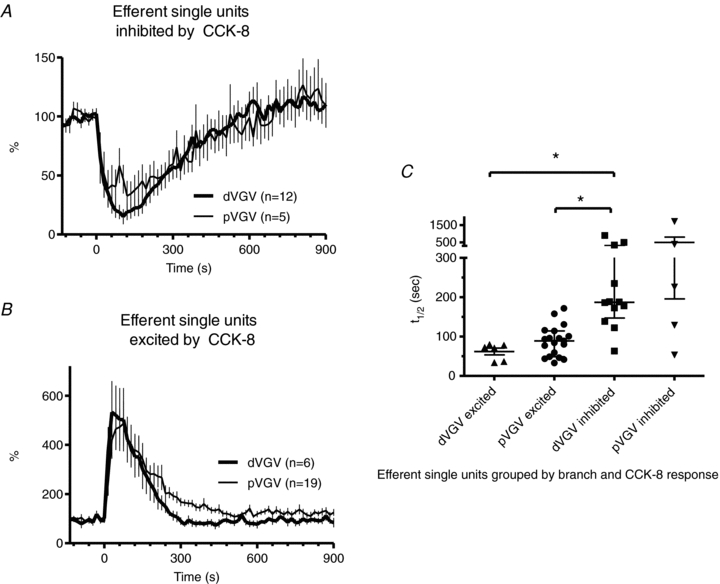
A, plot of aggregate activity of all efferent single-units inhibited by CCK-8 in the proximal vs. distal VGV. B, plot of aggregate activity of all efferent single-units excited by CCK-8 in the proximal vs. distal VGV inhibited. C, half-times determined by fitting a single-phase exponential function to the decay phase of the response for each efferent single unit, grouped by branch and response type (excited or inhibited) to CCK-8. The t1/2 calculated for units inhibited by CCK-8 did not differ significantly between distal and proximal VGV, but both differed significantly from that of proximal VGV units excited by CCK-8 (*P < 0.05, non-parametric ANOVA with Dunn's post hoc test).
Proximal and distal VGV afferent responses to i.v. CCK-8
Prior to any i.v. injections, basal afferent discharge in multi-unit bundles was 22.4 ± 6.1 Hz (n = 7, range: 6.4–42.2 Hz) in dVGV filaments, and 23.4 ± 8.2 Hz (n = 6, range: 4.9–52.2 Hz) in pVGV filaments. Ongoing multi-unit afferent activity often exhibited a distinct modulation by prevailing phasic gastric contractions, an effect that was more common and more pronounced in dVGV recordings. CCK-8 i.v. dose-dependently excited multi-unit afferent activity in both the dVGV and pVGV (Fig. 7), but the amplitude and temporal profile of afferent responses to CCK-8 differed between the two branches. In response to 1 nmol kg−1 CCK-8, dVGV afferent discharge rose to a peak of 170 ± 13% of pre-stimulus levels at 30 s, and the duration of the response (the time to 90% recovery) was 235 ± 46 s (n = 5). The decay half-time of the dVGV afferent response to CCK-8 at 1 nmol kg−1 was 86 s (95% confidence interval: 64 to 129 s, r2 = 0.63, n = 6). In contrast, pVGV afferent activity increased to a peak of 140 ± 8% of pre-stimulus levels at 45 s and declined slowly thereafter. The duration of pVGV afferent excitation, 1164 ± 186 s (n = 5), was significantly longer than the dVGV afferent response (t test, P < 0.05). At lower doses of CCK-8, duration measurements of pVGV afferent activity were unreliable, due to the relatively small magnitude of the excitation compared with the level of variability in the firing rates. However, the aggregate data (Fig. 7) clearly demonstrate that even at the 100 and 300 pmol kg−1 dose, total pVGV afferent activity remained above baseline for more than 20 min post-stimulus. An exponential curve could not be fitted to the decay portion of pVGV afferent responses (r2 < 0.1, 95% confidence interval 30 s to +∞, n = 6). In one pVGV recording, afferent inhibition, rather than excitation, was observed.
Figure 7. Aggregate multi-unit afferent activity recorded from the distal VGV (A and C, N = 6) and proximal VGV (B and D, N = 6) plotted both as mean discharge rate (A and B) and per cent activity relative to prestimulus levels (C and D).
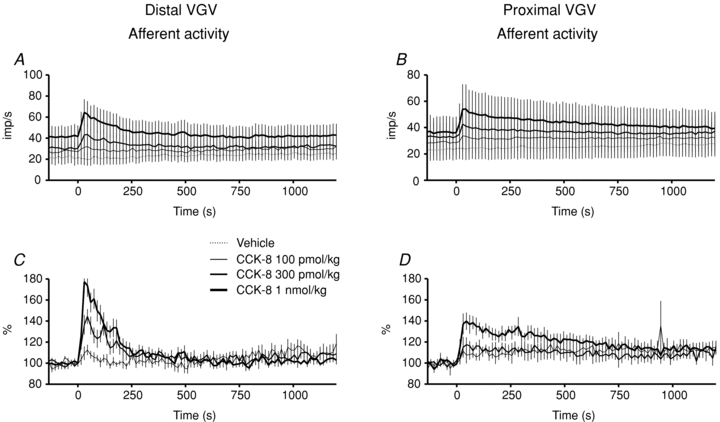
Vehicle traces have been excluded from normalized plots (C and D) for clarity. The large s.e.m.s observed in the mean discharge plots are due to wide variation in the initial discharge rates in bundles from different preparations.
Temporal relationships between vagal efferent and afferent activity
Response profiles and durations
In both dVGV and pVGV dual recordings, the temporal profile of CCK-8-evoked afferent excitation in each branch differed distinctively from that of the efferent responses in the same branch (Fig. 8A and B). Of interest, the temporal relationship of dVGV efferent to dVGV afferent activity bore a striking resemblance to that between dVGV efferent and pVGV efferent activity (Fig. 7A). This suggested that dVGV afferent activity might parallel pVGV efferent activity. Such a parallel was indeed observed (Fig. 8C and Supplemental Fig. S5). Linear regression of dVGV afferent vs. pVGV efferent responses yielded a coefficient of determination (r2) of 0.91 at the 1 nmol kg−1 dose and 0.88 for all doses combined, indicating a strong linear relationship between the two (slope = 1.7 ± 0.1 at 1 nmol kg−1 CCK-8). In contrast, r2 values for regressions of dVGV afferent vs. dVGV efferent responses were 0.31 at the 1 nmol kg−1 dose and 0.11 for all doses combined, and for pVGV afferent vs. pVGV efferent responses, r2 values were 0.46 at the 1 nmol kg−1 dose and 0.10 over all doses combined. Regression of pVGV afferent vs. dVGV efferent responses yielded r2 values of 0.22 both for the 1 nmol kg−1 dose and for all doses combined. Thus, only the dVGV afferent and pVGV efferent responses showed a strong linear relationship. The duration (time to 90% recovery) of dVGV afferent and pVGV efferent responses did not differ (unpaired t test, P < 0.05), while the duration of afferent activity in each branch differed significantly from the duration of efferent activity in the same branch (paired t test, P < 0.5) (Fig. 8D). The response decay half-times for dVGV afferent multi-unit activity (t1/2 = 86 s, 95% confidence interval: 64 to 129 s, r2 0.63, n = 6) and pVGV efferent multi-unit activity (t1/2 = 73 s, 95% confidence interval: 63 to 87 s, r2 = 0.73, n = 8) did not differ significantly (F test).
Figure 8. Temporal correspondence of dVGV afferent and pVGV efferent responses to i.v. CCK-8.
