Abstract
In cat medial gastrocnemius (MG), fibres supplied by individual motoneurones (muscle units) distribute extensively along the muscle longitudinal axis. In the human MG, the size of motor unit territory is unknown. It is uncertain if the absolute size of muscle unit territory or the size relative to the whole muscle is most comparable with the cat. By comparing intramuscular and surface electromyograms we tested whether muscle units extend narrowly or widely along the human MG muscle. Due to the pennation of the MG, if individual motoneurones supply fibres scattered along the muscle, then action potentials of single motor units are expected to appear sparsely on the surface of the skin. In nine healthy subjects, pairs of wire electrodes were inserted in three locations along the MG muscle (MG60%, MG75% and MG90%). A longitudinal array of 16 surface electrodes was positioned alongside the intramuscular electrodes. While subjects stood quietly, 55 motor units were identified, of which, significantly more units were detected in the most distal sites. The surface action potentials had maximum amplitude at 4.40 ± 1.67 (mean ± s.d.), 8.02 ± 2.16 and 11.63 ± 2.09 cm (P < 0.001) from the most proximal surface electrode, for motor units in the MG60%, MG75% and MG90% locations, respectively. Single motor unit potentials were recorded by five consecutive surface electrodes, at most, indicating that muscle units extend shortly along the MG longitudinal axis. It is concluded that relative to the whole muscle, and compared with the cat, muscle units in human MG are localised. The localisation of muscle units might have implications for the regional control of muscle activity.
Non-technical summary
In the medial gastrocnemius muscle of cats, the longitudinal size (3–4 cm) of the territory of motor units is large (∼60%) relative to the muscle length. In the human medial gastrocnemius, the size of motor unit territories is unknown. By comparing intramuscular and surface electromyograms we show that, when subjects stand at ease, the motor units active in the medial gastrocnemius have small territories. They extend no longer than 4 cm and no less than 1 cm along the longitudinal axis (∼25 cm long) of the muscle. Physiologically, the small territories of motor units give the nervous system a mechanism for the independent activation of sub-volumes of the medial gastrocnemius muscle.
Introduction
The medial gastrocnemius (MG), the lateral gastrocnemius and the soleus muscles are separated heads of the triceps surae (calf muscles), the chief extensor of the ankle. Early investigations on cats focused on the physiological, morphological and histochemical characterisation of muscle fibres in individual motor units in the triceps surae (Eccles & Sherrington, 1930; Wuerker et al. 1965; Burke, 1968; Burke & Tsairis, 1973; Burke et al. 1973). As a common outcome, the motor units in the gastrocnemius muscles proved to be widely diversified with respect to the contraction–tension capacity, contraction time, maximum tetanic tension, innervation ratio, diameter of motor and muscle fibres, conduction velocity along the axons, firing patterns and concentration of mitochondrial enzymes in the muscle fibres. While the general features of motor units are comparable between humans and cats (Burke & Edgerton, 1975; Garnett et al. 1979), important differences might exist in muscle organisation. The spatial localisation of muscle fibres innervated by single motoneurones (the muscle unit), for example, remains unclear.
Direct localisation of muscle fibres revealed that muscle units extend extensively along the cat MG muscle. By staining muscle fibres contained in different cross sections of the MG muscle with periodic acid-Schiff, Burke & Tsairis (1973) showed that the muscle fibres of a glycogen-depleted unit distribute widely (∼4 cm) along the muscle (Fig. 1A). This result suggests that individual motoneurones innervate scattered gastrocnemius fibres (Fig. 1B) rather than localised fibres (Fig. 1C) along the muscle. When comparing the territory of muscle units in humans and cats, a key question is whether its longitudinal size is more similar in absolute value or relative to the muscle length. The territory of muscle units along the cat MG (3–4 cm; Burke & Tsairis, 1973; Rafuse & Gordon, 1996) represents ∼14% of the length of the human MG muscle at rest (∼25 cm; Narici et al. 1996). If the absolute rather than the relative size of the territory of muscle units in cat and human is more similar, then single muscle units are localised in the human MG muscle.
Figure 1. Distribution of muscle units within the medial gastrocnemius muscle.
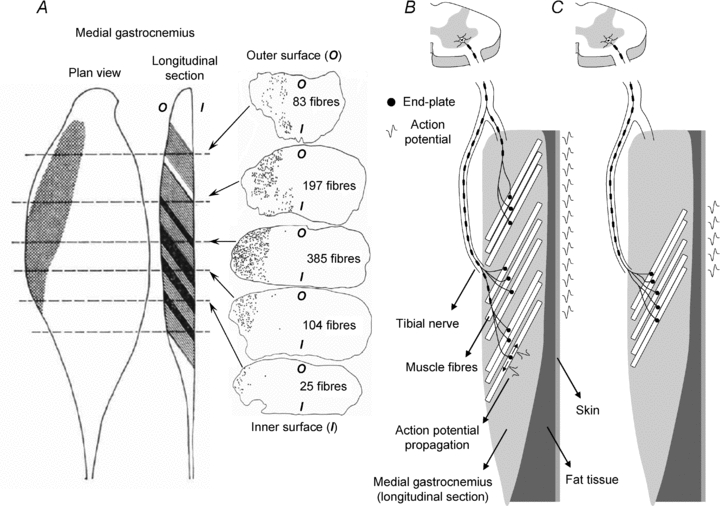
A, shows the localisation of muscle fibres innervated by a single motoneuron (muscle unit; fast fatiguing). The muscle unit was identified by direct counting of glycogen-depleted muscle fibres (grey bands in the longitudinal section) at several cross-sections taken along the longitudinal axis of the cat medial gastrocnemius (MG) muscle. Dark bands denote muscle fibres appearing in two cross sections, due to the pennation of gastrocnemius fibres. Cross-hatchings in the plane view indicate the territory of the muscle unit. The number of muscles fibre identified in five cross-sections of the MG muscle is shown in the right column. B, illustrates the hypothesis of muscle units extending widely along the human MG muscle. The body of one motoneuron is located at the ventral root in the spinal cord, while its axon runs through the tibial nerve and branches to supply muscle fibres scattered along the whole MG muscle. C, shows the hypothesis of spatially localised muscle units. Axonal branching occurs close to the muscle and supplies muscle fibres distributed locally. Due to the pennate arrangement of MG muscle fibres, and since action potentials propagate along muscle fibres and not along the muscle, intramuscular potentials might appear locally or widely on the surface of the skin, depending on how extensive are the MG muscle units (compare the schematic representation of action potentials on the surface for case B and case C).
