Abstract
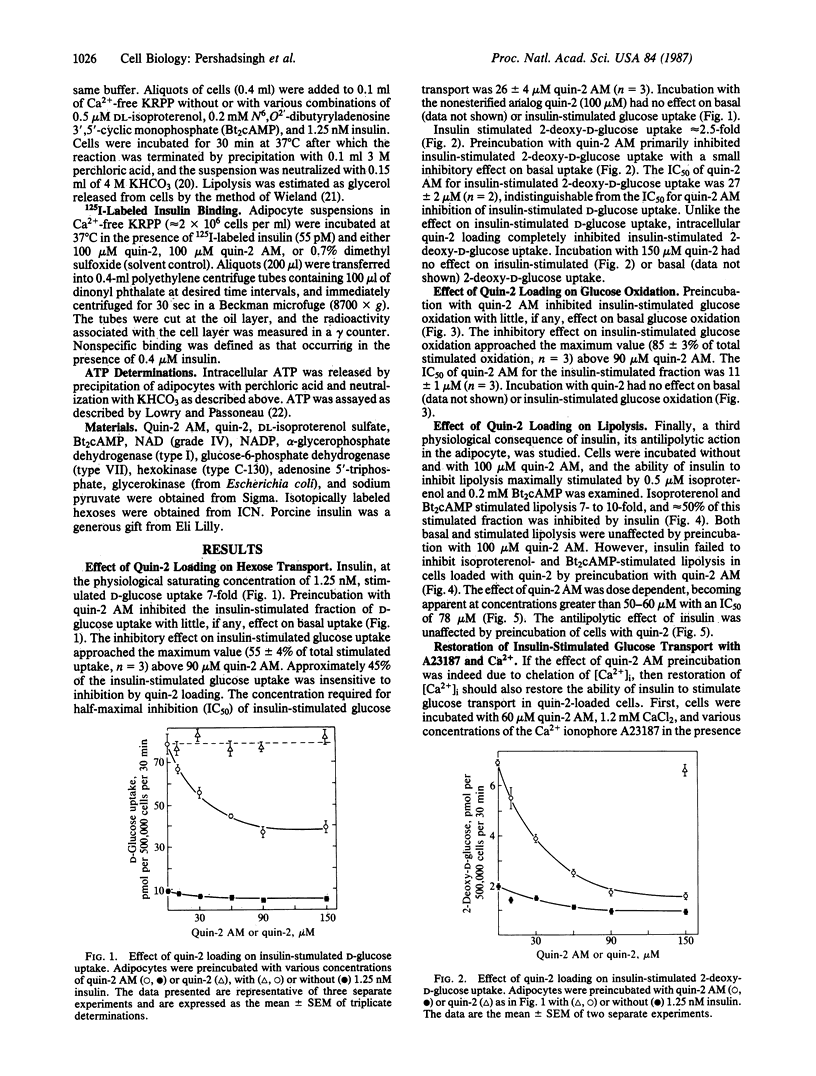
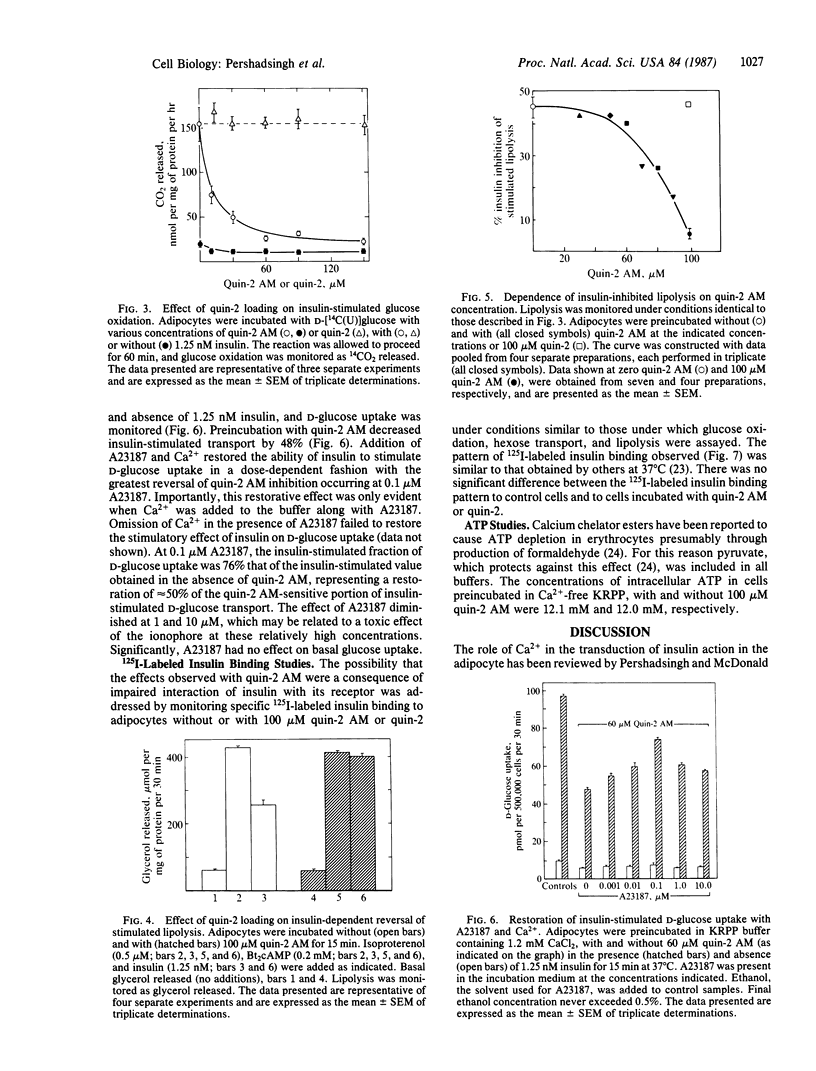
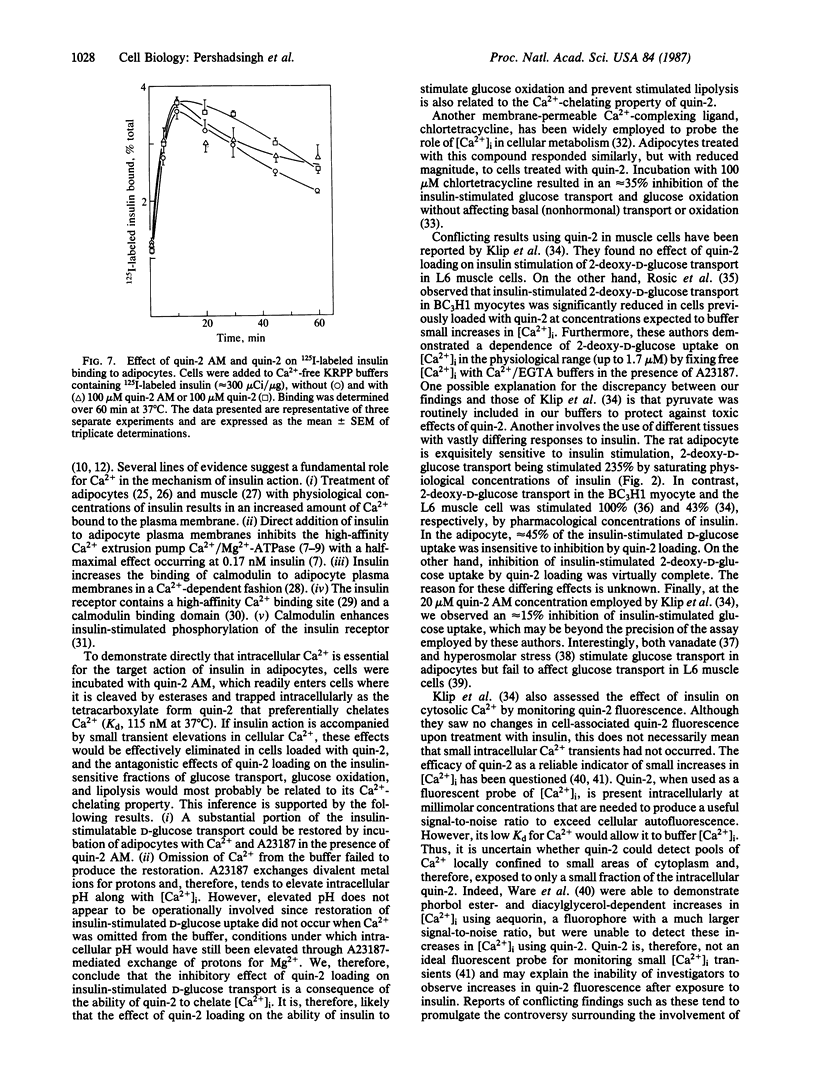
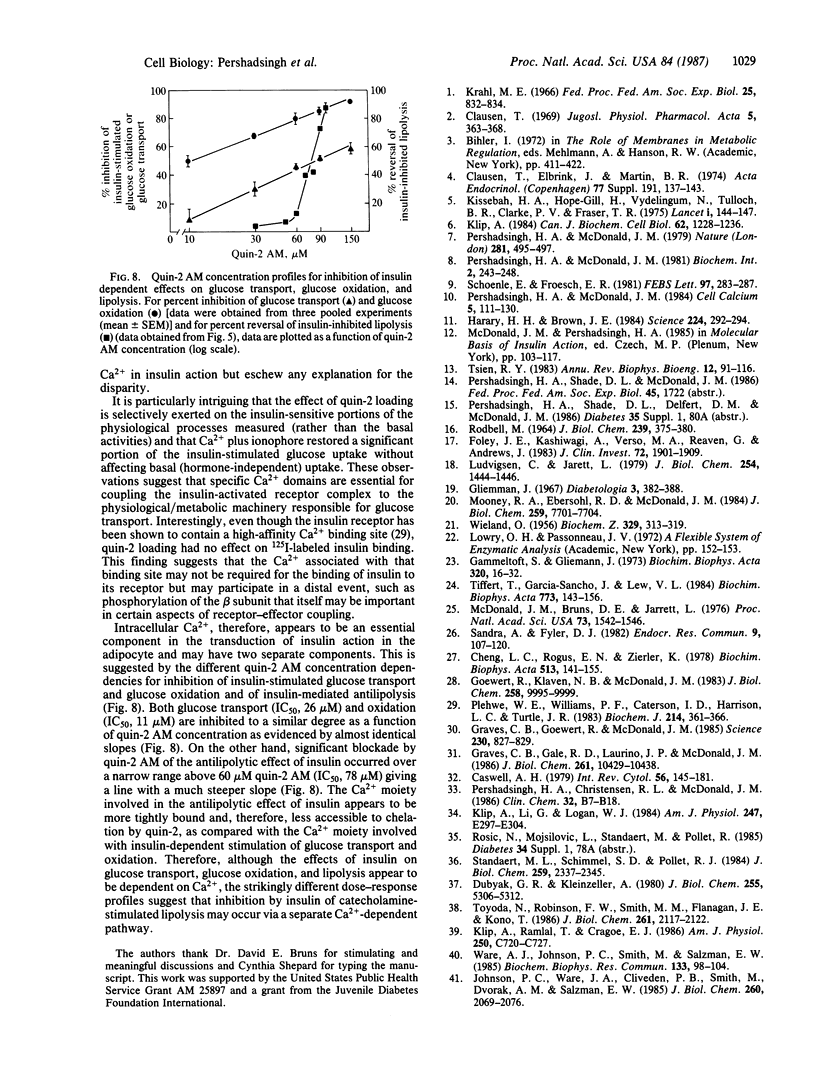
The hypothesis that intracellular Ca2+ is an essential component of the intracellular mechanism of insulin action in the adipocyte was evaluated. Cells were loaded with the Ca2+ chelator quin-2, by preincubating them with quin-2 AM, the tetrakis(acetoxymethyl) ester of quin-2. Quin-2 loading inhibited insulin-stimulated glucose transport (IC50, 26 microM quin-2 AM) without affecting basal activity. The ability of insulin to stimulate glucose uptake in quin-2-loaded cells could be partially restored by preincubating cells with buffer supplemented with 1.2 mM CaCl2 and the Ca2+ ionophore A23187. These conditions had no effect on basal activity and omission of CaCl2 from the buffer prevented the restoration of insulin-stimulated glucose uptake by A23187. Quin-2 loading also inhibited insulin-stimulated glucose oxidation (IC50, 11 microM quin-2 AM) and the ability of insulin to inhibit cAMP-stimulated lipolysis (IC50, 78 microM quin-2 AM), without affecting their basal activities. Incubation of cells with 100 microM quin-2 or quin-2 AM had no effect on intracellular ATP concentration or the specific binding of 125I-labeled insulin to adipocytes. These findings suggest that intracellular Ca2+ is an essential component in the coupling of the insulin-activated receptor complex to cellular physiological/metabolic machinery. Furthermore, differing quin-2 AM dose-response profiles suggest the presence of dual Ca2+-dependent pathways in the adipocyte. One involves insulin stimulation of glucose transport and oxidation, whereas the other involves the antilipolytic action of insulin.