Abstract
Steroidal sex hormones play an important role in the neural control of breathing. Previous studies in our laboratory have shown that gonadectomy in young male rats (3 months) eliminates a form of respiratory plasticity induced by intermittent hypoxia, known as long term facilitation (LTF). Testosterone replenishment restores LTF in gonadectomized male rats, and this is dependent on the conversion of testosterone to oestradiol by aromatase. By middle age (12 months), male rats no longer exhibit LTF of hypoglossal motor output; phrenic LTF is significantly reduced, and this persists into old age. We tested the hypothesis that LTF can be restored in old male rats by administration of testosterone. Intact Fischer 344 rats (>20 months) were implanted with Silastic tubing containing testosterone (T), T plus an aromatase inhibitor (T+ADT), or 5α-dihydrotestosterone (DHT), a form of testosterone not converted to oestradiol. One week post-surgery, LTF of hypoglossal and phrenic motor output was measured. By comparison with control rats, hypoglossal LTF was increased in testosterone-treated rats, with levels approaching that of normal young rats. LTF was not restored in T+ADT or DHT-treated rats. Aromatase levels in hypoglossal and phrenic nuclei did not change with age. As serum testosterone levels did not decline with age, local bioavailability of testosterone in old rats may be a limiting factor in the expression of this form of respiratory plasticity. Our findings suggest that testosterone supplementation could potentially be used to enhance upper airway control in the elderly.
Non-technical summary
Steroidal sex hormones (testosterone, oestradiol and progesterone) play an important role in the neural control of breathing. Hormone levels typically change throughout life. Testosterone levels increase during puberty in boys, but from ∼30 years of age levels decline gradually. The typical age of onset for obstructive sleep apnoea, a prominent breathing disorder of older humans, is ∼50 years of age in men. In a study in old male rats, we show that testosterone supplementation can reverse the age-associated decrease in one measurement of the neural control of breathing. We conclude that testosterone supplementation can potentially be used to enhance upper airway function in the elderly.
Introduction
Obstructive sleep apnoea (OSA) is a respiratory pathology characterized by frequent closures of the airway during sleep, resulting in periods of hypoxia and asphyxia (Kapsimalis & Kryger, 2002a,b; Lee et al. 2008). The long-term health consequences of OSA include diminished neurocognitive function, increased risk of motor vehicle accidents, reduced quality of life, hypertension, insulin resistance and cardiovascular disease (Young et al. 2004; White, 2006; George, 2007; Lee et al. 2008; Dempsey et al. 2010). The prevalence of OSA in the general population increases with age, with a plateau around 65 years of age (Bixler et al. 1998, 2001). A striking feature of OSA is that it occurs more frequently in men than in women, with estimates of the male:female ratio between 2:1 and 4:1 (Young et al. 1993; Bixler et al. 2001). The prevalence of OSA in postmenopausal women approximates that of age-matched men, but postmenopausal women taking hormonal supplements have a much lower incidence of the disease (Bixler et al. 2001; Shahar et al. 2003). Taken together these findings point to sex hormones as playing a role in maintenance of airway patency. Age-associated changes in levels of testosterone in men and in oestrogen and progesterone in women have been proposed as significantly contributing to disease pathology although the mechanisms remain unclear (Lin et al. 2008).
Sex steroid hormones have profound effects on breathing (Dempsey & Skatrud, 1986; Fukuda, 1991, 1992; Tatsumi et al. 1994; Behan et al. 2003; Behan & Wenninger, 2008). Studies in humans have reported sex differences in resting ventilation and ventilatory responses to hypoxia and hypercapnia at different ages (White et al. 1983; Saaresranta & Polo, 2002; Jensen et al. 2005a). Ventilatory metrics also change with the menstrual cycle and in pregnancy (Driver et al. 2005; Jensen et al. 2005b; da Silva et al. 2006). Although the data from animal studies are not always in agreement, it is clear that age-associated changes in ventilatory responses to hypoxia and hypercapnia are different in male and female rats (Wenninger et al. 2009).
Sex hormones have been shown to directly influence a form of respiratory plasticity that occurs after exposure to intermittent hypoxia called long term facilitation (LTF). LTF is a sustained enhancement of respiratory motor output following intermittent hypoxia (Millhorn et al. 1980a,b;). LTF has been documented in humans and animals, in wakefulness and sleep, as well as in anaesthetized animals (Mitchell & Johnson, 2003; Mateika & Narwani, 2009). LTF of upper airway dilator muscles including the genioglossus muscle of the tongue can be induced in rats by episodes of upper airway negative pressure or airway occlusion (Ryan & Nolan, 2009; Tadjalli et al. 2010). It has been suggested that LTF is a protective mechanism to maintain respiratory homeostasis during sleep, engaged by the periods of upper airway collapse and intermittent hypoxia associated with OSA (Mateika & Narwani, 2009). Previously we showed that in young male rats, LTF of hypoglossal motor output was reduced by gonadectomy (Zabka et al. 2005), and could be restored by supplementing gonadectomized rats with testosterone (Zabka et al. 2006). In both of these studies, there was a positive relationship between the magnitude of LTF and serum testosterone levels.
Testosterone can act directly on androgen receptors (ARs) or be metabolized by 5α-reductase to a more potent androgen receptor ligand, 5α-dihydrotestosterone (DHT), present in neurons and glia (Celotti et al. 1991). Testosterone can also be metabolized by aromatase to 17β-oestradiol (E2) and exert its effects via oestrogen receptors (ERs). Previous studies in our laboratory showed that restoration of LTF in castrated young male rats is dependent on the conversion of testosterone to oestrogen in the brain by aromatase (Zabka et al. 2006). Circulating testosterone levels generally decline with age (Smith et al. 1992; Leifke et al. 2000; Tenover, 2003; Wu & Gore, 2009), and LTF of respiratory motor output also decreases with age in male rats (Zabka et al. 2001a). We hypothesized that old male rats retain their capacity for plasticity in respiratory motor output, and that the decline in LTF in old male rats could be reversed by supplementing with testosterone. We further hypothesized that supplemental testosterone exerts its effect on LTF by further promoting local conversion to oestradiol by aromatase. The results indicate that testosterone can enhance hypoglossal LTF in old rats by an aromatase-dependent mechanism, and we further show that aromatase levels do not appear to decline with age in these respiratory brain regions. The ability of testosterone to enhance respiratory plasticity in old male rats suggests such treatments may be considered in age-related human breathing disorders, including OSA.
Methods
Ethical approval
All experimental procedures were approved by the University of Wisconsin School of Veterinary Medicine Animal Care and Use Committee and are in compliance with the policies and regulations of The Journal of Physiology (Drummond, 2009).