Although recent results indicate the local activation of human gastrocnemius muscles (Wolf et al. 1998; Kinugasa et al. 2005; Segal & Song, 2005; Staudenmann et al. 2009), compelling evidence on the localisation of gastrocnemius muscle units is still missing. In a previous study, we used a matrix of surface electrodes to investigate the synchronisation of activity across the surface of the gastrocnemius muscles in standing (Vieira et al. 2010). Strikingly, the longitudinal, temporal sequence of activation was not constant. On occasion, proximal regions of medial gastrocnemius were the first to be activated (Figs 2 and 5 in Vieira et al. 2010); on other occasions, activation started in the distal regions (Fig. 9 in Vieira et al. 2010). Comparison between intramuscular and surface EMGs excluded the conduction of action potentials as the cause of the sequential activation. These results are in agreement with the controversial possibility that activation is organised regionally.
Figure 2. Spatial distribution of intramuscular and surface EMG recordings during standing.
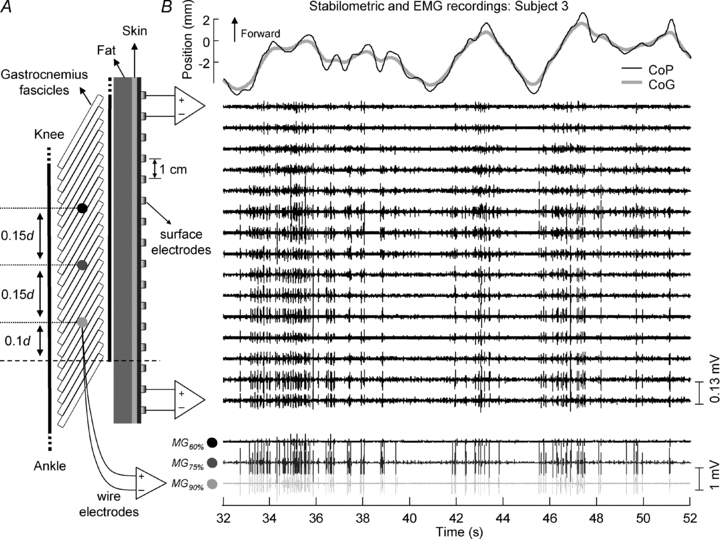
A, the positioning of the surface array of 16 electrodes and the approximate locations (MG60%, MG75% and MG90%) for insertion of the wire electrodes along the MG muscle. Surface electrodes were spaced by 1 cm and intramuscular recording locations were spaced by 15% of the distance (d) between the popliteal crease and the superficial extremity of the most distal MG fascicle (dashed line). B, the position of body centre of pressure (thin black line) and centre of gravity (thick grey line) (top), the surface (middle) and the intramuscular electromyograms (bottom) recorded from MG muscle during 20 s out of 60 s of quiet standing (subject 3). Intramuscular signals detected by electrodes inserted in different MG locations (as shown in A) are depicted in shades of grey (MG60%: black; MG75%: dark grey; MG90%: light grey).
Figure 5. Local manifestation of surface motor unit action potentials.
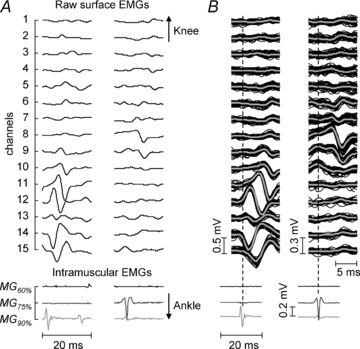
A, a typical example of surface (top) and intramuscular (bottom) electromyograms recorded from the MG muscle of subject 7. The grey level of intramuscular traces denotes from which location these signals were recorded (see Fig. 2). Note the clear correspondence of the intramuscular potentials in the MG75% and MG90% locations with the surface action potentials on the middle (channels 8 and 9) and on the distal (from channel 9 to 15) portions of the array of electrodes, respectively. B, the surface potentials (top, black lines) and their average (thick grey line), triggered with the discharge pattern of intramuscular potentials shown at the bottom. Consistent and local representation of intramuscular action potentials is evident on the surface.
For the regional organisation of postural activity in the MG muscle to be possible, the motor units activated during standing must have small territory with respect to the muscle length. Therefore, in this study we ask: Are muscle fibres in individual muscle units localised in the human MG muscle? If a motoneurone stimulates fibres throughout the muscle, we would expect the activity of one motor unit to be represented at different locations on the surface of the MG muscle (Fig. 1B). If a motoneurone stimulates fibres distributed closely in one location, then given the geometry of the MG muscle, we expect its activity to appear locally in the surface EMG (Fig. 1C). Figure 1 illustrates the hypothesis of spatially extensive or spatially localised muscle units and how the territory of single motor units might be associated with the appearance of action potentials on the surface of the skin. We test for these two hypotheses with an innovative method for estimating the size of the motor unit territory in pennate muscles, whose methodological validation is also provided in this study.
Methods
Subjects and the experimental protocol
Nine healthy subjects (seven men and two women; age range: 27–33 years; stature: 160–178 cm; body mass: 56–90 kg) participated in the study after providing written informed consent. The experiment was approved by the ethics committee of the Region North Jutland, Denmark, and conformed to the latest version of the Declaration of Helsinki.
Subjects were asked to stand at ease on a force platform (OR6-7, AMTI) for 60 s, with their feet in comfortable position, their arms alongside the body and facing forward. At least four trials were performed, half of them with eyes closed. If spontaneous abnormal movements were perceived by the experimenter, the trial was repeated. Eyes open and eyes closed data were pooled, since the effect of visual input was not relevant for the analysis of the territory of single motor units.