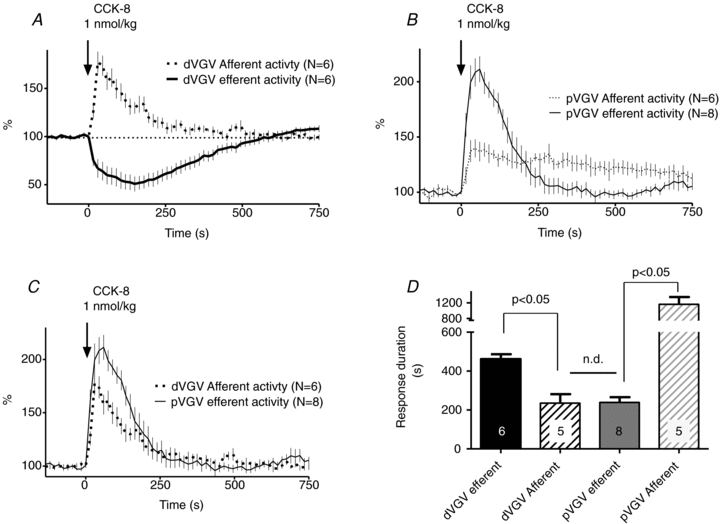
Comparing aggregate response profiles of efferent and afferent activity recorded in the distal VGV (A) and proximal VGV (B), respectively, demonstrates temporal mismatches between the two in each branch. C, distal VGV afferent responses parallel proximal VGV efferent responses, suggesting a potential causal (vago-vagal) link between the two. The relationships shown were consistent at all doses tested (see Supplemental material for comparisons at each dose). D, the durations of response of dVGV efferents and pVGV afferents to 1 nmol kg−1 CCK-8 did not differ (unpaired t test), while the duration of efferent response in each branch differed significantly from the duration of afferent response recorded in the same branch (*P < 0.05, paired t test).
Onset times
To examine the relationship between the onset times of CCK-8-evoked changes in afferent and efferent activity, multi-unit afferent and efferent activity traces were compared in individual preparations. In 4 of 6 dVGV preparations, changes in CCK-8-evoked efferent activity clearly preceded afferent excitation by 3 to 20 s at the 1 nmol kg−1 dose (Supplemental Fig. S6). In the remaining two preparations afferent excitation appeared to lag the onset of the efferent response slightly, but the prevailing variations in ongoing activity made it difficult to definitively specify the beginning of each response. In no case did the onset of CCK-8-evoked dVGV afferent excitation precede the onset of changes in dVGV efferent activity. In contrast to the situation in the dVGV, in two of four pVGV preparations afferent excitation lagged the efferent response, and in two the opposite relation occurred, i.e. no consistent relationship between efferent and afferent response onset times was found in the four preparations in which we were able to perform dual recording in the pVGV.
The observed temporal relationships between efferent and afferent activity suggested that while CCK-8-evoked excitation of dVGV afferents might drive pVGV efferent excitation (given the marked similarity of the response profiles), dVGV afferent excitation alone could not account for dVGV efferent responses that began prior to and persisted longer than such dVGV afferent excitation. We therefore considered the possibility that excitation of vagal afferent fibres innervating the intestines via other subdiaphragmatic branches might contribute to efferent inhibition in the dVGV.
To examine the viability of this hypothesis, four additional experiments were performed in which afferent activity was recorded in the accessory celiac branch of the vagus (ACV). ACV afferent responses to i.v. CCK-8 (1 nmol kg−1) varied considerably more than did dVGV afferent responses (Fig. 9A). The duration (time to 90% recovery) of ACV afferent excitation in response to 1 nmol kg−1 CCK-8 was 485 ± 74 s (n = 4), which did not differ from that of dVGV efferent responses (461 ± 25 s, n = 6), while both were significantly longer than the duration of dVGV afferent responses (235 ± 46 s, n = 5) (P < 0.05, one-way ANOVA). Despite the wide variation in ACV afferent response amplitudes, the temporal profile of aggregate ACV excitation appeared to mirror that of dVGV efferent inhibition, while both differed from that of dVGV afferent excitation (Fig. 9A). An exponential curve could not be fitted to the decay portion of ACV afferent responses (r2 < 0.2, 95% confidence interval 40 s to +∞, n = 4), but a strong linear relationship between ACV afferent and dVGV efferent responses was reflected in a coefficient of determination (r2) of 0.91 at 1 nmol kg−1 CCK-8 for the linear regression between the two. Figure 9B illustrates simultaneously recorded ACV afferent and dVGV afferent responses to i.v. CCK-8, and demonstrates that the ACV afferent response in this recording begins before, and persists longer than simultaneously recorded afferent activity in the distal VGV.
Figure 9. Temporal relationship of ACV afferent to dVGV responses to i.v. CCK-8.
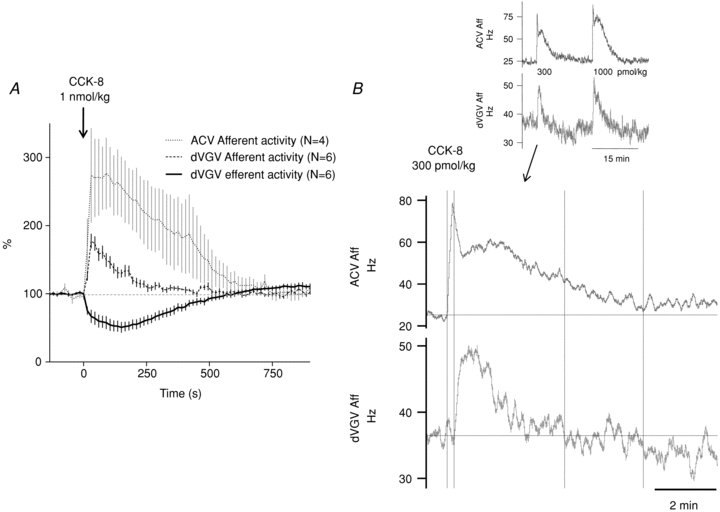
A, aggregate ACV afferent responses to i.v. CCK-8 (1 nmol kg−1), plotted along with dVGV efferent and dVGV afferent responses (recorded in separate experiments). ACV afferent responses mirror dVGV efferent responses, both of which decay more slowly and persist longer than dVGV afferent response. B, a comparison of simultaneously recorded ACV afferent and dVGV afferent activity demonstrating a more rapid onset and longer durations of ACV afferent excitation compared to dVGV afferent excitation in response to i.v. CCK-8. The main figure (lower frame) shows the response to 300 pmol kg−1 CCK-8. Vertical lines denote onset and recovery of each trace, respectively. Upper inset: records from the same recording showing the responses to successive 300 pmol kg−1 and 1000 pmol kg−1 injections.
Proximal and distal efferent and afferent responses to mild gastric distention
The subdiaphragmatic vagal recording sites used in these studies place limits on the volume of gastric inflation that can be achieved without disturbing the recording conditions. We therefore used a moderate distention volume of 1 ml. Gastric distention with 1 ml saline delivered via the intragastric cannula at a rate of 2 ml per min evoked significant increases in afferent excitation in both the dVGV and the pVGV, although the amplitude of the increase in activity was of the order of 2.5-fold greater in the pVGV compared to the dVGV (Fig. 10). Further, pVGV afferents adapted to a substantially greater degree than did dVGV afferents during the hold period of the distention trial. Gastric distention at this volume also evoked a significant increase in pVGV multi-unit efferent activity, but did not significantly alter dVGV multi-unit efferent activity (Fig. 10).
Figure 10. Multi-unit efferent and afferent activity recorded in the distal (A and C) and proximal (B and D) VGV in response to moderate (1 ml) gastric distention.