Experimental groups
Initially eight groups of male rats (Fischer 344; National Institute of Aging colony) were used for this study: intact young male (Young, 3–4 months; n (phrenic) = 10, n (XII) = 10); old control male (Old-C, >20 months; n (phrenic) = 4, n (XII) = 4); old male with sham surgery (Old-S, n (phrenic) = 3, n (XII) = 3); old male with sham implants (Old-SI, n (phrenic) = 3, n (XII) = 3); old male with testosterone (Old+T, n (phrenic) = 7, n (XII) = 8); old male with testosterone and one aromatase inhibitor implant (Old+T+ADT, n (phrenic) = 3, n (XII) = 3); old male with testosterone and two aromatase inhibitor implants (Old+T+2xADT, n (phrenic) = 5, n (XII) = 5); old male with DHT pellets (Old+DHT, n (phrenic) = 5, n (XII) = 5). We based the use of one aromatase inhibitor implant on earlier studies in young male rats (Zabka et al. 2006), but found that one implant was insufficient to reduce the effects of supplemental testosterone (Old+T+ADT) in the heavier, old rats. Therefore we increased the dosage to two implants in a new group of old rats (Old+T+2×ADT). There were no statistical differences between groups Old-C, Old-S and Old-SI in physiological measures, and therefore all of these animals were grouped together (Old), yielding a total of five groups of rats. In some rats, only one neurogram could be obtained; thus, n (phrenic) and n (XII) differed in some groups.
Hormone implants
Silastic laboratory tubing (Dow Corning, Midland, MI, USA) was used for implants. Testosterone implants consisted of a Silastic tube (i.d. 0.16 cm, o.d. 0.32, length 3 cm) packed with testosterone (crystalline testosterone (T), Steraloids, Inc., Newport, RI, USA). Aromatase implants consisted of a Silastic tube (i.d. 0.15 cm, o.d. 0.20 cm, length 3.03 cm) packed with aromatase inhibitor (crystalline 1,4,9-androstatriene-3,17-dione (ADT), Steraloids, Inc.). Prior to implantation, T and ADT implants were submerged in 0.1 M phosphate buffered saline at room temperature for 24 h. Dihydrotestosterone (DHT) implants were in time-released pellet form (5α-dihydrotestosterone, 10.0 mg per pellet, 21 day release, Innovative Research of America, Sarasota, FL, USA). The number of tubes/pellets implanted was based on a previous study (Zabka et al. 2006).
Anaesthesia was introduced with isoflurane in an induction chamber and maintained (3.0–3.5% isoflurane in 100% O2) using a nose cone. Six centimetres distal to the shoulder blades, the skin was clipped and cleaned with an antiseptic detergent in an area 2 cm × 2 cm. A skin incision of 1 cm was made parallel to the spine. The skin was undermined bluntly and implants/pellets inserted subcutaneously. The incision was closed using wound clips. Seven days post-surgery and following the LTF protocol, blood was collected to assess serum levels of testosterone, oestrogen and progesterone.
Experimental preparation
Methods have been described in previous publications (Zabka et al. 2005, 2006). Briefly, animals were anaesthetized with urethane, subjected to neuromuscular blockade (pancuronium bromide, 2.5 mg kg−1i.v.), bilaterally vagotomized and pump ventilated. Blood samples (∼60 μl in a 125 μl capillary tube) were drawn to determine arterial blood gases (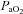 and
and 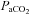 ), pH and base excess (ABL 810, Radiometer, Copenhagen, Denmark). Body temperature was maintained at approximately 37°C using a heated table. End-tidal CO2 was measured with a flow-through capnograph (Capnogard, Novametrix, Wallingford, CT, USA). The right phrenic and XII nerves were isolated via a dorsal approach, cut distally, de-sheathed, submerged in mineral oil and placed on bipolar, silver wire electrodes. Nerve activities were amplified (×10,000), band pass filtered (100 Hz to 10 kHZ) (Model 1700, A-M Systems, Inc., Carlsborg, WA, USA) and integrated (time constant = 50 ms, Model MA-821RSP, CWE Inc., Ardmore, PA, USA).
), pH and base excess (ABL 810, Radiometer, Copenhagen, Denmark). Body temperature was maintained at approximately 37°C using a heated table. End-tidal CO2 was measured with a flow-through capnograph (Capnogard, Novametrix, Wallingford, CT, USA). The right phrenic and XII nerves were isolated via a dorsal approach, cut distally, de-sheathed, submerged in mineral oil and placed on bipolar, silver wire electrodes. Nerve activities were amplified (×10,000), band pass filtered (100 Hz to 10 kHZ) (Model 1700, A-M Systems, Inc., Carlsborg, WA, USA) and integrated (time constant = 50 ms, Model MA-821RSP, CWE Inc., Ardmore, PA, USA).
Experimental protocol
Recording began approximately 60 min following surgery. The phrenic and XII nerves were positioned on the electrodes and allowed to stabilize for approximately 5 min under hyperoxia and normocapnia (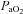 > 150 mmHg). Subsequently the CO2 apnoeic/recruitment threshold was determined and baseline nerve activities were established 2 mmHg above this threshold. Baseline blood gas values were assessed before starting the protocol. All subsequent blood samples were compared to this initial baseline value. Strict isocapnia (±1 mmHg from baseline
> 150 mmHg). Subsequently the CO2 apnoeic/recruitment threshold was determined and baseline nerve activities were established 2 mmHg above this threshold. Baseline blood gas values were assessed before starting the protocol. All subsequent blood samples were compared to this initial baseline value. Strict isocapnia (±1 mmHg from baseline 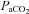 ) was maintained throughout an experiment. A typical tracing of hypoglossal motor output of a Young rat, an Old rat and an Old+T rat is shown in Fig. 1. An LTF protocol started with three 5 min hypoxic episodes (
) was maintained throughout an experiment. A typical tracing of hypoglossal motor output of a Young rat, an Old rat and an Old+T rat is shown in Fig. 1. An LTF protocol started with three 5 min hypoxic episodes (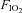 = 0.11–0.14, target
= 0.11–0.14, target 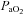 35–45 mmHg) separated by 5 min intervals of hyperoxia, and followed by 60 min of isocapnic–hyperoxic baseline conditions. A protocol ended with 5 min of hypercapnia (
35–45 mmHg) separated by 5 min intervals of hyperoxia, and followed by 60 min of isocapnic–hyperoxic baseline conditions. A protocol ended with 5 min of hypercapnia (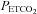 > 80 mmHg) to assess maximal hypercapnia-stimulated nerve activity. Arterial blood samples were drawn during the last minute of the first hypoxic episode to determine the severity of hypoxia, and 15, 30 and 60 min after the final hypoxic episode to confirm isocapnic conditions. Rats were excluded from the analysis if
> 80 mmHg) to assess maximal hypercapnia-stimulated nerve activity. Arterial blood samples were drawn during the last minute of the first hypoxic episode to determine the severity of hypoxia, and 15, 30 and 60 min after the final hypoxic episode to confirm isocapnic conditions. Rats were excluded from the analysis if 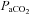 deviated from baseline by more than 3 mmHg during hypoxia or 1 mmHg during post-hypoxic episodes from baseline. Therefore, changes in
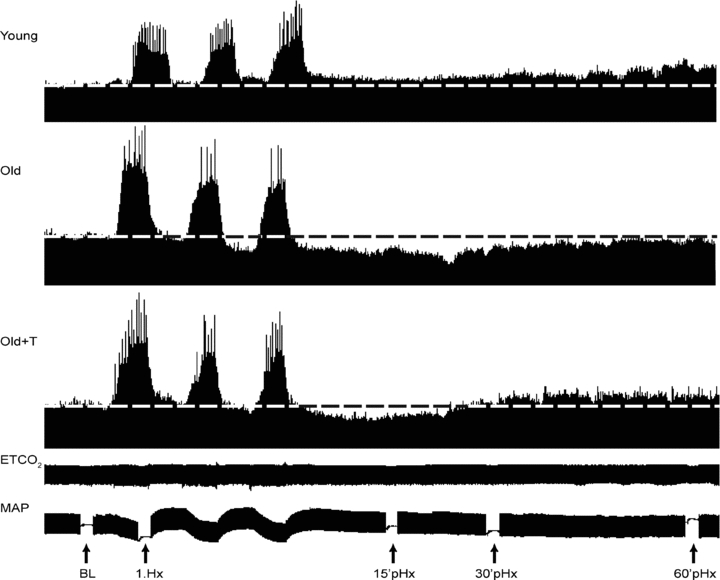
deviated from baseline by more than 3 mmHg during hypoxia or 1 mmHg during post-hypoxic episodes from baseline. Therefore, changes in 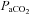 had minimal impact on the results of this study. Experiments were also excluded if arterial blood pressure dropped by more than 30 mmHg from baseline at the end of a protocol. At the end of each experiment, rats were killed with an overdose of urethane (i.v.) or decapitated for tissue harvest.