EMG recordings
Intramuscular EMGs were recorded in bipolar configuration using pairs of Teflon-coated stainless-steel wire electrodes (0.2 mm diameter; A-M Systems, Carlsborg, WA, USA), inserted with a 25-gauge hypodermic needle. One needle comprised three wire electrodes, thus allowing for the acquisition of two bipolar EMGs in the same location. Only one of the two EMGs was further considered for analysis, based on visual inspection of signal quality. To minimise the detection volume of the intramuscular recordings, the wire electrodes were cut to expose their cross section only (Stålberg, 1980). Surface EMGs were recorded using a linear array of 16 silver electrodes (10 × 1 mm size), with 10 mm inter-electrode distance, after cleansing of the skin with alcohol and water. The electrode array was fixed on the skin using double adhesive foam. Gel between electrodes and the skin ensured low impedance of the electrode–skin interface.
An EMG amplifier (10 Hz to 5 kHz bandwidth; EMG-USB2, OT Bioelettronica, Turin, Italy, and Center for Sensory-Motor Interaction, Aalborg, Denmark) was used to record both the intramuscular and surface EMGs concurrently. All signals were sampled at 10 kHz after adjusting the amplification factor to 5000–10,000 for the surface EMGs and to 2000–5000 for the intramuscular EMGs. The highest gain not resulting in saturation of the recorded EMGs was used. Intramuscular and surface EMGs were offline band-pass filtered (15–1500 Hz and 15–350 Hz, respectively) with a second order zero-lag Butterworth filter.
Electrode location
The distal extremity of the superficial aponeurosis of the MG muscle was identified with ultrasound imaging and marked on the skin (see dashed line in Fig. 2A). Then, the longitudinal distance (d) between the marking location and the popliteal crease was measured with a ruler. Three needles were used to place the wire electrodes in three locations along the MG muscle. The first needle (two pairs of wire electrodes) was inserted at a distance of 0.9d distally from the popliteal crease (light grey circle in Fig. 2A; MG90% location). The second and the third needles were inserted, respectively, at 0.15d and 0.3d proximally to the location in which the first pairs of wire electrodes were inserted (dark grey and black circles in Fig. 2A; MG75% and MG60% locations).
The mean ± s.d. value of d was 18.1 ± 1.9 cm (n = 9 subjects), which resulted in an average distance of 2.7 ± 0.3 cm between the locations of intramuscular electrodes. Because of the pennate architecture of gastrocnemius muscles, the wire electrodes inserted in the MG60%, MG75% and MG90% locations did not record from the same MG fascicles.
The array of surface electrodes was located immediately beside the wire electrodes, so that the eighth surface electrode was approximately aligned with the MG75% location (Fig. 2A). The direction of the array was parallel to the direction of the line passing by the three sites of intramuscular recordings. Since the needles were inserted in the transverse direction and slightly oblique with respect to the skin, with a depth of ∼2.2 cm, the surface electrodes were directly above the pick-up regions of the wire electrodes, as confirmed by identifying the needle with ultrasound. Figure 2B shows representative intramuscular and surface EMGs recorded during standing.
Estimating the longitudinal size of motor unit territories
Individual trains of motor unit action potentials were discriminated by automatic decomposition of intramuscular EMGs using the software tool EMGlab (McGill et al. 2005). All signals were inspected visually to correct for any missed action potential, or for any action potential identified improperly by the algorithm (e.g. because of the superimposition of action potentials of different motor units). Once the trains of action potentials were identified and inspected, the instants of discharge of each motor unit were used as triggers for the averaging of the 15 bipolar surface EMGs (Fig. 3A). This procedure (spike triggered averaging) provides the multi-channel surface action potentials (Farina et al. 2002). Figure 3A shows the surface triggered potentials (superimposed black traces) and their average (thick grey traces), calculated with the discharge pattern of one motor unit identified in the MG60% location.
Figure 3. Method of localising intramuscular potentials in the surface EMG.
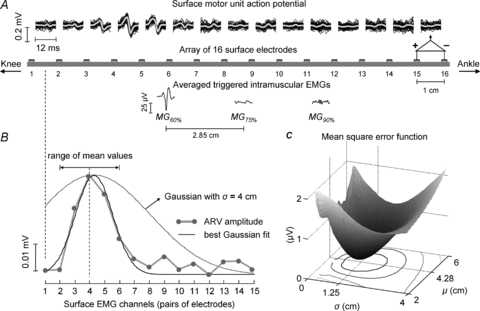
A, the triggered (black lines) and the averaged surface potentials (grey lines) of one motor unit identified from the intramuscular EMG in the MG60% location. The discharge pattern of this motor unit was used to trigger and then average the intramuscular EMGs recorded from each MG location (traces below the array). B, the distribution of the averaged rectified value (ARV) amplitude (•) across channels (grey lines). The experimental distribution of ARV values was fitted with a Gaussian function (black line) by minimising the mean squared error function (shown in C). The theoretical mean (μ) was allowed to vary from –2 to +2 cm (see double arrows) with respect to the position of maximal ARV value, while the standard deviation (σ) value changed from 0.1 to 8 cm (see dotted Gaussian). C, the bi-dimensional distribution of the mean squared error. The optimal Gaussian had mean and standard deviation equal to 4.28 and 1.25 (cm), respectively. Note that a single minimum is clearly defined in the error function.
The amplitude of surface triggered EMGs was calculated to quantify the surface localisation of the intramuscular action potentials. Firstly, surface EMGs (epochs of 20 ms, 200 samples) centred on each of the discharge times of a single motor unit were averaged. After that, the surface signals were rectified (absolute value) and then averaged across the 20 ms epoch. Finally, the average rectified value (ARV) of surface triggered potentials was calculated for each of the signals recorded with the array of surface electrodes. In the example of Fig. 3B, the values of ARV calculated from the third to the sixth channel are greater with respect to those computed for the other channels. The average position and the extent to which the surface action potential is distributed over the skin were estimated by adjusting a theoretical Gaussian curve to the experimental ARV distribution. The optimal mean (μ) and the standard deviation (σ) minimised the mean square error between Gaussians and the empirical ARV amplitude (Fig. 3C). It was assumed that the motor unit territory was proportional to the σ of the fitting Gaussian function. This assumption was validated by simulations.