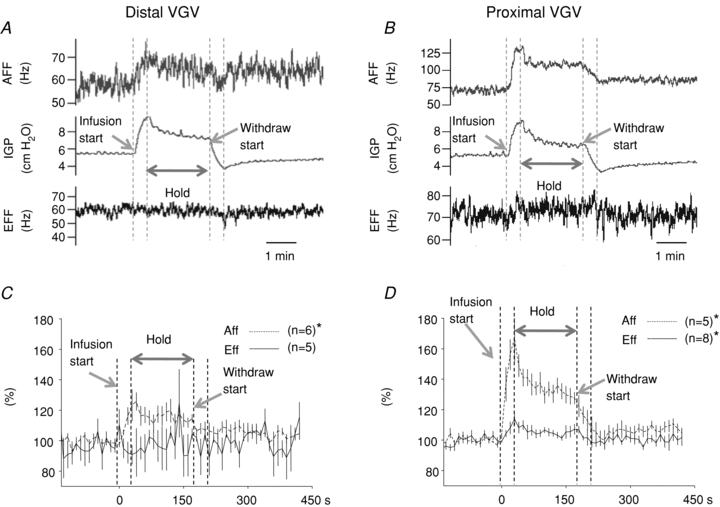
A and B illustrate recordings from individual experiments in each branch. C and D illustrate the aggregate multi-unit responses. Moderate gastric distention caused a significant increase in proximal VGV afferent activity, proximal VGV efferent activity, and distal VGV afferent activity, but not in distal VGV efferent activity.
Discussion
The existence of an ‘inhibitory’ vagal pathway involved in the control of accommodation of the proximal stomach is well established (Langley, 1898; Davison & Grundy, 1978; Andrews et al. 1980; Andrews & Scratcherd, 1980; Grundy et al. 1981; Takahashi & Owyang, 1997; Hermann et al. 2006), but the activity of this fibre population has not been reflected in previous recordings from the gastric vagus nerve. The present study provides the first electrophysiological characterization of the fibre population selectively supplying the proximal stomach via the proximal VGV. The excitation of a majority of proximal VGV efferent fibres indicates that i.v. CCK-8 increases fundic compliance under these experimental conditions in part by activating a population of ‘inhibitory’ vagal preganglionic fibres. Single-unit analysis revealed a diversity of response types in both branches of the VGV, suggesting that a combination of excitatory and inhibitory effects are responsible for the net change in gastric activity in each region.
The most surprising finding of this study was that substantial, statistically significant differences existed between the time courses of i.v. CCK-8-evoked efferent inhibition in the dVGV and efferent excitation in the pVGV. This indicates that different pathways are responsible for driving vagal regulation of the proximal stomach and distal stomach function in response to CCK. The time course of CCK-8-evoked afferent excitation in the dVGV paralleled that of pVGV efferent excitation, but began later and decayed more rapidly than dVGV efferent output. In contrast, the time course of CCK-8 afferent excitation in the ACV was similar to that of dVGV efferent inhibition. Thus, the results suggest that vagal afferent input from the intestines may be primarily responsible for the inhibition of distal gastric contractile activity by CCK, while afferent input from the hindstomach may be primarily responsible for the vagally-mediated influence of CCK on gastric compliance. If so, then endogenous CCK released postprandially may influence distal and proximal gastric motor functions with substantially different time courses, via distinct vagal circuits. CCK released from intestinal I cells in the vicinity of intestinal vagal afferent terminals may immediately cause inhibitory effects on excitatory drive primarily to the distal stomach, while CCK-mediated effects on proximal gastric compliance may occur only after CCK levels in the systemic circulation rise to a level capable of influencing gastric vagal afferent excitation.
In the discussion below, we first consider the differences in the types of efferent and afferent responses to CCK-8 occurring in each branch of the VGV, after which we address the relationships between afferent and efferent responses within and between branches, and the implications of these temporal patterns.
Efferent responses to i.v. CCK-8 in the distal vs. proximal VGV
The time course of CCK-8-evoked dVGV efferent inhibition roughly paralleled the inhibition of phasic IGP (which primarily reflects the contractile activity of the distal corpus and antrum) (Fig. 4). This inhibitory effect of CCK-8 on dVGV motor output is qualitatively similar to that observed in two prior studies, each of which used recording sites in either the distal or common VGV, although this distinction was not made (Niijima et al. 1996; Bucinskaite et al. 2000). At either location, the aggregate efferent activity observed would reflect primarily activity in efferent fibres innervating the distal stomach.
In the dVGV, the majority of efferent fibres inhibited by CCK-8 are likely to comprise excitatory cholinergic fibres involved in regulating the amplitude of phasic contractions in the hindstomach (Sarna & Daniel, 1975; Daniel & Sarna, 1976; Andrews & Scratcherd, 1980; Fox et al. 1982; Lloyd et al. 1992; Zhou et al. 2008), as well as relaxatory fibres controlling pyloric tone (Mir et al. 1979; Telford et al. 1979; Lingenfelser et al. 1997). Other inhibited dVGV efferents may include those involved in the inhibition of gastric acid secretion by CCK-8 (Raybould & Lloyd, 1994), although under urethane anaesthesia, acid secretion is profoundly inhibited already due substantially to the pronounced release of somatostatin induced by urethane (Yang et al. 1990).
The minority of dVGV efferent single-units that were excited by CCK-8 may include motor fibres contributing to CCK-8-evoked tonic narrowing of the antrum (Allescher et al. 1989; Adelson et al. 2004) or excitation of duodenal phasic contractions (Allescher et al. 1989; Giralt & Vergara, 1999), while others probably project to the pancreas and contribute to the vagally driven component of CCK-8-evoked pancreatic secretion (Li et al. 1997; Wan et al. 2007). Due to the diversity of physiological functions mediated by dVGV efferent fibres, in order to permit reasonable inference of fibre type it will be necessary to test responses to multiple stimuli that differentially influence each of these functions.
CCK-8-evoked pVGV efferent excitation was significantly shorter in duration than was dVGV efferent inhibition, and roughly paralleled the time course of the reduction in tonic IGP. In the pVGV it is likely that most or all efferent fibres participate in the control of forestomach tone, given that this is the primary, if not sole, function of this region of the rat stomach. Presumably pVGV excitation reflects activation of the so-called vagal inhibitory pathway to the proximal stomach (Martinson, 1965; Jansson, 1969; Abrahamsson & Jansson, 1973). Consistent with this assumption, a significant increase in pVGV efferent activity was observed in response to moderate gastric inflation, coincident with a steady fall in IGP indicative of adaptive accommodation, throughout the inflation trial (Fig. 10). (A more extended discussion of the interpretation of the phasic and tonic components of IGP changes evoked by CCK-8 is contained in Supplemental material available online.) Proximal VGV efferent fibres inhibited by i.v. CCK-8 are most probably excitatory fibres whose inhibition may complement the excitation of the majority of inhibitory fibres in producing forestomach relaxation. It is of note that the proportion of efferent single units inhibited by CCK-8 in the pVGV in this study (18%) corresponds closely to the 17% of fundic-projecting vagal dorsal motor nucleus (DMV) neurons that are believed to comprise an excitatory preganglionic projection to cholinergic postganglionic neurons (Pearson et al. 2007; Herman et al. 2008).