had minimal impact on the results of this study. Experiments were also excluded if arterial blood pressure dropped by more than 30 mmHg from baseline at the end of a protocol. At the end of each experiment, rats were killed with an overdose of urethane (i.v.) or decapitated for tissue harvest.
Figure 1. Neurograms of representative LTF protocols showing integrated XII motor output in Young, Old and Old+T rats.
After establishing baseline conditions, three episodes of hypoxia (11–14%) were applied. Arterial blood samples for blood gas analysis (arrows) were taken under baseline conditions (BL), during the last minute of the first hypoxic episode (1.Hx) and at 15, 30 and 60 min after the last hypoxic episode (15′pHx, 30′pHx, 60′pHx). The upper panel shows a compressed tracing from a Young rat showing LTF typically seen in young male rats. The second panel shows the absence of LTF in an Old male rat. The third panel shows LTF in an Old male rat supplemented with testosterone (Old+T). The fourth panel shows the stability of end tidal CO2 (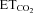 ) throughout the protocol. The lower panel shows mean arterial blood pressure (MAP) throughout the protocol.
) throughout the protocol. The lower panel shows mean arterial blood pressure (MAP) throughout the protocol.
Measurement of sex hormone levels
Arterial blood samples (1 ml) were taken following the hypercapnic challenge. Blood samples were centrifuged, and serum collected and immediately frozen at −80°C. Total testosterone, oestradiol and progesterone levels were analysed using enzyme-linked immunosorbent assay (ELISA; Immuno-Biological Laboratories Inc., Minneapolis, MN, USA; testosterone cat. no. IB79106, range: 0.2–16 ng ml−1, sensitivity: 0.083 ng ml−1; 17β-oestradiol, cat. no. IB79103, range: 25–2000 pg ml−1, sensitivity: 9.714 pg ml−1; progesterone, cat. no. IB79105, range: 0.3–40 ng ml−1, sensitivity: 0.045 ng ml−1).
Measurement of aromatase activity
Cervical spinal cord segments C3–C6 containing phrenic motor nuclei, XII motor nuclei and hypothalamus were harvested from rats at the end of each experiment. Using a freezing microtome, the dorsal half of the C3–C6 sections was shaved off, leaving the ventral cord containing the phrenic motor nuclei. XII motor nuclei were dissected from 500 μm sections through the brainstem cut with a McIlwain Tissue Chopper. The hypothalamus was dissected from the diencephalon. Tissues were frozen and stored at −80°C. Aromatase activity was quantified using a further-optimized variant of a previously validated radiometric ‘water’ assay (Trainor et al. 2003) that measures stereospecific loss of tritium from the C-1β position of [1β-3H]androstenedione; tritium is liberated to the aqueous phase in direct proportion to the amount of steroid (oestradiol) formed by aromatase (Lephart & Simpson, 1991). Due to activity being low relative to testis, brain tissue samples were pooled (3 animals per sample) and homogenized in microfuge tubes on ice in a total of 250 μl of buffer (0.1 m potassium phosphate buffer; 1 mm EDTA, 0.5 mm PMSF and 20% glycerol; pH 7.4). Homogenate was briefly centrifuged to remove remaining tissues fragments (3000 rpm = 704 × gravity; 3 min; 4°C) and the supernatant removed for storage at −70°C. Aliquots of homogenate (100–190 μl) were made up to 200 μl volume with assay buffer (50 mm potassium phosphate, 1 mm EDTA; pH 7.4) and substrate and cofactors added to a total of 250 μl volume in Pyrex glass tubes. Additions were made to achieve final concentrations of [1β-3H]androstenedione (1.25 μCi per tube) with 200 nm added ‘cold’ androstenedione, and a NADP/NADPH regenerating system, with final concentrations 17 mm glucose-6-phosphate, 1 mm NADPH, 2 mm NADP and 1 unit glucose-6-phosphate dehydrogenase (all chemicals obtained from Sigma-Aldrich). Incubation was continued for 6 h at 37°C, after which tubes were snap frozen in liquid nitrogen prior to extraction of unused steroid substrate. Samples were thawed by addition of 300 μl ddH2O and the thawed aqueous phase extracted using 1 ml chloroform (CHCl3) with strong vortexing (∼10 s). Phases were separated by centrifugation (Beckman GPR Centrifuge, 1000 rpm = 212 × gravity; 5 min) and 300 μl of the upper aqueous phase removed to a microfuge tube. A further 300 μl ddH2O was added to each glass tube to back-extract the remaining aqueous radioactivity, and following brief mixing and centrifugation, another 300 μl of the upper aqueous phase removed and pooled with the first aliquot (total 600 μl). To each microfuge tube, 600 μl of 5% charcoal, 0.5% dextran (MW 144,000) solution was added, and mixed by strong vortexing. The tubes were then centrifuged (3000 rpm = 704 × gravity; 15 min; 4°C) and 1 ml of each upper aqueous phase sample was transferred to scintillation vials followed by addition of 6 ml Insta-Gel plus (Perkin Elmer, Waltham, MA, USA) for quantification of radioactivity using a Packard Tricarb 2900 scintillation counter (counts 20 min each or to 1% accuracy). Results were corrected for background (from ‘no tissue blanks’ processed in parallel), and activity for each sample calculated by comparison to total radioactivity added (counted in separate vials). We validated the aromatase activity assay by running both testis controls (>14 000 dpm conversion) and no tissue blanks (<500 dpm conversion) in each assay. We also measured time-dependent increases in radioactivity for testis homogenate which was blocked by the aromatase inhibitor letrozole (Bhatnagar et al. 1990; gift from Novartis, Basel, Switzerland). Total protein in each sample was measured using a bicinchoninic acid (BCA) assay against BCA standards (Sigma-Aldrich). Data are expressed as both femtomoles of product per hour, and as femtomoles h−1 (mg protein)−1.