Surface EMGs were simulated with the model proposed by Mesin & Farina (2004) to test for the association between the size of motor unit territory and the σ of the fitting Gaussian function. The simulation parameters are reported in Table 1. Two libraries of single fibre action potentials were created. Each library comprised the surface potentials of 3200 fibres (40 fibre mm−1), distributed normally or uniformly along the muscle region simulated. The longitudinal position of each simulated fibre was set by extracting a random number from a Gaussian (standard deviation: 2 cm) or a uniform (range: 8 cm) distribution. As the number of muscle fibres composing the small, postural motor units in the human MG muscle is uncertain, territories were simulated with three densities. Motor unit territories included 200, 300 and 400 fibres (about 400 fibres compose a single, large muscle unit in the cat MG muscle; Burke & Tsairis, 1973). For each motor unit, the ARV amplitude of its simulated surface action potential was computed, and then fitted with a Gaussian curve, as for the experimental signals. The standard deviation of the optimal Gaussian was compared with the longitudinal size of the territories simulated to test for the validity of our method (i.e. to test for a systematic association between the standard deviation of the Gaussian fitting curve and the size of the motor unit territory).
Table 1.
Description of all parameters used to simulate the surface representation of action potentials generated in pennate fibres
| Parameter | Value |
|---|---|
| Pennation angle | 30 deg |
| Skin thickness | 1 mm |
| Subcutaneous tissue thickness | 2 mm |
| Fibre length | Gaussian distribution with 50 mm mean and 2 mm s.d. |
| Location of end-plates | Gaussian distribution with the mean corresponding to fibre centre and 1 mm s.d. |
| Fibre density (surface) | 40 fibre mm−1 |
| Number of motor units | 300 |
| Skin conductivity | 4.3 × 10−4 S m−1 |
| Fat conductivity | 4.0 × 10−2 S m−1 |
| Muscle longitudinal conductivity | 40 × 10−2 S m−1 |
| Muscle axial conductivity | 9.0 × 10−2 S m−1 |
| Conduction velocity (CV) | Gaussian distribution with 4 m s−1 mean and 0.3 m s−1s.d. |
The model we used is an extension of that proposed by Mesin & Farina (2004), to further include the adipose and the skin layers separating the muscle tissue from the recording electrodes.
Statistical analysis
The peak position (μ) and the dispersion (σ) of the ARV distribution of surface action potentials were compared for motor units identified in different MG locations. The parametric one-way ANOVA was applied for comparisons (one factor with three levels; MG60%, MG75% and MG90% locations of intramuscular recordings). Fisher's least significant difference test was used for multiple comparisons. Kruskal–Wallis ANOVA was used to test for the number of motor units detected across recording locations. Values are presented as mean and standard deviation or as median values. The level of statistical significance was set to 5%.
Results
A total of 55 motor units were identified from the three locations in which the pairs of wire electrodes were inserted in the MG muscle of seven subjects. Of these motor units, 11, 25 and 19 were found to be in the MG60%, MG75% and MG90% recording location (Fig. 2A), respectively. The number of detected motor units was significantly greater in the MG75% (median value per subject, 4 motor units) and MG90% (3 motor units) than in the MG60% location (1 motor unit; Kruskal–Wallis ANOVA, P = 0.001, n = 7 subjects). The representation of 46 motor units in the surface EMGs was confined to five consecutive channels, at most. From two subjects it was not possible to detect any motor unit activity throughout the whole standing tasks, although surface and intramuscular potentials were observed for all subjects when they were asked to contract their MG muscle voluntarily. The data from these two subjects were thus not included in the analysis.
Estimated territory reflects the simulated territory of pennate motor units
The method proposed to estimate the size of the motor unit territory was validated with extensive simulations. From the simulations, regardless of whether muscle fibres distributed uniformly or normally within the territory of simulated motor units, the standard deviation of Gaussian fittings was significantly correlated with the size of the motor unit territories (Pearson R > 0.9, P < 0.0001; n = 900 units; Fig. 4). Regression analysis between the standard deviation (σ) of the optimal Gaussians and the territory longitudinal size yielded slopes equal to 0.26 and 0.32, when the simulated muscle fibres were distributed normally and uniformly within the unit territory, respectively. These values for the slope of the linear regression indicate that the motor unit territory corresponds to approximately 3 to 4 times (1/0.26 or 1/0.32 for the two distributions) the standard deviation of the Gaussian fitting.
Figure 4. Surface representation of action potentials in pennate motor units.
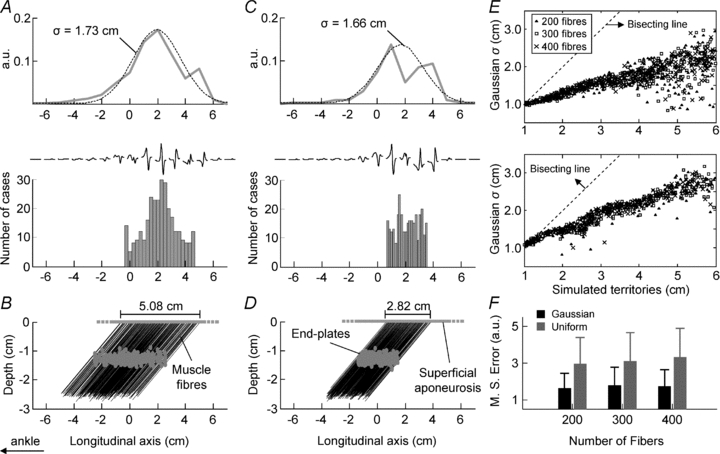
A, the distribution of the ARV amplitude (grey line), its optimal Gaussian curve (dashed line), the histogram created with the position of the superficial aponeuroses of all muscle fibres of a single, simulated motor unit and its surface potentials. Note how closely the amplitude and the location of surface potentials relate to the distribution of muscle fibres. B, the location of end-plates and muscle fibres simulated for this motor unit. The location of the superficial aponeuroses was distributed normally along the simulated territory (5.08 cm). C and D, same as in A and B, for a simulated unit whose muscle fibres were distributed uniformly along its territory (2.82 cm long). E, plots of the standard deviations of Gaussians against the actual, longitudinal size of the territories of simulated motor units, for different territory densities (▴: 200 fibres; □: 300 fibres; ×: 400 fibres; n = 300 motor units). The upper and lower graphs show that the distribution of muscle fibres along the territory of each unit was Gaussian and uniform, respectively. Both distributions of fibres resulted in Gaussian curves significantly correlated with the size of the motor unit territories (Pearson R > 0.9, P < 0.0001). F, shows the mean error values (whiskers: standard deviation; n = 300 units) obtained by fitting the Gaussian curves to the ARV distributions, for motor units with 200, 300 and 400 fibres. When the fibres were distributed normally (black bars) within the unit territory the mean error was significantly smaller than that obtained for fibres distributed uniformly (grey bars) along the territory (ANOVA, P < 0.0001, n = 1800 units).