The pVGV ‘inhibitory’ fibres excited by i.v. CCK-8 may comprise two or more distinct classes. Inhibition of fundic smooth muscle could result from (1) excitation of cholinergic preganglionic fibres that project to postganglionic inhibitory non-adrenergic, non-cholinergic (NANC) neurons (Aimi et al. 1993; Takahashi & Owyang, 1995, 1997; Nakamura et al. 1998; Jarvinen et al. 1999; Zhou et al. 2008), and/or (2) excitation of preganglionic inhibitory nitrergic neurons (Krowicki et al. 1997; Zheng et al. 1999) that project to postganglionic excitatory cholinergic neurons. In either case, the net effect of excitation of the preganglionic fibre would be a reduction in smooth muscle tone. Zheng et al. (1999) found in the rat that 12% of DMV cells labelled by retrograde tracers applied to the fundus express nitric oxide synthase. This proportion is small enough that it cannot account for the entirety of the 68% (19/28) of pVGV fibres excited by i.v. CCK-8, unless there is a much larger population of fibres within the nerve that were silent throughout the experiment, a possibility we believe unlikely. Thus, the majority of excited fibres are likely to comprise cholinergic preganglionic fibres that drive NANC inhibitory neurons in the myenteric plexus. Finally, centrifugally directed CCK-8-evoked impulse activity might be the result of descending impulse traffic in axon collaterals of afferent fibres innervating both the oesophagus and stomach. It has previously been shown that approximately 10% of centrifugal impulse activity recorded in the VGV is attributable to activity in axon collaterals with this type of branching (Wei et al. 1992, 1995).
The diversity of efferent response types within each branch are consistent with work in the ferret demonstrating that both proximal and distal regions of the stomach each receive a combination of excitatory and inhibitory vagal motor inputs (Andrews & Scratcherd, 1980), and provides support for earlier proposals, based on recordings of efferent activity made at the cervical level, that gastric smooth muscle activity in each region is adjusted by reciprocal changes in excitatory and inhibitory drive (Grundy et al. 1981; Blackshaw & Grundy, 1989; Chang et al. 2003). This type of ‘push–pull’ regulation of gastric accommodation via reciprocal changes in opposing fibre types is similar to that suggested by Hermann et al. (2006) in the case of the oesophagogastric reflex, although the degree to which excitation of ‘inhibitory’ preganglionic fibres contributes to accommodation during the oesophagogastric reflex has been disputed (Herman et al. 2008). It is likely that the relative role of differing neuronal subpopulations in driving accommodation varies substantially with physiological condition, and thus we make no assertion that the particular balance of excitatory and inhibitory effects of i.v. CCK-8 observed in these experiments represents a stereotypical pattern.
In this regard, it should be noted that the role of vagal efferent pathways in producing forestomach relaxation in response to CCK-8 has been questioned (Takahashi & Owyang, 1999). Initial studies investigating the motor mechanisms by which i.v. CCK-8 reduces IGP in rats determined that the pathways involved comprised a combination of a vagal, hexamethonium-sensitive, non-adrenergic pathway and a splanchnic, hexamethonium-insensitive α-adrenergic pathway (Raybould et al. 1987; Raybould & Taché, 1988). However, the drop in IGP cannot be considered as an unambiguous reflection of changes in forestomach tone, since i.v. CCK-8 also causes relaxation of the lower oesophageal sphincter (Behar & Biancani, 1977; Adelson et al. 2004), and so the drop in tonic IGP is also influenced by the resulting common cavity effects. A study examining the effects of i.v. CCK-8 in the proximal stomach specifically, using strain gauges acutely sutured to serosal surface of ‘the gastric body’ in a circular orientation in rats anaesthetized with a combination of xylazine and ketamine, found that circular relaxation in response to CCK-8 was entirely mediated by a splanchnic motor pathway (Takahashi & Owyang, 1999). The data obtained in the present study clearly indicate that i.v. CCK-8 alters the activity of a majority of vagal efferent fibres supplying the proximal stomach in rats under urethane anaesthesia. While we cannot exclude the possibility that this vagal drive to the forestomach does not drive proximal gastric relaxation, we consider it more likely that other factors may account for the apparent contradictions between the present results and those of this earlier study. One possibility is that the use of acutely implanted strain gauges in the gastric body might affect subsequent accommodation responses and/or that circularly oriented strain gauges might not capture all accommodative effects exerted by i.v. CCK-8 in the proximal stomach, i.e. those acting primarily on longitudinal rather than circular muscle. Alternatively, specific aspects of the preparation we have used, such as the high circulating concentrations of somatostatin resulting from the use of urethane anaesthesia (Yang et al. 1990), may cause a greater degree of vagal efferent output to the stomach in response to CCK-8 than occurs under other conditions, or may alter the extent to which this outflow influences gastric tone.
Afferent responses to i.v. CCK-8 in the distal vs. proximal VGV
A large number of prior studies in the rat and ferret have investigated vagal afferent responses to CCK in various branches of the subdiaphragmatic vagus nerve (Niijima, 1981, 1983; Davison & Clarke, 1988; Blackshaw & Grundy, 1990; Schwartz et al. 1994, 1995, 1997; Schwartz & Moran, 1994; Grundy et al. 1995; Richards et al. 1996; Yoshida-Yoneda et al. 1996; Cox & Randich, 1997; Eastwood et al. 1998; Li et al. 1999, 2004; Lal et al. 2001; Glatzle et al. 2003; Darcel et al. 2005; Date et al. 2005). These studies have established (1) the role of CCK-A receptors in mediating the CCK-induced excitation of gastric and intestinal vagal afferent fibres, and resulting effects on satiation and vago-vagally mediated gastric motility responses, (2) the role of duodenal intraluminal stimuli in evoking vagal afferent discharge via a CCK-A receptor-mediated mechanism, and (3) the interaction of diverse hormonal and mechanical stimuli in shaping CCK-mediated afferent signalling and vice versa, including the impact of mechanical loading of the stomach on CCK-8-evoked vagal afferent excitation.
In the dVGV, multi-unit afferent activity comprised a combination of mechanoreceptive and chemoreceptive components, since a clear modulation of afferent activity by ongoing phasic contractions was often apparent, while following CCK-8 injection, multi-unit firing increased rapidly, coincident with an inhibition of phasic contractions and a drop in tone. The temporal profile of the dVGV afferent response to i.v. CCK-8 comprised a dose-related, rapid rise to peak excitation followed by a rapid decay in activity, consistent with excitation of a contingent of chemosensitive afferent fibres by circulating levels of CCK-8 post-injection. In addition, the net afferent response also presumably includes a contribution from the activity of gastric tension receptors excited by CCK-8-mediated tonic circular contraction of the antral region (Blackshaw & Grundy, 1990), as well as reductions in activity of gastric tension receptors responsive to phasic contractile activity.
In the pVGV, afferent responses were much weaker and longer-lived than in the dVGV, often lasting in excess of 20 min post-stimulus. The early phase of the pVGV multi-unit afferent response may result from excitation of fibres directly sensitive to CCK-8, but the latter portion of the response presumably reflects indirect excitation resulting from longer-lived physiological changes. Such changes may include changes in fundic muscle tension and/or length (stretch). While a great deal of experimental work has focused on fundic intramural tension receptors, which have been elegantly identified with the intralaminar ganglionic endings of vagal afferents (Zagorodnyuk et al. 2001; Blackshaw et al. 2007), it also appears that stretch, rather than intramural tension per se, may be an effective stimulus activating a minority of mechanoreceptors in the proximal stomach (Blackshaw et al. 1987; Phillips & Powley, 2000). The use of a recording site in the pVGV is likely to improve the chance of sampling this potentially important class of fibres.