Data analysis
Phrenic and XII nerve activities were recorded throughout the protocol. Peak integrated amplitude (ΔPhr and ΔXII), burst frequency (bursts min−1), and mean arterial blood pressure (MAP) were measured at the following time points: baseline, last minute of first hypoxic episode (short-term hypoxic response), 15, 30 and 60 min after the final hypoxic episode, and the last minute of the hypercapnic response (max CO2 response). Nerve activity was averaged over 60 s at each measurement. Changes in amplitude from baseline were normalized as a percentage of baseline nerve activity (% baseline), and as a percentage of the hypercapnic response (% maximum). All conclusions were the same, regardless of the normalization used. Thus, only the percentage baseline data are presented in this paper. Changes in burst frequency were expressed as a difference from baseline in bursts per minute. Depending on the variable, either a one-way or a two-way ANOVA with a repeated measures design (SigmaStat v. 2.0, Systat Software Inc., San Jose, CA, USA) was performed, followed by post hoc inferences for individual comparisons using Fisher's least significant difference (LSD) method. If the normality test failed, a Kruskal–Wallis one-way ANOVA on ranks was performed followed by an all pair-wise multiple comparisons test (Dunn's method) if differences were significant. Differences were considered significant if P < 0.05. All data reported are means ± s.e.m. Serum levels of testosterone, oestradiol and progesterone in individual rats were related to the magnitude of the phrenic and XII LTF via multiple and/or simple linear regressions. A variable was considered to contribute significantly to the model if P < 0.05.
Results
Experimental animals
The mean weight of young rats (range: 313–390 g) was significantly lower than that of old rats (range: 396–506 g; P < 0.001). Absolute weight changes between the time of implantation and recording were very small, with an average loss of ∼1% total body weight over this week-long period for any group.
Mean arterial blood pressure (MAP)
MAP did not differ between experimental groups before, during or following hypoxic episodes. MAP decreased during hypoxic episodes when compared to all time points before and after hypoxia, which is typically seen in hypoxic, anaesthetized rats. Although MAP decreased significantly over the duration of the experimental protocol in all treatment groups, the change in MAP in all rats was within the acceptable range (Zabka et al. 2005, 2006).
Baseline conditions, apnoeic/recruitment threshold, and CO2 regulation
Baseline conditions were standardized through individual determination of the CO2 apnoeic/recruitment threshold and establishing a baseline 2 mmHg above this level (apnoeic/recruitment  (mmHg): Young, 39.9 ± 0.7/41.4 ± 0.7; Old, 42.5 ± 0.7/44.6 ± 0.8; Old+T, 44.3 ± 1.6/46.3 ± 1.8; Old+T+2xADT, 44.0 ± 0.4/46.0 ± 0.3; Old+DHT, 45.3 ± 1.0/48.0 ± 0.8). Apnoeic and recruitment thresholds were essentially identical for the two nerves, and they did not differ between young and old rats. Apnoeic and recruitment thresholds were similar to those previously reported (Zabka et al. 2001a, 2005, 2006). The ratio of both phrenic and XII baseline/maximal CO2 response was not different among the groups, indicating a similar dynamic range and baseline ventilatory drive (Phrenic: P = 0.069; XII: P = 0.174).
(mmHg): Young, 39.9 ± 0.7/41.4 ± 0.7; Old, 42.5 ± 0.7/44.6 ± 0.8; Old+T, 44.3 ± 1.6/46.3 ± 1.8; Old+T+2xADT, 44.0 ± 0.4/46.0 ± 0.3; Old+DHT, 45.3 ± 1.0/48.0 ± 0.8). Apnoeic and recruitment thresholds were essentially identical for the two nerves, and they did not differ between young and old rats. Apnoeic and recruitment thresholds were similar to those previously reported (Zabka et al. 2001a, 2005, 2006). The ratio of both phrenic and XII baseline/maximal CO2 response was not different among the groups, indicating a similar dynamic range and baseline ventilatory drive (Phrenic: P = 0.069; XII: P = 0.174).
Short-term hypoxic response
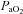 levels during hypoxia were significantly different between treatment groups although all values fell within the target range for hypoxic challenge (mmHg: Young, 44.6 ± 1.5; Old, 43.6 ± 1.2; Old+T, 42.6 ± 1.7; Old+T+2xADT, 37.0 ± 1.4; Old+DHT, 38.1 ± 1.9; P = 0.007). Phrenic and XII short term hypoxic response amplitude expressed as a percent change from baseline was significantly greater in Old+T+2xADT rats compared to all other treatment groups (P < 0.05; data not shown). Phrenic and XII short term hypoxic response frequency during the last minute of the first hypoxic episode, when expressed as a percent change from baseline (bursts min−1), was greater in Young rats than in all the other groups (Phrenic: P < 0.05; XII, P < 0.05). Phrenic and XII short term hypoxic response frequency during the last minute of the second and third hypoxic episodes did not differ among groups.
levels during hypoxia were significantly different between treatment groups although all values fell within the target range for hypoxic challenge (mmHg: Young, 44.6 ± 1.5; Old, 43.6 ± 1.2; Old+T, 42.6 ± 1.7; Old+T+2xADT, 37.0 ± 1.4; Old+DHT, 38.1 ± 1.9; P = 0.007). Phrenic and XII short term hypoxic response amplitude expressed as a percent change from baseline was significantly greater in Old+T+2xADT rats compared to all other treatment groups (P < 0.05; data not shown). Phrenic and XII short term hypoxic response frequency during the last minute of the first hypoxic episode, when expressed as a percent change from baseline (bursts min−1), was greater in Young rats than in all the other groups (Phrenic: P < 0.05; XII, P < 0.05). Phrenic and XII short term hypoxic response frequency during the last minute of the second and third hypoxic episodes did not differ among groups.
Phrenic long term facilitation
At 15 min post-hypoxia all groups had phrenic nerve amplitudes below baseline levels (P < 0.001; Fig. 2A). At 30 min post-hypoxia there was no phrenic LTF (ΔPhr significantly increased vs. baseline; Δ%BL) within any of the treatment groups (P = 0.895). At 60 min post-hypoxia, four of the five groups of rats showed phrenic LTF (P < 0.001). Old rats did not show a significant difference from baseline. There were no significant differences in the magnitude of LTF between any of the rat groups at 15, 30 or 60 min post-hypoxia (P > 0.05; Fig. 2A). Phrenic LTF values for Young rats at 60 min post-hypoxia were similar to those previously reported in our lab (Zabka et al. 2001b, 2005, 2006).
Figure 2. Phrenic and hypoglossal long-term facilitation (LTF) in Young, Old and treated rats.
LTF was measured as an amplitude increase in integrated phrenic (A) and XII (B) nerve activity from baseline (Δ%BL) at 15, 30 and 60 min after the final hypoxic episode. A, phrenic amplitude was similar in all treatments groups at 15, 30 and 60 min post-hypoxia. Phrenic motor activity was significantly elevated above baseline at 60 min in Young (*), Old+T (#), Old+T+2xADT (†) and Old +DHT (‡) groups of rats (P < 0.001), but not in Old rats. B, XII amplitude was similar in all treatment groups at 15 and 30 min post-hypoxia. At 60 min, XII amplitude was significantly elevated above baseline in Young (*) and Old+T (#), but not in Old, Old+T+2xADT and Old+DHT rats (P < 0.05). C, phrenic and XII amplitude at 60 min after the final hypoxic episode. XII amplitude was significantly elevated above baseline in Young and Old+T, but not in Old, Old+T+2xADT and Old+DHT rats (P < 0.05).