Localisation of muscle units in the medial gastrocnemius
From the experimental data, visual inspection of raw surface EMGs revealed that action potentials were detected only with a few surface channels and in specific locations on the skin (Figs 3 and 5). Figure 5A shows 20 ms of the 15 single differential surface signals and of the three intramuscular EMGs recorded from one of the subjects tested. The intramuscular action potentials observed in the MG90% (light grey line) and MG75% (dark grey line) locations were associated with large surface potentials recorded with the most distal (from channel 9 to 15) and central (from channel 8 to 9) surface electrodes, respectively. When using the discharge times of motor units to trigger the surface EMGs, the local association of the intramuscular action potentials with the surface potentials was evident (see Figs 3A and 5B).
Considering all participants, Fig. 6 shows that intramuscular potentials have spatially localised representation in the surface EMGs. On the surface of the skin, motor unit action potentials were clearly observed in up to five consecutive surface channels and were centred, significantly, on the locations of intramuscular recordings (see filled circles, open circles and crosses in Fig. 6A). Peaks of ARV amplitude were centred at 4.40 ± 1.67 cm (mean ± s.d.; n = 11 motor units), 8.02 ± 2.16 cm (n = 19 motor units) and 11.63 ± 2.09 cm (n = 25 motor units) longitudinal distance from the position of the most proximal surface channel, when considering the surface potentials triggered with the intramuscular action potentials in the MG60%, MG75% and MG90% locations, respectively (ANOVA, P < 0.001; left panel in Fig. 6B). Surface electrodes provided selective recordings on the central and proximal portions of the MG muscle, with ∼68% of ARV amplitude being distributed, respectively, within ±1.01 cm and ±1.39 cm (mean values; right panel in Fig. 6B).
Figure 6. Localisation and spread of surface action potentials in the MG muscle.
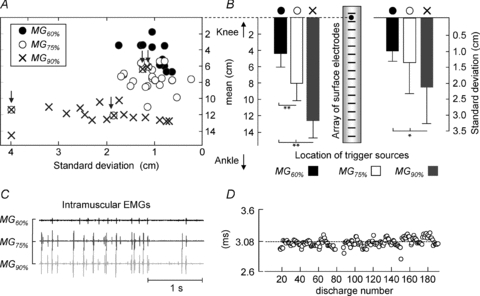
A, the scatter plot of mean versus standard deviation values for the Gaussians optimally fitted to the distribution of surface ARV amplitude (n = 7 subjects), computed for intramuscular potentials in the three recording locations (•: MG60%; ○: MG75%; ×: MG90%). B, averaged values (whiskers indicate standard deviation) of the mean (left panel) and standard deviation (right panel) of the fitted ARV distributions. C, a short epoch (from 45 to 48 s) of raw intramuscular EMGs recorded from subject 3. The largest potentials in the MG75% location appear after the largest potentials in the most distal location, with a small and consistent delay, shown in D. This indicates that wire electrodes in both locations recorded from the same motor unit, resulting in Gaussians with the same mean and standard deviation (see arrows in A). The median delay of 3.08 ms is presumably due to small differences in the location of end-plates between different fibres of the same motor unit. Statistical significance for the multiple comparisons is reported as: *P < 0.05; **P < 0.001. n = 55 motor units.
As an exception to the general finding, in four out of 55 cases (two subjects), the interval between action potentials detected in the MG75% and MG90% locations was small and constant throughout the whole standing test. Figure 6C shows a short epoch (from 45 to 48 s) of raw intramuscular signals recorded from the three locations for subject 3. The largest motor unit action potentials in the MG75% location, for example, appeared 3.08 ms (median value, n = 180 discharges) after the largest potentials in the MG90% location, consistently (Fig. 6D). The Gaussian curves representing the amplitude distribution of surface potentials triggered with the discharge patterns computed for the two locations had the same mean and standard deviation (see the left most arrow in Fig. 6A). Consistency of the timing between intramuscular potentials in the central and distal locations was also observed for the other three cases (Fig. 6A). Therefore, the arrows shown in Fig. 6A indicate, presumably, the four cases for which the wire electrodes in the MG75% and MG90% locations recorded from the same motor unit.
In the distal recording location, intramuscular action potentials produced amplitude distributions significantly more scattered on the surface of the skin with respect to those in the other two locations (ANOVA, P < 0.05; right panel in Fig. 6B; see also the values shown by the crosses in the abscissa of Fig. 6A). This result can be explained by the effect of propagation of action potentials on the detection of surface EMGs.
Propagation of action potentials influences the spatial distribution of surface EMGs
Considering the oblique orientation of the gastrocnemius fibres, the spread of triggered surface potentials is expected to be associated with the size of the territory of MG motor units, as schematically explained in Fig. 1. Conversely, if consecutive surface electrodes detect the same propagating action potentials, the spread of surface triggered potentials would indicate the length of muscle fibres rather than the territory of motor units.