Temporal relationships between afferent and efferent activity evoked by i.v. CCK-8 in dVGV, pVGV and ACV
Simultaneous recording of afferent and efferent activity in the dVGV made it possible to note that dVGV afferent excitation developed in most cases after a delay of a number of seconds relative to the prompt drop in efferent activity in the same branch, and never prior to it. Also, dVGV afferent excitation decayed well before dVGV efferent activity recovered to pre-stimulus levels, at all doses tested. Thus, dVGV afferent excitation is unlikely to drive the inhibition of dVGV efferent activity, at least during the initial and the late phase of the response. We noted that the relationship of dVGV efferent to dVGV afferent activity strongly resembled the relationship between efferent activity evoked by CCK-8 in the dVGV vs. the pVGV, which suggested a potential parallel between dVGV afferent and pVGV efferent responses to CCK-8. Direct comparison of the aggregate response profiles, linear regression analysis, and statistical comparisons of response duration and response decay half-times all confirmed the similarity of dVGV afferent responses and pVGV efferent responses. The correspondence suggests that a vago-vagal reflex arc regulating changes in forestomach capacity in response to CCK may run from afferent fibres supplying the distal stomach to inhibitory motor fibres supplying the proximal stomach, although other pathways may also play a role. This hypothesis is consistent with findings in the ferret demonstrating that removal of the antrum changes the response to exogenous CCK-8 from a reduction in tone to an increase in tone (Blackshaw & Grundy, 1991). Similarly, in humans distal gastrectomy impairs fundic accommodation in response to a meal (Le Blanc-Louvry et al. 2003). In contrast to the consistent temporal correlations seen between dVGV afferent activity and pVGV efferent activity in response to i.v. CCK-8, no similar correspondence existed in the reverse direction, i.e. between pVGV afferent excitation and dVGV efferent inhibition. Indeed, the pattern of pVGV afferent excitation showed no obvious relation to any other profile recorded in this study.
The temporal mismatch between CCK-8-evoked gastric afferent excitation and dVGV efferent inhibition raises two questions. What inputs could drive dVGV efferent inhibition, and why does the time course differ from that of dVGV afferent excitation (and, in parallel, pVGV efferent excitation)? With respect to the first question, it seems unlikely that the more rapidly developing and longer lasting efferent inhibition is due to a humoral route of influence of CCK-8. A humoral route has been shown to be responsible for some component of i.v. CCK-8-mediated gastric vagal efferent inhibition at the doses used in the present study, but that humorally-mediated component appeared to develop more slowly and decay more quickly than did the vagal- afferent-mediated component (Bucinskaite et al. 2000). It is also possible that splanchnic afferent input drives some component of dVGV efferent inhibition, but it has been shown, under similar experimental conditions, that vagal afferents play a role in the inhibition of phasic activity by CCK-8 (Raybould & Taché, 1988). Since CCK released from intestinal endocrine cells inhibits gastric emptying via excitation of intestinal vagal afferent fibres (Forster et al. 1990; Raybould & Lloyd, 1994; Glatzle et al. 2003), and since removal of the small intestine in the ferret has been shown to blunt the gastric motor inhibitory response of exogenous CCK-8 (Blackshaw & Grundy, 1991), it seemed possible that intestinal vagal afferent input might be responsible for inhibiting dVGV efferent outflow. We therefore recorded from ACV afferent fibres to determine whether the time course of their response to CCK-8 differed from that of dVGV afferents, and whether it was comparable to that of dVGV efferent inhibition. Indeed, this is what was found. The mean duration of afferent ACV responses to CCK-8 was nearly identical to that of dVGV efferent responses, and both were significantly longer than that of dVGV afferent responses. Further, regression analysis and qualitative comparisons of aggregate response profiles indicated a strong correspondence between ACV afferent and dVGV efferent activity. Additionally, a direct comparison of simultaneously recorded ACV afferent and dVGV afferent profiles showed that CCK-8-evoked excitation of the former began before and lasted longer than that of the latter (Fig. 9). Thus, some proportion of intestinal vagal afferent fibres could well be responsible for driving the observed pattern of inhibition of dVGV efferent inhibition.
This leaves the question of why the intestinal afferent input lasted longer than gastric afferent input, when both were initiated by the same i.v. CCK-8 injection. At least four potential explanations exist. First, differences in receptor desensitization, internalization and recycling as well as intracellular signal transduction mechanisms in intestinal vs. gastric afferents may play a role in the differing response kinetics. Second, the prolonged afferent response in the ACV might result from activation of intestinal vagal mechanoreceptors evoked by long-lived, CCK-8-evoked increases in intestinal motility. However, this seems unlikely, since although duodenal vagal mechanoreceptors are indeed excited by CCK-8 as a result of an increase in duodenal motility, the duodenal motility changes appear shorter-lived than the gastric effects (Blackshaw & Grundy, 1990). Third, intestinal vagal afferents might express a different CCK-receptor subtype, with different response properties, than is present on dVGV afferent fibres. This possibility has some experimental support (Li et al. 1997, 1999, 2004). The CCK-A receptor has two pharmacologically-defined binding sites, or states: a high-affinity (Kd = 26 pm), low-capacity site, or state (CCK-A-ha), and a low-affinity (Kd = 2.2 nm), high-capacity state (CCK-A-la) (Sankaran et al. 1982). Individual vagal afferents are excited by CCK either via CCK-A-la or via CCK-A-ha, but not both (Li et al. 1999). Other things being equal, CCK-8, injected i.v. over a ∼20 s interval, would activate vagal afferent fibres bearing CCK-A-ha slightly before it would activate fibres bearing the ∼100-fold less sensitive CCK-A-la, and could continue to excite the former population after the concentration of CCK-8 fell below the level capable of exciting the latter. However, activation of CCK-la, not CCK-ha, is responsible for CCK-8-mediated inhibition of liquid gastric emptying (Moran et al. 1994). Of course, this inhibitory effect on liquid emptying could result primarily from vagally-mediated changes in proximal gastric motor function and from direct effects on pyloric and/or antral muscle, rather than from effects on distal gastric efferent drive and phasic contractions. Still, the absence of any inhibitory effect of JMV-180, a selective CCK-A-ha agonist and CCK-A-la antagonist, on gastric emptying (Moran et al. 1994) suggests that it may also fail to substantially inhibit phasic contractile activity of the distal stomach, a conclusion supported by preliminary motility studies we have performed (D.W.A., unpublished observations).
Finally, unique characteristics of the intestinal vascular supply, particularly the structure of intestinal fenestrated capillaries, and/or the positioning of intestinal vagal afferent terminals relative to the intestinal vasculature, may be such that, as it is injected i.v., CCK-8 reaches intestinal chemoreceptor terminals more quickly than it reaches gastric chemoreceptor terminals, and persists in their terminal microenvironment for a longer period of time. Consistent with this possibility, it has recently been demonstrated that glucgon-like peptide-1 (GLP-1) is cleared from the intestinal extracellular space via the lymphatic system more slowly than it is cleared from the portal circulation (D’Alessio et al. 2007). The action of CCK-8 on different populations of afferent fibres, in different compartments with different clearance characteristics, could account for the several-fold longer decay half-times for CCK-8-evoked dVGV efferent inhibition compared to that for pVGV efferent excitation. Of note, the 73 s decay half-time for pVGV efferent excitation is comparable to the 78 s half-time for clearance of CCK-8 from the systemic circulation measured in dogs (Hoffmann et al. 1993).
The role of vago-vagal reflex arcs between the duodenum and stomach in limiting gastric emptying in response to luminal nutrients is a long-established principle (Hunt, 1956), and it is well known that intestinal nutrients alter gastric emptying in part via vago-vagal reflexes involving CCK signalling (Forster et al. 1990). Based on our results, we suggest that the excitation of intestinal vagal afferent terminals by CCK released postprandially from nearby intestinal endocrine cells primarily drives inhibition of vagal excitatory motor drive to the hindstomach. This negative feedback loop would act rapidly, due to the high local concentrations of CCK achieved following release from adjacent I-cells, and would operate continuously to match delivery of nutrients to the intestine with its digestive and absorptive capacity as has been suggested. In contrast, effects of postprandially released CCK on gastric accommodation may occur only later, as CCK reaches the systemic circulation in amounts sufficient to influence the activity of gastric afferent fibres and/or other sensors with access to the systemic circulation. Such circulating concentrations of CCK, of course, are necessarily much lower than the levels achieved in the intestinal interstitium. Direct effects of circulating CCK on gastric mucosal chemoreceptors, combined with synergistic and/or antagonistic influences of local mechanical forces and paracrine signals, as well as other endocrine signals, on these and other types of distal gastric afferent fibres would then shape the level of inhibitory efferent drive to the proximal stomach, so as to adjust proximal gastric capacity to accommodate longer-term storage of stomach contents and reduce gastro-duodenal pressure gradients. Despite the attractiveness of this proposal, it is possible that there exist other populations of afferent fibres or central neurons with access to the systemic circulation that have CCK-8 response profiles comparable to the responses recorded here, and so a definitive demonstration of the inputs responsible for the observed differences in efferent outflow to the proximal vs. distal stomach awaits further studies.