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caswell A. H. Methods of measuring intracellular calcium. Int Rev Cytol. 1979;56:145–181. doi: 10.1016/s0074-7696(08)61822-7. [DOI] [PubMed] [Google Scholar]
- Cheng L. C., Rogus E. M., Zierler K. Specific D-glucose transport in sarcolemma vesicles. Biochim Biophys Acta. 1978 Oct 19;513(1):141–155. doi: 10.1016/0005-2736(78)90119-0. [DOI] [PubMed] [Google Scholar]
- Clausen T., Elbrink J., Martin B. R. Insulin controlling calcium distribution in muscle and fat cells. Acta Endocrinol Suppl (Copenh) 1974;191:137–143. doi: 10.1530/acta.0.077s0137. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Kleinzeller A. The insulin-mimetic effects of vanadate in isolated rat adipocytes. Dissociation from effects of vanadate as a (Na+-K+)ATPase inhibitor. J Biol Chem. 1980 Jun 10;255(11):5306–5312. [PubMed] [Google Scholar]
- Foley J. E., Kashiwagi A., Verso M. A., Reaven G., Andrews J. Improvement in in vitro insulin action after one month of insulin therapy in obese noninsulin-dependent diabetics. Measurements of glucose transport and metabolism, insulin binding, and lipolysis in isolated adipocytes. J Clin Invest. 1983 Dec;72(6):1901–1909. doi: 10.1172/JCI111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gliemann J. Assay of insulin-like activity by the isolated fat cell method. I. Factors influencing the response to crystalline insulin. Diabetologia. 1967 Aug;3(4):382–388. doi: 10.1007/BF02342631. [DOI] [PubMed] [Google Scholar]