XII long term facilitation
At 15 min post-hypoxia all groups had XII nerve amplitudes below baseline levels (Fig. 2B). At 30 min post-hypoxia XII nerve amplitudes were below baseline levels in the Old, Old+T+2xADT and Old+DHT rat groups. At 60 min post-hypoxia only Young and Old+T rats showed XII LTF (ΔXII significantly increased vs. baseline; Δ%BL; P < 0.05; Fig. 3B). Old, Old+T+2xADT and Old+DHT treatment groups did not show any change in amplitude by comparison with baseline at 60 min post-hypoxia. There were no differences in XII LTF magnitude between any of the treatment groups at 15 and 30 min post-hypoxia (P > 0.05; Fig. 2B). XII LTF values for Young rats at 60 min post-hypoxia were somewhat lower than those previously reported in our lab (Zabka et al. 2001b, 2005, 2006).
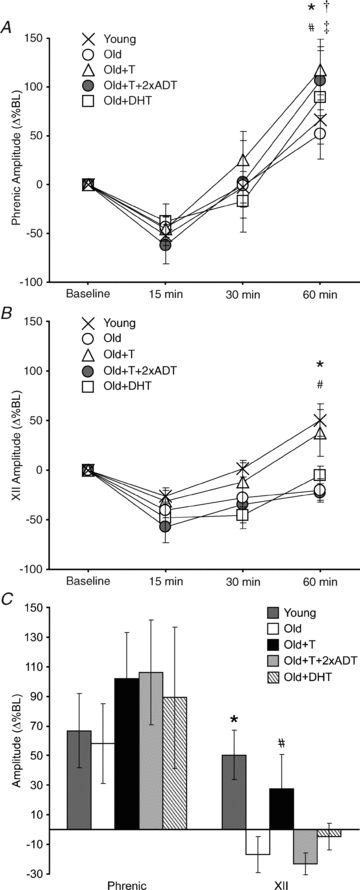
Figure 3. Serum levels of testosterone, oestradiol and progesterone.
Testosterone levels (ng ml−1) were significantly greater in Old+T (#) and Old+T+2xADT (†) rats than in Young, Old and Old+DHT rats (P < 0.05). Oestradiol levels (pg ml−1) were significantly greater in Old+T+2xADT (†) than in all other groups. Progesterone levels (ng ml−1) were similar in all groups. Values are means ± s.e.m.
Frequency long term facilitation
Phrenic and XII burst frequencies were similar in each treatment group at 60 min after the last hypoxic episode when compared to baseline burst frequency (P > 0.05).
Sex hormone levels
Serum testosterone levels were not significantly different in Young and Old rats (ng ml−1; 1.14 ± 0.08; 1.19 ± 0.16; P > 0.05) (Fig. 3). Testosterone levels in Old +DHT rats (0.58 ± 0.06 ng ml−1; P > 0.05) were not significantly different from that of Young and Old rats. Following testosterone implantation, serum testosterone levels increased significantly in the Old+T and Old+T+2xADT groups compared to all other treatment groups (ng ml−1: Old+T, 7.22 ± 0.50; O+T+2xADT, 4.78 ± 0.41; P < 0.001). Testosterone levels in treated rats were similar to that of previous studies in our laboratory (Zabka et al. 2006).
Serum oestradiol levels in all groups were low (pg ml−1: Young, 14.70 ± 0.39; Old, 17.58 ± 1.04; Old+T, 13.58 ± 1.29; Old+T+2xADT, 27.92 ± 4.67; Old+DHT, 10.19 ± 0.49). There was a significant difference between oestradiol levels in Old+T+2xADT and the other groups (P < 0.001) (Fig. 3), but as oestradiol levels were at the low end of the detectable range, this was not considered to be biologically significant.
Serum progesterone levels showed no significant differences between the groups (ng ml−1: Young, 18.24 ± 1.09; Old, 27.29 ± 6.55; O+T, 32.58 ± 12.37; O+T+2xADT, 16.52 ± 2.33; O+DHT, 21.50 ± 3.61; P = 0.456) (Fig. 3).
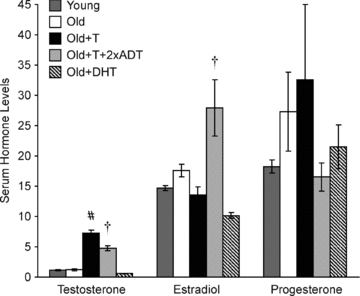
There was a slightly positive but non-significant correlation between the magnitude of phrenic LTF and serum testosterone levels in groups in which testosterone was available for conversion to oestradiol (Young, Old, Old+T) (r2 = 0.127; P = 0.068; Fig. 4A). Similarly, there was a slightly positive but non-significant correlation between the magnitude of XII LTF and serum testosterone levels (r2 = 0.042; P = 0.286; Fig. 4B).
Figure 4. Relationship between the magnitude of phrenic and XII LTF and serum levels of testosterone.
A, the magnitude of phrenic LTF measured in Young, Old and Old+T rats was positively but not significantly correlated with serum testosterone levels (P = 0.068, r2 = 0.127). B, the magnitude of XII LTF measured in Young, Old and Old+T rats was not significantly correlated with serum testosterone levels (P = 0.286, r2 = 0.042).
Aromatase activity levels
Aromatase activity was detected in XII, the C3–C6 ventral spinal cord and the hypothalamus, but was at the low end of the detectable range (fmol h−1: XII: Young, 0.87; Old, 1.17; Old+T, 0.79; Phrenic: Young, 2.88; Old, 2.41; Old+T, 2.48; Hypothalamus: Young, 5.05; Old, 4.46; Old+T, 5.61). Despite the low values detected in the brain samples, higher values in testis control tissues (199.42 fmol h−1) validated the assay. Aromatase activity could be blocked with the aromatase inhibitor, letrozole, also confirming the specificity of the assay (data not shown). When expressed as fmol h−1 (mg protein)−1, aromatase activity was similar in the hypothalamus and XII, but lower in the C3–C6 ventral spinal cord (Fig. 5). Higher levels were measured in testis control tissues (23.19 fmol h−1 (mg protein)−1). Comparison of aromatase activity levels in young vs. old animals and following treatment with testosterone did not suggest major changes in activity, but there was insufficient power for statistical analysis of the small changes observed due to the need to pool samples for sensitivity.
Figure 5. Aromatase activity does not change with age.
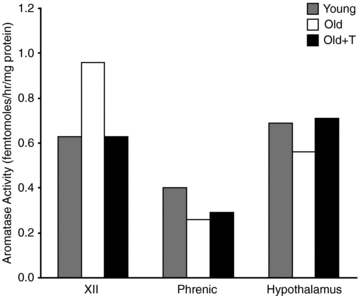
Aromatase activity (fmol h−1 (mg protein)−1) was measured in the hypoglossal nucleus (XII), C3–C6 ventral spinal cord (Phrenic) and the hypothalamus. Comparison of aromatase activity levels in young and old animals did not suggest major age-related changes in activity, but there was insufficient power for statistical analysis. Bars show the mean of two samples, each containing pooled tissues from 3 animals. Range (fmol h−1 (mg protein)−1): XII: Young, 0.48–0.79; Old, 0.92–1.01; Old+T, 0.55–0.71; Phrenic: Young, 0.37–0.43; Old, 0.20–0.31; Old+T, 0.28–0.30; Hypothalamus: Young, 0.54–0.85; Old, 0.53–0.58; Old+T, 0.59–0.84).