To ascertain whether the propagation of action potentials along MG fibres could account for the larger spread of ARV amplitude in the distal surface electrodes (Fig. 6A and B), the delay between surface triggered potentials in successive channels was estimated for intramuscular action potentials recorded from the three locations (e.g. between averaged potentials in channels 11 and 12 and between averaged potentials in channels 8 and 9 shown in the left and right panels of Fig. 5B, respectively). Delay values were estimated in the frequency domain and thus with resolution not limited to the sampling interval (Farina et al. 2001). In the distal portion of the MG muscle, intramuscular potentials corresponded to consecutive surface potentials with delays similar to those produced by intramuscular potentials in the other MG locations (ANOVA, P = 0.54; Fig. 7).
Figure 7. Delay values estimated between consecutive surface potentials.
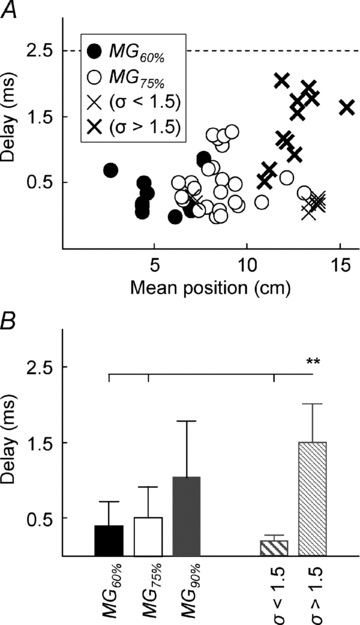
A, plot of the delay between surface potentials recorded from adjacent channels versus the mean value of ARV distribution. Note that the delay estimated for surface potentials with highly dispersed ARV distribution (thicker crosses; σ > 1.5), is close to the delay expected for propagating potentials (2.5 ms is expected for electrodes spaced by 10 mm; propagation velocity of action potentials along muscle fibres range from 3 to 6 m s−1, Andreassen & Arendt-Nielsen, 1987). Therefore, on the most distal MG location, the amplitude of surface potentials reflects the length of muscle fibres rather than the territory of motor units (see Fig. 8 and Discussion). B, the mean values (whiskers correspond to the standard deviation) of the delay data shown in A. The delay values estimated for the MG90% location are higher (P = 0.0002) than those estimated for the other locations.
On average, surface potentials with ARV amplitude widely distributed on the skin (optimal Gaussian with σ > 1.5 cm; Fig. 3) produced significantly higher delay values (1.51 ± 0.49 ms; n = 11 motor units; Kruskal–Wallis ANOVA, P = 0.0002), when compared to those less diffused on the skin (optimal Gaussian with σ < 1.5 cm; 0.2 ± 0.08 ms; n = 8 motor units) and to those in the central (0.52 ± 0.49 ms; n = 25) and proximal (0.40 ± 0.32 ms; n = 11) portions of the surface array (Fig. 7B). Delay estimates were grouped for Gaussians with σ higher and smaller than 1.5 cm because, on average, σ was smaller than 1.5 cm for the Gaussians representing the amplitude distribution of surface potentials in the MG75% and MG60% locations (Fig. 6B).
Discussion
Surface and intramuscular EMGs were recorded from different locations along the human MG to address the question of whether the territories of its motor units extend extensively along the muscle longitudinal axis or occupy a relatively small muscle region. Given the pennation of gastrocnemius fibres, the extent to which motor unit action potentials appear along the MG surface is associated with the size of the territory of individual motor units along the longitudinal axis of the muscle. Action potentials of single motor units appeared locally on the surface of the skin (within ∼2 cm) and centred close to the sites of intramuscular recording. The possibility of independent control of gastrocnemius sub-volumes is the main physiological implication following the evidence of spatially localised muscle units.
Methodology for assessing the territory of individual motor units
The territory of motor units in the human MG muscle was indirectly estimated by spike-triggered averaging of multi-channel surface EMG. It is worth mentioning that the estimation of the territory of single motor units is usually obtained directly with the scanning EMG technique (Stålberg & Antoni, 1980), which has been used for the morphological study of motor units in normal and pathological muscles (Stålberg & Dioszeghy, 1991; Roeleveld et al. 1997a). However, the scanning EMG technique requires all the fibres of one motor unit to be located within a single muscle cross section. At least for the cat calf muscles, the muscle fibres of individual motor units are not confined within single cross sections of the muscle (Burke & Tsairis, 1973; Burke & Edgerton, 1975; English & Weeks, 1984; Bodine et al. 1988; English & Weeks, 1989; Rafuse & Gordon, 1996). To our knowledge, previously available techniques do not suffice for the quantification of motor unit territory in the human gastrocnemius muscles. The method we propose and apply in this study was validated (Fig. 4) and constitutes a new approach for assessing motor unit territories in pennate muscles.
Surface distribution of motor unit action potentials in the pennate gastrocnemius muscle
The surface amplitude of motor unit action potentials in the pennate MG muscle depends, chiefly, on the distribution of MG fibres within the motor unit territory. Our simulations indicate that the surface representation of each single fibre action potential concentrates mainly at the superficial end of the pennate fibres, where the distance between the muscle fibres and the surrounding, surface electrodes is minimal. This observation is consistent with the fact that the amplitude of surface, motor unit action potentials decreases steeply when the absolute distance between the recording electrodes and the action potentials increases (Roeleveld et al. 1997a,b; see Fig. 2 in Roeleveld et al. 1997c). Then, the higher the number of muscle fibres located beneath a single electrode, the higher the amplitude of the surface motor unit potential detected by this electrode. Consequently, the amplitude distribution of surface potentials of a single motor unit reflects the distribution of its muscle fibres across the electrode array (Fig. 4A–D). On this view, the spread of the Gaussian curves fitting the amplitude of the surface potentials of individual motor units provides an indication of the region within which most of their muscle fibres reside (Fig. 4E); this corresponds to a ‘probabilistic’ definition of the motor unit territory. Therefore, analysing the ‘electrical territory’ of MG motor units does not depend on fitting the amplitude distribution of surface potentials (Fig. 4F) but on how dispersed this distribution is.