- Goewert R. R., Klaven N. B., McDonald J. M. Direct effect of insulin on the binding of calmodulin to rat adipocyte plasma membranes. J Biol Chem. 1983 Aug 25;258(16):9995–9999. [PubMed] [Google Scholar]
- Graves C. B., Gale R. D., Laurino J. P., McDonald J. M. The insulin receptor and calmodulin. Calmodulin enhances insulin-mediated receptor kinase activity and insulin stimulates phosphorylation of calmodulin. J Biol Chem. 1986 Aug 5;261(22):10429–10438. [PubMed] [Google Scholar]
- Graves C. B., Goewert R. R., McDonald J. M. The insulin receptor contains a calmodulin-binding domain. Science. 1985 Nov 15;230(4727):827–829. doi: 10.1126/science.3904001. [DOI] [PubMed] [Google Scholar]
- Harary H. H., Brown J. E. Spatially nonuniform changes in intracellular calcium ion concentrations. Science. 1984 Apr 20;224(4646):292–294. doi: 10.1126/science.6710144. [DOI] [PubMed] [Google Scholar]
- Johnson P. C., Ware J. A., Cliveden P. B., Smith M., Dvorak A. M., Salzman E. W. Measurement of ionized calcium in blood platelets with the photoprotein aequorin. Comparison with Quin 2. J Biol Chem. 1985 Feb 25;260(4):2069–2076. [PubMed] [Google Scholar]
- Kissebah A. H., Tulloch B. R., Hope-Gill H., Clarke P. V., Vydelingum N., Fraser T. R. Mode of insulin action. Lancet. 1975 Jan 18;1(7899):144–147. doi: 10.1016/s0140-6736(75)91435-x. [DOI] [PubMed] [Google Scholar]
- Klip A. Is intracellular Ca2+ involved in insulin stimulation of sugar transport? Fact and prejudice. Can J Biochem Cell Biol. 1984 Nov;62(11):1228–1236. doi: 10.1139/o84-157. [DOI] [PubMed] [Google Scholar]