Discussion
The major finding of this study is that XII LTF is restored in old male rats by supplementation with testosterone. This effect can be blocked by an aromatase inhibitor (ADT), or by supplementation with DHT, a form of testosterone that cannot be converted to oestradiol, indicating that the conversion of testosterone to oestradiol in the brain by aromatase is necessary for restoration of LTF. The latter conclusion is supported by a study in gonadectomized, young male rats that showed recovery of LTF when supplemented with testosterone (Zabka et al. 2006). The second finding of this study is that aromatase activity in XII and C3–C6 ventral spinal cord does not appear to change with age. As testosterone levels also did not decline with age in this strain of rats, local bioavailability of testosterone may be a limiting factor in the expression of this form of respiratory plasticity in old rats.
Aromatase activity levels
This is the first report of aromatase activity in respiratory motor regions of the central nervous system. Aromatase activity in the XII, when normalized to protein levels, was similar to that of the hypothalamus, although lower levels were measured in C3–C6 ventral spinal cord where the phrenic nucleus is located. Greater aromatase activity in XII than in the C3–C6 ventral spinal cord is consistent with the differential effects of supplemental testosterone on LTF: XII > phrenic. We previously showed that oestrogen receptors (ERα, ERβ) are present in hypoglossal and phrenic motoneurons in young and old rats (Behan & Thomas, 2005), potentially providing a substrate for XII and phrenic LTF. Whereas blocking aromatase activity in the presence of supplemental testosterone in old rats abolished XII LTF (Fig. 2B, Old+T+2xADT), it did not completely abolish phrenic LTF (Fig. 2A), indicating that testosterone might also act directly on androgen receptors (ARs) in phrenic motoneurons (Behan & Thomas, 2005).
We were unable to detect any significant age-related change in aromatase activity in XII, C3–C6 ventral spinal cord or the hypothalamus (Fig. 5). The assay replicate size was small: two samples, each consisting of pooled tissues from three rats. However, our data for hypothalamus are consistent with two previous reports in male Sprague–Dawley rats and F344 rats showing no age-associated change in aromatase activity (Roselli et al. 1986; Chambers et al. 1991). Although the Sprague–Dawley and F344 rats in the previous studies had a significant age-related decrease in serum testosterone, levels did not decline appreciably with age in the F344 rats that we used in the present study. This was somewhat surprising, as a previous study with similarly sourced F344 rats (National Institute on Aging colony) showed a decline in testosterone levels (Zabka et al. 2005). This may reflect rat strain/substrain, genetic drift, or methodological differences in hormone measurement (radioimmunoassay vs. enzyme immunoassay). Indeed, testosterone levels have been shown to increase in middle aged Sprague–Dawley rats (12 months) by comparison with young rats (4 months) (Wu & Gore, 2009), although other studies have reported a decline in serum testosterone at middle age (Gruenewald et al. 2000). Despite reports of an age-associated decline in testosterone levels in humans (Ferrini & Barrett-Connor, 1998; Leifke et al. 2000), many older men have serum testosterone levels in the normal range. This population has been shown to be as responsive as younger men to the anabolic effects of high, physiological doses of supplemental testosterone on skeletal muscle (Bhasin et al. 2005).
Bioavailability of testosterone
Measurements of total serum testosterone levels include both bioavailable (free) testosterone, and testosterone bound to proteins such as albumin and steroid hormone binding globulin (SHBG), the latter having a high affinity for testosterone. It is estimated that only 2% of serum testosterone is present in an unbound form (Sodergard et al. 1982). Changes in levels of SHBG greatly affect the amount of sex hormones available to tissues. In humans, serum total and free testosterone decrease linearly with age, whereas SHBG increases with age (Harman et al. 2001). Consequently, with increasing age, less testosterone is biologically available. As testosterone is the primary substrate for bioavailable oestradiol in males, not surprisingly this too has been shown to decline with age (Ferrini & Barrett-Connor, 1998). In the present study, by supplementing old rats with ‘new’ testosterone, more bioavailable (free) testosterone may have been produced in the circulation. Additionally, as high androgen levels are known to decrease SHBG (Plymate et al. 1983; Selby, 1990; Kicman, 2010), abruptly raising serum testosterone levels may have also resulted in a greater local proportion of bioavailable testosterone that contributed to the restoration of XII LTF and enhancement of phrenic LTF.
Previously, Zabka et al. (2006) reported a weak but significant positive correlation between testosterone levels and XII LTF in groups of young, middle-aged and gonadectomized male rats. Although there was a positive slope, we did not find a statistically significant correlation between total serum testosterone levels and XII and phrenic LTF in Young, Old and Old+T groups (Fig. 4). However, measurement of serum testosterone is, at best, a crude estimate of the amount of testosterone in the central nervous system that can be aromatized to oestrogen and affect the expression of LTF.
An important question is whether neurons in an aging brain have the same capacity as neurons in a young brain to respond to exogenous testosterone. Wu & Gore (2010) showed that middle aged intact rats had significantly impaired sexual behaviour by comparison with young rats. After castration and testosterone implantation, sexual behaviours were comparable to that of young rats. They found that hypothalamic expression of AR and ERα was also affected by ageing, and essentially restored by testosterone supplementation (Wu & Gore, 2010). The rats in the Wu and Gore study were middle-aged, castrated and subsequently supplemented with testosterone, whereas the rats in the present study were old when they received testosterone. Nonetheless, our data suggest that respiratory motoneurons in old rats can respond to exogenous testosterone: hypoglossal motoneurons show an augmented response to hypoxia (increased LTF), similar to that seen in young rats.