Acknowledgments
The authors wish to thank Dr Jen Yu Wei, whose innovative work formed the foundation for the approaches used here. The work was supported by NIH R21 DK074736-01 (D.W.A.), DK 41301 (Animal Core, Y.T., D.W.A.), a VA Research Career Scientist Award (Y.T.) and by Takeda Pharmaceutical Co., Ltd, Pharmaceutical Research Division.
Glossary
Abbreviations
- ACV
accessory celiac branch of the vagus
- CCK-8
sulfated cholecystokinin octapeptide
- DMV
dorsal motor nucleus of the vagus
- dVGV
distal subdivision of the ventral gastric vagus
- GLP-1
glucagon like peptide-1
- IGP
intragastric pressure
- LES
lower oesophageal sphincter
- NANC
nonadrenergi-noncholinergic
- phIGP
root mean square amplitude of phasic component of intragastric pressure
- pVGV
proximal subdivision of the ventral gastric vagus
- SU
single unit
- tIGP
tonic component of intragastric pressure
- VGV
ventral gastric vagus
Author contributions
The experiments were performed in the laboratories of D.W.A. and Y.F.T. at the West Los Angeles campus of the VA Greater Los Angeles Area Healthcare System (VAGLAHS). D.W.A. conceived and designed the experiments. S.O.M. and D.W.A. conducted the experiments. S.O.M., J.A.M. and D.W.A. analysed and interpreted the data. D.W.A. drafted the manuscript, and all authors participated in the preparation of the final version of the manuscript, which all approved.
Supporting information
References
- Abrahamsson H, Jansson G. Vago-vagal gastro-gastric relaxation in the cat. Acta Physiol Scand. 1973;88:289–295. doi: 10.1111/j.1748-1716.1973.tb05457.x. [DOI] [PubMed] [Google Scholar]
- Adelson DW, Kosoyan HP, Wang Y, Steinberg JZ, Taché Y. Gastric vagal efferent inhibition evoked by intravenous CRF is unrelated to simultaneously recorded vagal afferent activity in urethane-anesthetized rats. J Neurophysiol. 2007;97:3004–3014. doi: 10.1152/jn.01143.2006. [DOI] [PubMed] [Google Scholar]
- Adelson DW, Million M, Kanamoto K, Palanca T, Taché Y. Coordinated gastric and sphincter motility evoked by intravenous CCK-8 as monitored by ultrasonomicrometry in rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G321–G332. doi: 10.1152/ajpgi.00057.2003. [DOI] [PubMed] [Google Scholar]
- Aimi Y, Kimura H, Kinoshita T, Minami Y, Fujimura M, Vincent SR. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993;53:553–560. doi: 10.1016/0306-4522(93)90220-a. [DOI] [PubMed] [Google Scholar]
- Allescher HD, Daniel EE, Fox JE, Kostolanska F, Rovati LA. Effect of the novel cholecystokinin receptor antagonist CR-1392 on cholecystokinin-induced antroduodenal and pyloric motor activity in vivo. J Pharmacol Exp Ther. 1989;251:1134–1141. [PubMed] [Google Scholar]
- Andrews PL, Grundy D, Lawes IN. The role of the vagus and splanchnic nerves in the regulation of intragastric pressure in the ferret. J Physiol. 1980;307:401–411. doi: 10.1113/jphysiol.1980.sp013442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Scratcherd T. The gastric motility patterns induced by direct and reflex excitation of the vagus nerves in the anaesthetized ferret. J Physiol. 1980;302:363–378. doi: 10.1113/jphysiol.1980.sp013248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz F, Malagelada JR. Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am J Physiol Gastrointest Liver Physiol. 1986;251:G727–G735. doi: 10.1152/ajpgi.1986.251.6.G727. [DOI] [PubMed] [Google Scholar]
- Behar J, Biancani P. Effect of cholecystokinin-octapeptide on lower esophageal sphincter. Gastroenterology. 1977;73:57–61. [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol Respir Physiol. 1991a;260:R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Fox EA, Powley TL. Abdominal pathways and central origin of rat vagal fibers that stimulate gastric acid. Gastroenterology. 1991b;100:627–637. doi: 10.1016/0016-5085(91)80006-u. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Responses of vagal efferent fibres to stimulation of gastric mechano- and chemoreceptors in the anaesthetized ferret. J Auton Nerv Syst. 1989;27:39–45. doi: 10.1016/0165-1838(89)90127-6. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Locally and reflexly mediated effects of cholecystokinin-octapeptide on the ferret stomach. J Auton Nerv Syst. 1991;36:129–137. doi: 10.1016/0165-1838(91)90109-g. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D, Scratcherd T. Vagal afferent discharge from gastric mechanoreceptors during contraction and relaxation of the ferret corpus. J Auton Nerv Syst. 1987;18:19–24. doi: 10.1016/0165-1838(87)90130-5. [DOI] [PubMed] [Google Scholar]
- Bucinskaite V, Kurosawa M, Lundeberg T. Exogenous cholecystokinin-8 reduces vagal efferent nerve activity in rats through CCKA receptors. Br J Pharmacol. 2000;129:1649–1654. doi: 10.1038/sj.bjp.0703270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Spiller D, Jiang W, Thompson D, Attwood S, Saeed S, Grundy D, Varro A, Dimaline R, Dockray GJ. Localization of orexin-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology. 2003;124:129–139. doi: 10.1053/gast.2003.50020. [DOI] [PubMed] [Google Scholar]
- Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- Cox JE, Randich A. CCK-8 activates hepatic vagal C-fiber afferents. Brain Res. 1997;776:189–194. doi: 10.1016/s0006-8993(97)01036-6. [DOI] [PubMed] [Google Scholar]
- D’Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2163–R2169. doi: 10.1152/ajpregu.00911.2006. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Sarna SK. Distribution of excitatory vagal fibers in canine gastric wall to control motility. Gastroenterology. 1976;71:608–613. [PubMed] [Google Scholar]
- Darcel NP, Liou AP, Tome D, Raybould HE. Activation of vagal afferents in the rat duodenum by protein digests requires PepT1. J Nutr. 2005;135:1491–1495. doi: 10.1093/jn/135.6.1491. [DOI] [PubMed] [Google Scholar]
- Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146:3518–3525. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- Davison JS, Clarke GD. Mechanical properties and sensitivity to CCK of vagal gastric slowly adapting mechanoreceptors. Am J Physiol Gastrointest Liver Physiol. 1988;255:G55–G61. doi: 10.1152/ajpgi.1988.255.1.G55. [DOI] [PubMed] [Google Scholar]
- Davison JS, Grundy D. Modulation of single vagal efferent fibre discharge by gastrointestinal afferents in the rat. J Physiol. 1978;284:69–82. doi: 10.1113/jphysiol.1978.sp012528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood C, Maubach K, Kirkup AJ, Grundy D. The role of endogenous cholecystokinin in the sensory transduction of luminal nutrient signals in the rat jejunum. Neurosci Lett. 1998;254:145–148. doi: 10.1016/s0304-3940(98)00666-1. [DOI] [PubMed] [Google Scholar]
- Forster ER, Green T, Elliot M, Bremner A, Dockray GJ. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am J Physiol Gastrointest Liver Physiol. 1990;258:G552–G556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- Fox JE, Daniel EE, Jury J, Track NS, Chiu S. Cholinergic control of canine antral immunoreactive gastrin release and motility. Can J Physiol Pharmacol. 1982;60:893–901. doi: 10.1139/y82-126. [DOI] [PubMed] [Google Scholar]