- Klip A., Li G., Logan W. J. Role of calcium ions in insulin action on hexose transport in L6 muscle cells. Am J Physiol. 1984 Sep;247(3 Pt 1):E297–E304. doi: 10.1152/ajpendo.1984.247.3.E297. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Cragoe E. J., Jr Insulin-induced cytoplasmic alkalinization and glucose transport in muscle cells. Am J Physiol. 1986 May;250(5 Pt 1):C720–C728. doi: 10.1152/ajpcell.1986.250.5.C720. [DOI] [PubMed] [Google Scholar]
- Krahl M. E. Insulinlike and anti-insulin effects of chelating agents on adipose tissue. Fed Proc. 1966 May-Jun;25(3):832–834. [PubMed] [Google Scholar]
- Ludvigsen C., Jarett L. A kinetic analysis of D-glucose transport by adipocyte plasma membranes. J Biol Chem. 1979 Mar 10;254(5):1444–1446. [PubMed] [Google Scholar]
- McDonald J. M., Bruns D. E., Jarett L. Ability of insulin to increase calcium binding by adipocyte plasma membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1542–1546. doi: 10.1073/pnas.73.5.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. A., Ebersohl R. D., McDonald J. M. Insulin-mediated antilipolysis in permeabilized rat adipocytes. J Biol Chem. 1984 Jun 25;259(12):7701–7704. [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. Direct addition of insulin inhibits a high affinity Ca2+-ATPase in isolated adipocyte plasma membranes. Nature. 1979 Oct 11;281(5731):495–497. doi: 10.1038/281495a0. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. Hormone-receptor coupling and the molecular mechanism of insulin action in the adipocyte: a paradigm for Ca2+ homeostasis in the initiation of the insulin-induced metabolic cascade. Cell Calcium. 1984 Apr;5(2):111–130. doi: 10.1016/0143-4160(84)90011-3. [DOI] [PubMed] [Google Scholar]