The mean (μ) and standard deviation (σ) of Gaussian curves fitted to the distribution of surface EMG amplitude are, then, associated with the location and the size of the territory of single motor units, respectively. However, using the values of σ to make exact inferences regarding the size of the territory of MG motor units is not possible. The value of σ depends on how steeply the amplitude of surface potentials decreases from the peak location. The variations in amplitude (i.e. variations in σ) of the surface potentials reflect both the distance between the surface channels and the superficial extremities of muscle fibres (Roeleveld et al. 1997a,b,c;), and the distribution of muscle fibres within the motor unit territory (Fig. 4A–D). Therefore, very rarely the muscle fibres of an individual MG muscle unit would reside outside a region including 95% of the area of the Gaussian curves (i.e. 4σ; Fig. 4E).
Intramuscular recordings from the MG90% location cannot be used to ascertain the size of the territory of single motor units with surface EMG. For the values of ankle angle and plantar flexion torque observed during standing (Loram et al. 2005), the length of muscle fibres in the human MG muscle is ∼55 mm (Narici et al. 1996; Kawakami et al. 1998). As surface electrodes were spaced by 1 cm from each other, about five electrodes were positioned along the same muscle fibres on the most distal MG region (Fig. 8). Therefore, it is reasonable to expect the most distal surface electrodes to record the same action potential propagating along the most distal MG muscle fibres. In fact, only the most distal surface electrodes detected, frequently, the same surface potential with a delay proportional to the interelectrode distance. As a result, the spread of Gaussian curves centred on the MG90% location reflects the propagation of action potentials rather than the territory of motor units.
Figure 8. Propagation of action potentials along distal fibres influences the distribution of surface potentials.
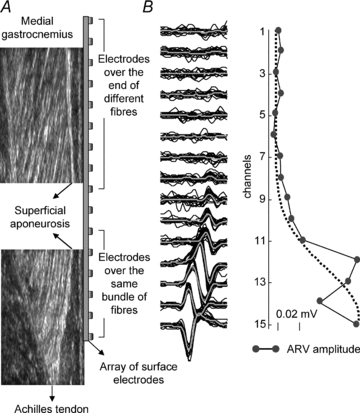
A illustrates how the fascicles of MG muscle were distributed below the array of surface electrodes. Electrodes in the distal portion of the array cover the same MG fibres, allowing the same intramuscular potential to be detected from different locations on the surface of the skin because of the propagation of action potentials. Proximal electrodes are located on the superficial extremity (aponeurosis) of different MG fascicles and are unlikely to have recorded from the same muscle fibres. B, raw surface action potentials (and their average; thick grey lines) triggered with the firing pattern of one motor unit identified in the MG90% location. Note the delay between potentials and the phase inversion for the potentials with similar amplitude in channels 14 and 15. C shows the sparse distribution of ARV amplitude for the averaged potentials shown in B.
How large is the territory of the ‘postural’ motor units with respect to the length of the human MG muscle?
Gaussian curves indicate that the muscle fibres supplied by single motoneurones distribute locally along the human MG muscle. Our results obtained for the motor units in the MG60% and MG75% locations indicate an average value of σ = 1.25 cm (Fig. 6). Considering a pennation angle of about 30 deg during standing (estimated from ultrasound images collected in this study; see also Loram et al. 2005), the distance (perpendicular to muscle fibres) between the most distal and the most proximal fibre of a single muscle unit would be approximately 2.5 cm (= 4σsin(π/6)). Interestingly, for two subjects, the intramuscular electrodes in the two most distal locations recorded from the same motor unit (see arrows in Fig. 6A). As the distance between the two locations was measured before inserting the wire electrodes, the smallest distance between two fibres of the same motor unit, one in each location, could be calculated precisely (1.2 and 1.1 cm for subject 2 and 3, respectively). Therefore, 1.1 and 2.5 cm define an approximate range for the territory of single, ‘postural’ motor units (i.e. motor units identified during standing) along an axis perpendicular to the MG fibres. These values are substantially smaller than the longitudinal dimension of the human MG muscle (∼25 cm; Narici et al. 1996; Loram et al. 2005).
The individual, ‘postural’ (small) muscle units seem to occupy a relatively small area of the human MG muscle. If the physiological cross sectional area of muscle units is circular (Stålberg & Antoni, 1980), the territory of single MG motor units would be expected to range from 3.8 to 19.6 cm2 (according to the range of 1.1–2.5 cm). In this case, with respect to the physiological cross sectional area of the human MG muscle at rest (46.5 cm2; Narici et al. 1996), the relative size of the territory of single motor units would range from 8.2 to 42.1%. However, single MG muscle units seem to occupy an elliptical rather than circular area, with its major axis extending mostly along the longitudinal axis of the muscle (Burke & Tsairis, 1973; Rafuse & Gordon, 1996). Furthermore, considering that the attenuation of recorded potentials is partly accounted for by their distance from the surface electrodes, a value of 42.1% would overestimate fairly the relative size of MG motor unit territory. Therefore, contrary to the cat, the territory of small motor units in the human MG muscle is relatively small with respect to the longitudinal size of the muscle.
How much the estimated size of the territory of motor units might be generalised for all motor units in the MG muscle is not predictable. The gastrocnemius is a pale muscle, with mixed proportions of fast fatiguing, fatiguing resistant and slow units (Wuerker et al. 1965; Burke et al. 1973; Garnett et al. 1979). Given the extremely small fluctuations in the plantar flexion torque resulting from the activation of the calf muscles in standing (∼5 N m; Jacono et al. 2004; Di Giulio et al. 2009), all the ‘postural’ motor units identified in this study were small, i.e. they had low recruitment thresholds. In virtue of the proportionality between the number of muscle fibres composing individual muscle units and the recruitment threshold of motor units (Henneman et al. 1965), one could expect that large motor units might have larger territories than the ‘postural’ motor units in the MG muscle. However, it is not clear whether the territory of motor units depends more on the number of muscle fibres per motor unit or more on how proximally the axonal branching occurs (Rafuse & Gordon, 1996). The current study focused exclusively on the motor units active in quiet standing, which are likely to be low-threshold units. The results positing spatially localised muscle units have implications for the regionalisation of postural activity within the gastrocnemius muscles.