- Giralt M, Vergara P. Both afferent and efferent nerves are implicated in cholecytokinin motor actions in the small intestine of the rat. Regul Pept. 1999;81:73–80. doi: 10.1016/s0167-0115(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Darcel N, Rechs AJ, Kalogeris TJ, Tso P, Raybould HE. Apolipoprotein A-IV stimulates duodenal vagal afferent activity to inhibit gastric motility via a CCK1 pathway. Am J Physiol Regul Integr Comp Physiol. 2004;287:R354–R359. doi: 10.1152/ajpregu.00705.2003. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Wang Y, Adelson DW, Kalogeris TJ, Zittel TT, Tso P, Wei JY, Raybould HE. Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J Physiol. 2003;550:657–664. doi: 10.1113/jphysiol.2003.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D, Bagaev V, Hillsley K. Inhibition of gastric mechanoreceptor discharge by cholecystokinin in the rat. Am J Physiol Gastrointest Liver Physiol. 1995;268:G355–360. doi: 10.1152/ajpgi.1995.268.2.G355. [DOI] [PubMed] [Google Scholar]
- Grundy D, Salih AA, Scratcherd T. Modulation of vagal efferent fibre discharge by mechanoreceptors in the stomach, duodenum and colon of the ferret. J Physiol. 1981;319:43–52. doi: 10.1113/jphysiol.1981.sp013890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JJ, Browning KN, Rogers RC, Travagli RA. Catecholaminergic neurons in rat dorsal motor nucleus of vagus project selectively to gastric corpus. Am J Physiol Gastrointest Liver Physiol. 2001;280:G361–G367. doi: 10.1152/ajpgi.2001.280.3.G361. [DOI] [PubMed] [Google Scholar]
- Harper AA, Kidd C, Scratcherd T. Vago-vagal reflex effects on gastric and pancreatic secretion and gastrointestinal motility. J Physiol. 1959;148:417–436. doi: 10.1113/jphysiol.1959.sp006297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Niedringhaus M, Alayan A, Verbalis JG, Sahibzada N, Gillis RA. Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in reflex control of fundus tone. Am J Physiol Regul Integr Comp Physiol. 2008;294:R720–R729. doi: 10.1152/ajpregu.00630.2007. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Travagli RA, Rogers RC. Esophageal-gastric relaxation reflex in rat: dual control of peripheral nitrergic and cholinergic transmission. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1570–R1576. doi: 10.1152/ajpregu.00717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Eberlein GA, Reeve JR, Jr, Bunte RH, Grandt D, Goebell H, Eysselein VE. Comparison of clearance and metabolism of infused cholecystokinins 8 and 58 in dogs. Gastroenterology. 1993;105:1732–1736. doi: 10.1016/0016-5085(93)91070-x. [DOI] [PubMed] [Google Scholar]
- Hunt JN. Some properties of an alimentary osmoreceptor mechanism. J Physiol. 1956;132:267–288. doi: 10.1113/jphysiol.1956.sp005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson G. Vago-vagal reflex relaxation of the stomach in the cat. Acta Physiol Scand. 1969;75:245–252. doi: 10.1111/j.1748-1716.1969.tb04376.x. [DOI] [PubMed] [Google Scholar]
- Jarvinen MK, Wollmann WJ, Powrozek TA, Schultz JA, Powley TL. Nitric oxide synthase-containing neurons in the myenteric plexus of the rat gastrointestinal tract: distribution and regional density. Anat Embryol (Berl) 1999;199:99–112. doi: 10.1007/s004290050213. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- Langley JN. On inhibitory fibres in the vagus for the end of the œsophagus and the stomach. J Physiol. 1898;23:407–414. doi: 10.1113/jphysiol.1898.sp000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc-Louvry I, Savoye G, Maillot C, Denis P, Ducrotte P. An impaired accommodation of the proximal stomach to a meal is associated with symptoms after distal gastrectomy. Am J Gastroenterol. 2003;98:2642–2647. doi: 10.1111/j.1572-0241.2003.08725.x. [DOI] [PubMed] [Google Scholar]
- Legros G, Griffith CA. The anatomic basis for the variable adequacy of incomplete vagotomy: II. The various responses to insulin of various anatomic types of incomplete vagotomy in dogs. Ann Surg. 1968;168:1035–1042. doi: 10.1097/00000658-196812000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros G, Griffith CA. The abdominal vagal system in rats. An anatomical study with emphasis upon the distribution of the gastric vagi to the stomach. J Surg Res. 1969;9:183–186. doi: 10.1016/0022-4804(69)90050-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Hao Y, Owyang C. High-affinity CCK-A receptors on the vagus nerve mediate CCK-stimulated pancreatic secretion in rats. Am J Physiol Gastrointest Liver Physiol. 1997;273:G679–G685. doi: 10.1152/ajpgi.1997.273.3.G679. [DOI] [PubMed] [Google Scholar]
- Li Y, Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology. 1994;107:525–531. doi: 10.1016/0016-5085(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu XY, Owyang C. Serotonin and cholecystokinin synergistically stimulate rat vagal primary afferent neurones. J Physiol. 2004;559:651–662. doi: 10.1113/jphysiol.2004.064816. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li Y, Zhu J, Owyang C. Electrical physiological evidence for high and low-affinity vagal CCK-A receptors. Am J Physiol Gastrointest Liver Physiol. 1999;277:G469–G477. doi: 10.1152/ajpgi.1999.277.2.G469. [DOI] [PubMed] [Google Scholar]
- Lingenfelser T, Blackshaw LA, Sun WM, Dent J. Pyloric motor response to central and peripheral nitric oxide in the ferret. Neurogastroenterol Motil. 1997;9:167–175. doi: 10.1046/j.1365-2982.1997.d01-39.x. [DOI] [PubMed] [Google Scholar]
- Lloyd KC, Raybould HE, Walsh JH. Cholecystokinin inhibits gastric acid secretion through type ‘A’ cholecystokinin receptors and somatostatin in rats. Am J Physiol Gastrointest Liver Physiol. 1992;263:G287–G292. doi: 10.1152/ajpgi.1992.263.3.G287. [DOI] [PubMed] [Google Scholar]
- McSwiney BA. The innervation of the stomach. Physiol Rev. 1931;11:478–514. [Google Scholar]
- Martinson J. Studies on the efferent vagal control of the stomach. Acta Physiol Scand Suppl. 1965;255:1–24. [PubMed] [Google Scholar]
- Mir SS, Telford GL, Mason GR, Ormsbee HS., 3rd Noncholinergic nonadrenergic inhibitory innervation of the canine pylorus. Gastroenterology. 1979;76:1443–1448. [PubMed] [Google Scholar]
- Moran TH, Kornbluh R, Moore K, Schwartz GJ. Cholecystokinin inhibits gastric emptying and contracts the pyloric sphincter in rats by interacting with low affinity CCK receptor sites. Regul Pept. 1994;52:165–172. doi: 10.1016/0167-0115(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Morgan KG, Schmalz PF, Go VL, Szurszewski JH. Electrical and mechanical effects of molecular variants of CCK on antral smooth muscle. Am J Physiol Endocrinol Metab. 1978;235:E324–E329. doi: 10.1152/ajpendo.1978.235.3.E324. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Takahashi T, Taniuchi M, Hsu CX, Owyang C. Nicotinic receptor mediates nitric oxide synthase expression in the rat gastric myenteric plexus. J Clin Invest. 1998;101:1479–1489. doi: 10.1172/JCI627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima A. Visceral afferents and metabolic function. Diabetologia. 1981;20:325–330. doi: 10.1007/BF00254499. [DOI] [PubMed] [Google Scholar]
- Niijima A. Glucose-sensitive afferent nerve fibers in the liver and their role in food intake and blood glucose regulation. J Auton Nerv Syst. 1983;9:207–220. doi: 10.1016/0165-1838(83)90142-x. [DOI] [PubMed] [Google Scholar]