The mechanisms whereby respiratory LTF can be influenced by testosterone are not well understood, but probably involve a combination of: (1) direct action via sex hormone receptors (androgen and oestrogen receptors) on respiratory motoneurons or premotor neurons, and (2) indirect action via sex hormone receptors on neuromodulatory neurons that synapse on respiratory motoneurons (Behan & Wenninger, 2008). When activated, these classical nuclear hormone receptors initiate changes in the rate of transcription of hormone-regulated genes and subsequent protein synthesis (Nilsson et al. 2001). There are multiple cell signalling pathways that have been implicated in long term facilitation (for review, see Dale-Nagle et al. 2010). For example, new synthesis of brain derived neurotrophic factor (BDNF) and subsequent binding to high-affinity TrkB receptors is necessary and sufficient for phrenic LTF (Baker-Herman et al. 2004). Oestrogen can also bind to a G protein-coupled cell surface receptor (GPR30) and activate signal transduction pathways resulting in a rapid calcium influx, thereby altering neuronal firing properties (Vasudevan & Pfaff, 2008). Increasing evidence also suggests that androgens can activate neuronal membrane receptors (Frye, 2010). Future studies will address whether androgen and oestrogen receptors, which are present on respiratory motoneurons (Behan & Thomas, 2005), are upregulated following testosterone supplementation in old rats. Restoration of XII LTF following testosterone supplementation could also be due to upregulation of sex hormone receptors on serotonergic neurons in the caudal raphe nuclear group, which also express sex hormone receptors (M. Behan, unpublished observations). Respiratory LTF has been shown previously to be strongly influenced by serotonergic inputs to hypoglossal and phrenic motoneurons (Mitchell & Johnson, 2003). In the present study, neither age nor testosterone supplementation significantly affected phrenic LTF in contrast with hypoglossal LTF (Fig. 2C). Some raphe neurons project to both hypoglossal and phrenic motor pools, whereas others project to only one nucleus (Manaker et al. 1992; Manaker & Tischler, 1993), thus providing a potential anatomical substrate for the differential effects seen in XII and phrenic LTF. Previous studies of LTF have also shown distinct differences in the responses of XII and phrenic motoneurons to hypoxia. For example, in young male rats gonadectomy abolished XII LTF but merely reduced phrenic LTF (Zabka et al. 2005). Similarly, middle-aged and old male rats showed a reduced phrenic LTF, but hypoglossal LTF was virtually abolished (Zabka et al. 2001a, 2005). Taken together, these data suggest that phrenic motoneurons innervating the diaphragm are influenced to a lesser degree by sex hormones than hypoglossal motoneurons innervating the tongue.
Clinical significance
One of the most striking respiratory pathologies associated with ageing is OSA, characterized by episodic closure of the upper airway during sleep (Young et al. 1993; Kapsimalis & Kryger, 2002a,b; White, 2005, 2006; Lee et al. 2008; Dempsey et al. 2010). A key muscle involved in upper airway patency is the tongue, innervated by the hypoglossal nerve (White, 2005, 2006). There are reports of low serum testosterone levels in men with OSA, independent of age (Grunstein et al. 1989; Luboshitzky et al. 2002; Kirbas et al. 2007). However, very few studies have investigated the impact of supplemental testosterone on breathing in asymptomatic men. White et al. (1985) measured ventilatory responses in 12 hypogonadal men before and after testosterone supplementation and showed that resting ventilation, metabolic rate and the hypoxic ventilatory response were all increased following hormone treatment. A similar finding was reported in castrated cats supplemented with testosterone (Tatsumi et al. 1994). In contrast, testosterone therapy was reported to exacerbate OSA in some men (Matsumoto et al. 1985; Saaresranta & Polo, 2002; Liu et al. 2003; Bhasin et al. 2006), although the evidence for this is weak (Saaresranta & Polo, 2002; Bhasin et al. 2006; Hanafy, 2007). In a study of healthy young men who were given leuprolide acetate, a gonadotropin-releasing hormone agonist that greatly reduces serum levels of testosterone, the ventilatory response threshold to CO2 during wakefulness and sleep was reduced (Mateika et al. 2004). The question remains as to whether testosterone therapy would be beneficial in patients with OSA, and whether the potential benefits outweigh the risks (Gruenewald & Matsumoto, 2003; Swerdloff & Wang, 2004). Our studies in rats show that aromatase is still present in the brains of older rats and that testosterone supplementation can reverse the age-associated decline in respiratory plasticity, suggesting that this approach may be of benefit to some patients.
Acknowledgments
We would like to thank Gordon Mitchell and members of the Mitchell laboratory for their help throughout this project. We would also like to thank Alan J. Conley and Jo Corbin (University of California–Davis) for their contributions to assay optimization for the study and to Mary Grummer for technical assistance. This study was supported by NIH grant AG18760 (M.B.).
Glossary
Abbreviations
- ADT
1,4,9-androstatriene-3,17-dione
- AR
androgen receptor
- BDNF
brain-derived neurotrophic factor
- DHT
5α-dihydrotestosterone
- E2
oestradiol
- ER
oestrogen receptor
- LTF
long term facilitation
- MAP
mean arterial pressure
- OSA
obstructive sleep apnoea
- SHBG
sex hormone binding globulin
- T
testosterone
- TrkB
tyrosine kinase B
- XII
hypoglossal
- Δ%BL
percentage change in peak integrated nerve amplitude from baseline
- ΔPhr
peak integrated phrenic amplitude
- ΔXII
peak integrated hypoglossal amplitude
Author contributions
N.N. performed the experiments, collected, analysed and interpreted data, and drafted the manuscript. I.B. designed the aromatase experiments, analysed and interpreted these data, and drafted this section of the manuscript. The aromatase measurements were done in his laboratory. M.B. conceived and designed the experiments, interpreted data, and wrote and revised the final manuscript critically for intellectual content. The LTF experiments were done in her laboratory.
References
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Behan M, Thomas CF. Sex hormone receptors are expressed in identified respiratory motoneurons in male and female rats. Neuroscience. 2005;130:725–734. doi: 10.1016/j.neuroscience.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol. 2008;164:213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136:249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- Bhatnagar AS, Hausler A, Schieweck K, Lang M, Bowman R. Highly selective inhibition of estrogen biosynthesis by CGS 20267, a new non-steroidal aromatase inhibitor. J Steroid Biochem Mol Biol. 1990;37:1021–1027. doi: 10.1016/0960-0760(90)90460-3. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- Celotti F, Melcangi RC, Negri-Cesi P, Poletti A. Testosterone metabolism in brain cells and membranes. J Steroid Biochem Mol Biol. 1991;40:673–678. doi: 10.1016/0960-0760(91)90289-h. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Thornton JE, Roselli CE. Age-related deficits in brain androgen binding and metabolism, testosterone, and sexual behavior of male rats. Neurobiol Aging. 1991;12:123–130. doi: 10.1016/0197-4580(91)90050-t. [DOI] [PubMed] [Google Scholar]
- da Silva SB, de Sousa Ramalho Viana E, de Sousa MB. Changes in peak expiratory flow and respiratory strength during the menstrual cycle. Respir Physiol Neurobiol. 2006;150:211–219. doi: 10.1016/j.resp.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol. 2010;669:225–230. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Skatrud JB. A sleep-induced apneic threshold and its consequences. Am Rev Respir Dis. 1986;133:1163–1170. doi: 10.1164/arrd.1986.133.6.1163. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver HS, McLean H, Kumar DV, Farr N, Day AG, Fitzpatrick MF. The influence of the menstrual cycle on upper airway resistance and breathing during sleep. Sleep. 2005;28:449–456. doi: 10.1093/sleep/28.4.449. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- Frye CA. Effects and mechanisms of progestogens and androgens in ictal activity. Epilepsia. 2010;51(Suppl 3):135–140. doi: 10.1111/j.1528-1167.2010.02628.x. [DOI] [PubMed] [Google Scholar]