- Plehwe W. E., Williams P. F., Caterson I. D., Harrison L. C., Turtle J. R. Calcium-dependence of insulin receptor phosphorylation. Biochem J. 1983 Aug 15;214(2):361–366. doi: 10.1042/bj2140361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Sandra A., Fyler D. J. Effects of liposome-adipocyte interaction on calcium binding and insulin action. Endocr Res Commun. 1982;9(2):107–120. doi: 10.1080/07435808209045757. [DOI] [PubMed] [Google Scholar]
- Standaert M. L., Schimmel S. D., Pollet R. J. The development of insulin receptors and responses in the differentiating nonfusing muscle cell line BC3H-1. J Biol Chem. 1984 Feb 25;259(4):2337–2345. [PubMed] [Google Scholar]
- Tiffert T., Garcia-Sancho J., Lew V. L. Irreversible ATP depletion caused by low concentrations of formaldehyde and of calcium-chelator esters in intact human red cells. Biochim Biophys Acta. 1984 Jun 13;773(1):143–156. doi: 10.1016/0005-2736(84)90559-5. [DOI] [PubMed] [Google Scholar]
- Toyoda N., Robinson F. W., Smith M. M., Flanagan J. E., Kono T. Apparent translocation of glucose transport activity in rat epididymal adipocytes by insulin-like effects of high pH or hyperosmolarity. J Biol Chem. 1986 Feb 15;261(5):2117–2122. [PubMed] [Google Scholar]
- Tsien R. Y. Intracellular measurements of ion activities. Annu Rev Biophys Bioeng. 1983;12:91–116. doi: 10.1146/annurev.bb.12.060183.000515. [DOI] [PubMed] [Google Scholar]
- WIELAND O. Eine enzymatische Methode zur Bestimmung von Glycerin. Biochem Z. 1957;329(4):313–319. [PubMed] [Google Scholar]
- Ware J. A., Johnson P. C., Smith M., Salzman E. W. Aequorin detects increased cytoplasmic calcium in platelets stimulated with phorbol ester or diacylglycerol. Biochem Biophys Res Commun. 1985 Nov 27;133(1):98–104. doi: 10.1016/0006-291x(85)91846-7. [DOI] [PubMed] [Google Scholar]