- Niijima A, Hatanaka S, Furuhama K. Effects of DQ-2511 on neutral activity in afferent and efferent loops of gastric vago-vagal reflex pathways in the rat. J Auton Pharmacol. 1996;16:49–53. doi: 10.1111/j.1474-8673.1996.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Pearson RJ, Gatti PJ, Sahibzada N, Massari VJ, Gillis RA. Ultrastructural evidence for selective noradrenergic innervation of CNS vagal projections to the fundus of the rat. Auton Neurosci. 2007;136:31–42. doi: 10.1016/j.autneu.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Prechtl JC, Powley TL. The fiber composition of the abdominal vagus of the rat. Anat Embryol (Berl) 1990;181:101–115. doi: 10.1007/BF00198950. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Lloyd KC. Integration of postprandial function in the proximal gastrointestinal tract. Role of CCK and sensory pathways. Ann N Y Acad Sci. 1994;713:143–156. doi: 10.1111/j.1749-6632.1994.tb44061.x. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Roberts ME, Dockray GJ. Reflex decreases in intragastric pressure in response to cholecystokinin in rats. Am J Physiol Gastrointest Liver Physiol. 1987;253:G165–G170. doi: 10.1152/ajpgi.1987.253.2.G165. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Taché Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol Gastrointest Liver Physiol. 1988;255:G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Zittel TT, Holzer HH, Lloyd KC, Meyer JH. Gastroduodenal sensory mechanisms and CCK in inhibition of gastric emptying in response to a meal. Dig Dis Sci. 1994;39:41S–43S. doi: 10.1007/BF02300368. [DOI] [PubMed] [Google Scholar]
- Reeve JR, Jr, Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol. 2003;285:G255–G265. doi: 10.1152/ajpgi.00523.2002. [DOI] [PubMed] [Google Scholar]
- Richards W, Hillsley K, Eastwood C, Grundy D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. J Physiol. 1996;497:473–481. doi: 10.1113/jphysiol.1996.sp021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran H, Goldfine ID, Bailey A, Licko V, Williams JA. Relationship of cholecystokinin receptor binding to regulation of biological functions in pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 1982;242:G250–G257. doi: 10.1152/ajpgi.1982.242.3.G250. [DOI] [PubMed] [Google Scholar]
- Sarna SK, Daniel EE. Vagal control of gastric electrical control activity and motility. Gastroenterology. 1975;68:301–308. [PubMed] [Google Scholar]
- Scheurer U, Varga L, Drack E, Burki HR, Halter F. Measurement of cholecystokinin octapeptide-induced motility of rat antrum, pylorus, and duodenum in vitro. Am J Physiol Gastrointest Liver Physiol. 1983;244:G261–G265. doi: 10.1152/ajpgi.1983.244.3.G261. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH. Pharmacological dissociation of responses to CCK and gastric loads in rat mechanosensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol. 1994;267:R303–R308. doi: 10.1152/ajpregu.1994.267.1.R303. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Moran TH. CCK elicits and modulates vagal afferent activity arising from gastric and duodenal sites. Ann N Y Acad Sci. 1994;713:121–128. doi: 10.1111/j.1749-6632.1994.tb44058.x. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Moran TH, White WO, Ladenheim EE. Relationships between gastric motility and gastric vagal afferent responses to CCK and GRP in rats differ. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1726–R1733. doi: 10.1152/ajpregu.1997.272.6.R1726. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Tougas G, Moran TH. Integration of vagal afferent responses to duodenal loads and exogenous CCK in rats. Peptides. 1995;16:707–711. doi: 10.1016/0196-9781(95)00033-g. [DOI] [PubMed] [Google Scholar]
- Skandalakis JE, Gray SW, Soria RE, Sorg JL, Rowe JS., Jr Distribution of the vagus nerve to the stomach. Am Surg. 1980;46:130–139. [PubMed] [Google Scholar]
- Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol Regul Integr Comp Physiol. 1985;249:R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526–535. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J Physiol. 1995;484:481–492. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Mechanism of cholecystokinin-induced relaxation of the rat stomach. J Auton Nerv Syst. 1999;75:123–130. doi: 10.1016/s0165-1838(98)00181-7. [DOI] [PubMed] [Google Scholar]
- Telford GL, Mir SS, Mason GR, Ormsbee HS., 3rd Neural control of the canine pylorus. Am J Surg. 1979;137:92–98. doi: 10.1016/0002-9610(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Van Der Schaar PJ, Bremer Y, Lamers CB, Masclee AA. Role of cholecystokinin in relaxation of the proximal stomach. Scand J Gastroenterol. 2001;36:361–366. doi: 10.1080/003655201300051117. [DOI] [PubMed] [Google Scholar]
- Walter GC, Phillips RJ, Baronowsky EA, Powley TL. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J Neurosci Methods. 2009;178:1–9. doi: 10.1016/j.jneumeth.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Coleman FH, Travagli RA. Cholecystokinin-8s excites identified rat pancreatic-projecting vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007;293:G484–G492. doi: 10.1152/ajpgi.00116.2007. [DOI] [PubMed] [Google Scholar]
- Wang L, Barachina MD, Martinez V, Wei JY, Taché Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept. 2000;92:79–85. doi: 10.1016/s0167-0115(00)00153-1. [DOI] [PubMed] [Google Scholar]
- Wei JY, Adelson DW, Taché Y, Go VL. Centrifugal gastric vagal afferent unit activities: another source of gastric ‘efferent’ control. J Auton Nerv Syst. 1995;52:83–97. doi: 10.1016/0165-1838(94)00146-b. [DOI] [PubMed] [Google Scholar]
- Wei JY, Taché Y, Kruger L. Sources of anterior gastric vagal efferent discharge in rats: an electrophysiological study. J Auton Nerv Syst. 1992;37:29–37. doi: 10.1016/0165-1838(92)90142-4. [DOI] [PubMed] [Google Scholar]
- Whited KL, Tso P, Raybould HE. Involvement of apolipoprotein A-IV and cholecystokinin1 receptors in exogenous peptide YY3 36-induced stimulation of intestinal feedback. Endocrinology. 2007;148:4695–4703. doi: 10.1210/en.2006-1665. [DOI] [PubMed] [Google Scholar]
- Yang H, Wong H, Wu V, Walsh JH, Taché Y. Somatostatin monoclonal antibody immunoneutralization increases gastrin and gastric acid secretion in urethane-anesthetized rats. Gastroenterology. 1990;99:659–665. doi: 10.1016/0016-5085(90)90952-w. [DOI] [PubMed] [Google Scholar]
- Yoshida-Yoneda E, O-Leei TJ, Wei JY, Vigna SR, Taché Y. Peripheral bombesin induces gastric vagal afferent activation in rats. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1584–R1593. doi: 10.1152/ajpregu.1996.271.6.R1584. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZL, Rogers RC, Travagli RA. Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience. 1999;90:685–694. doi: 10.1016/s0306-4522(98)00586-7. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Lu YX, Yao H, Owyang C. Spatial organization of neurons in the dorsal motor nucleus of the vagus synapsing with intragastric cholinergic and nitric oxide/VIP neurons in the rat. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1201–G1209. doi: 10.1152/ajpgi.00309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