- Fukuda Y. Maintenance of ventilatory control by CO2 in the rat during growth and aging. Pflugers Arch. 1991;419:38–42. doi: 10.1007/BF00373745. [DOI] [PubMed] [Google Scholar]
- Fukuda Y. Changes in ventilatory response to hypoxia in the rat during growth and aging. Pflugers Arch. 1992;421:200–203. doi: 10.1007/BF00374827. [DOI] [PubMed] [Google Scholar]
- George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–956. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. discussion 115. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl. 2000;21:72–84. [PubMed] [Google Scholar]
- Grunstein RR, Handelsman DJ, Lawrence SJ, Blackwell C, Caterson ID, Sullivan CE. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. 1989;68:352–358. doi: 10.1210/jcem-68-2-352. [DOI] [PubMed] [Google Scholar]
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med. 2007;4:1241–1246. doi: 10.1111/j.1743-6109.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Jensen D, Wolfe LA, O’Donnell DE, Davies GA. Chemoreflex control of breathing during wakefulness in healthy men and women. J Appl Physiol. 2005a;98:822–828. doi: 10.1152/japplphysiol.01208.2003. [DOI] [PubMed] [Google Scholar]
- Jensen D, Wolfe LA, Slatkovska L, Webb KA, Davies GA, O’Donnell DE. Effects of human pregnancy on the ventilatory chemoreflex response to carbon dioxide. Am J Physiol Regul Integr Comp Physiol. 2005b;288:R1369–1375. doi: 10.1152/ajpregu.00862.2004. [DOI] [PubMed] [Google Scholar]
- Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: Clinical features. Sleep. 2002a;25:412–419. [PubMed] [Google Scholar]
- Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 2: mechanisms. Sleep. 2002b;25:499–506. [PubMed] [Google Scholar]
- Kicman AT. Biochemical and physiological aspects of endogenous androgens. Handb Exp Pharmacol. 2010:25–64. doi: 10.1007/978-3-540-79088-4_2. [DOI] [PubMed] [Google Scholar]
- Kirbas G, Abakay A, Topcu F, Kaplan A, Unlu M, Peker Y. Obstructive sleep apnoea, cigarette smoking and serum testosterone levels in a male sleep clinic cohort. J Int Med Res. 2007;35:38–45. doi: 10.1177/147323000703500103. [DOI] [PubMed] [Google Scholar]
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: A population-based perspective. Expert Rev Respir Med. 2008;2:349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifke E, Gorenoi V, Wichers C, Von Zur Muhlen A, Von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Simpson ER. Assay of aromatase activity. Methods Enzymol. 1991;206:477–483. doi: 10.1016/0076-6879(91)06116-k. [DOI] [PubMed] [Google Scholar]
- Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12:481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Yee B, Wishart SM, Jimenez M, Jung DG, Grunstein RR, Handelsman DJ. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003;88:3605–3613. doi: 10.1210/jc.2003-030236. [DOI] [PubMed] [Google Scholar]
- Luboshitzky R, Aviv A, Hefetz A, Herer P, Shen-Orr Z, Lavie L, Lavie P. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab. 2002;87:3394–3398. doi: 10.1210/jcem.87.7.8663. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ, Morrison AR. Raphespinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. J Comp Neurol. 1992;322:68–78. doi: 10.1002/cne.903220106. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol. 2009;94:279–296. doi: 10.1113/expphysiol.2008.045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Omran Q, Rowley JA, Zhou XS, Diamond MP, Badr MS. Treatment with leuprolide acetate decreases the threshold of the ventilatory response to carbon dioxide in healthy males. J Physiol. 2004;561:637–646. doi: 10.1113/jphysiol.2004.071811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto AM, Sandblom RE, Schoene RB, Lee KA, Giblin EC, Pierson DJ, Bremner WJ. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin Endocrinol (Oxf) 1985;22:713–721. doi: 10.1111/j.1365-2265.1985.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980a;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980b;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Plymate SR, Leonard JM, Paulsen CA, Fariss BL, Karpas AE. Sex hormone-binding globulin changes with androgen replacement. J Clin Endocrinol Metab. 1983;57:645–648. doi: 10.1210/jcem-57-3-645. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Kaler LW, Resko JA. Hypothalamic aromatase activity in young and old male rats. Neurobiol Aging. 1986;7:121–125. doi: 10.1016/0197-4580(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Ryan S, Nolan P. Long-term facilitation of upper airway muscle activity induced by episodic upper airway negative pressure and hypoxia in spontaneously breathing anaesthetized rats. J Physiol. 2009;587:3343–3353. doi: 10.1113/jphysiol.2009.169698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaresranta T, Polo O. Hormones and breathing. Chest. 2002;122:2165–2182. doi: 10.1378/chest.122.6.2165. [DOI] [PubMed] [Google Scholar]
- Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27:532–541. doi: 10.1177/000456329002700603. [DOI] [PubMed] [Google Scholar]
- Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O’Connor GT, Rapoport DM, Robbins JA. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav. 1992;26:110–135. doi: 10.1016/0018-506x(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C. Androgens and the ageing male. Best Pract Res Clin Endocrinol Metab. 2004;18:349–362. doi: 10.1016/j.beem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Tadjalli A, Duffin J, Peever J. Repeated obstructive apneas induce long-term facilitation of genioglossus muscle tone. Adv Exp Med Biol. 2010;669:297–301. doi: 10.1007/978-1-4419-5692-7_61. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Hannhart B, Pickett CK, Weil JV, Moore LG. Effects of testosterone on hypoxic ventilatory and carotid body neural responsiveness. Am J Respir Crit Care Med. 1994;149:1248–1253. doi: 10.1164/ajrccm.149.5.8173766. [DOI] [PubMed] [Google Scholar]
- Tenover JS. Declining testicular function in aging men. Int J Impot Res. 2003;15(Suppl 4):S3–8. doi: 10.1038/sj.ijir.3901029. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology. 2003;78:36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Olson EB, Jr, Cotter CJ, Thomas CF, Behan M. Hypoxic and hypercapnic ventilatory responses in aging male vs. aging female rats. J Appl Physiol. 2009;106:1522–1528. doi: 10.1152/japplphysiol.90802.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- White DP. Sleep apnea. Proc Am Thorac Soc. 2006;3:124–128. doi: 10.1513/pats.200510-116JH. [DOI] [PubMed] [Google Scholar]
- White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128:984–986. doi: 10.1164/arrd.1983.128.6.984. [DOI] [PubMed] [Google Scholar]
- White DP, Schneider BK, Santen RJ, McDermott M, Pickett CK, Zwillich CW, Weil JV. Influence of testosterone on ventilation and chemosensitivity in male subjects. J Appl Physiol. 1985;59:1452–1457. doi: 10.1152/jappl.1985.59.5.1452. [DOI] [PubMed] [Google Scholar]
- Wu D, Gore AC. Sexual experience changes sex hormones but not hypothalamic steroid hormone receptor expression in young and middle-aged male rats. Horm Behav. 2009;56:299–308. doi: 10.1016/j.yhbeh.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Gore AC. Changes in androgen receptor, estrogen receptor α, and sexual behavior with aging and testosterone in male rats. Horm Behav. 2010;58:306–316. doi: 10.1016/j.yhbeh.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 2001a;531:509–514. doi: 10.1111/j.1469-7793.2001.0509i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol. 2001b;91:2831–2838. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005;563:557–568. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J Physiol. 2006;576:903–912. doi: 10.1113/jphysiol.2006.114850. [DOI] [PMC free article] [PubMed] [Google Scholar]