Regional control of sub-volumes of the MG muscle requires two conditions: (i) muscle fibres of individual muscle units are spatially localised (i.e. motor units with small territories), and (ii) the independent recruitment of populations of spatially localised muscle units where independence is demonstrated spatially and/or temporarily. Our results verify the former condition for the human MG muscle during standing (Figs 3–6), as discussed above. Confirmation or rejection of the second condition is not direct from our results. Nevertheless, if postural activity distributed evenly along the MG muscle, then, allowing for statistical variation, the number of detected motor units should be equal across muscle locations. Interestingly, significantly more motor units were detected in the MG75% and MG90% than in the MG60% location. Although this result has to be substantiated by further experiments, the uneven distribution of detected motor units, in addition to the variable timing of activation observed previously (Vieira et al. 2010), supports rather than rejects the hypothesis of regional control of localised, small muscle units in the human MG muscle during standing.
Conclusion
Intramuscular and surface EMGs were recorded concurrently during quiet standing to study the size of the territory of motor units in the human medial gastrocnemius muscle. The results indicate the localised distribution of muscle fibres innervated by single motoneurones along the longitudinal axis of the human gastrocnemius muscle. The localised occupation of muscle units makes it possible for the nervous system to activate sub-volumes of the gastrocnemius muscle, perhaps taking advantage of local variations in muscle architecture.
Acknowledgments
T.M.M.V. and S.M. wish to acknowledge their doctoral scholarships provided by the Brazilian Research Council (CNPq) and the Regione Autonoma della Sardegna, respectively. This study was also supported by the Compagnia di San Paolo and Fondazione Cassa di Risparmio di Torino (R.M.) and by the Danish Technical Research Council (D.F.).
Glossary
Abbreviations
- ARV
average rectified value
- MG
medial gastrocnemius
Author contributions
Conception and design of the experiments: T.M.M.V., S.M., R.M., D.F. Collection, analysis and interpretation of data: T.M.M.V., I.D.L., S.M., R.M., D.F. Drafting the article or revising it critically for important intellectual content: T.M.M.V., I.D.L., R.M., D.F. All authors approved the version to be published. Experiments were conducted at the Center for Sensory-Motor Interaction, Aalborg University, Denmark.
References
- Andreassen S, Arendt-Nielsen L. Muscle fibre conduction velocity in motor units of the human anterior tibial muscle: a new size principle parameter. J Physiol. 1987;391:561–571. doi: 10.1113/jphysiol.1987.sp016756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine SC, Garfinkel A, Roy RR, Edgerton VR. Spatial distribution of motor unit fibers in the cat soleus and tibialis anterior muscles: local interactions. J Neurosci. 1988;8:2142–2152. doi: 10.1523/JNEUROSCI.08-06-02142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Edgerton VR. Motor units properties and selective involvement in movement. Exerc Sport Sci Rev. 1975;3:31–81. [PubMed] [Google Scholar]
- Burke RE, Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973;234:749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Firing patterns of gastrocnemius motor units in the decerebrate cat. J Physiol. 1968;196:631–654. doi: 10.1113/jphysiol.1968.sp008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio I, Maganaris CN, Baltzopoulos V, Loram ID. The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J Physiol. 2009;587:2399–2416. doi: 10.1113/jphysiol.2009.168690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Sherrington CS. Number and contraction-values of individual motor-units examined in some muscles of the limb. Proc R Soc Lond. 1930;106:326–357. [Google Scholar]
- English AW, Weeks OI. Compartmentalization of single muscle units in cat lateral gastrocnemius. Exp Brain Res. 1984;56:361–368. doi: 10.1007/BF00236292. [DOI] [PubMed] [Google Scholar]
- English AW, Weeks OI. Electromyographic cross-talk within a compartmentalized muscle of the cat. J Physiol. 1989;416:327–336. doi: 10.1113/jphysiol.1989.sp017763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Arendt-Nielsen L, Merletti R, Graven-Nielsen T. Assessment of single motor unit conduction velocity during sustained contractions of the tibialis anterior muscle with advanced spike triggered averaging. J Neurosci Methods. 2002;115:1–12. doi: 10.1016/s0165-0270(01)00510-6. [DOI] [PubMed] [Google Scholar]
- Farina D, Muhammad W, Fortunato E, Meste O, Merletti R, Rix H. Estimation of single motor unit conduction velocity from surface electromyogram signals detected with linear electrode arrays. Med Biol Eng Comput. 2001;39:225–236. doi: 10.1007/BF02344807. [DOI] [PubMed] [Google Scholar]
- Garnett RA, O’Donovan MJ, Stephens JA, Taylor A. Motor unit organization of human medial gastrocnemius. J Physiol. 1979;287:33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Jacono M, Casadio M, Morasso PG, Sanguineti V. The sway-density curve and the underlying postural stabilization process. Motor Control. 2004;8:292–311. doi: 10.1123/mcj.8.3.292. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol. 1998;85:398–404. doi: 10.1152/jappl.1998.85.2.398. [DOI] [PubMed] [Google Scholar]
- Kinugasa R, Kawakami Y, Fukunaga T. Muscle activation and its distribution within human triceps surae muscles. J Appl Physiol. 2005;99:1149–1156. doi: 10.1152/japplphysiol.01160.2004. [DOI] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Active, non-spring-like muscle movements in human postural sway: how might paradoxical changes in muscle length be produced? J Physiol. 2005;564:281–293. doi: 10.1113/jphysiol.2004.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods. 2005;149:121–133. doi: 10.1016/j.jneumeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Mesin L, Farina D. Simulation of surface EMG signals generated by muscle tissues with inhomogeneity due to fiber pinnation. IEEE Trans Biomed Eng. 2004;51:1521–9. doi: 10.1109/tbme.2004.827551. [DOI] [PubMed] [Google Scholar]
- Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol. 1996;496:287–97. doi: 10.1113/jphysiol.1996.sp021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. II. Analysis of the mechanisms and significance of fiber type grouping in reinnervated muscles. J Neurophysiol. 1996;75:282–297. doi: 10.1152/jn.1996.75.1.282. [DOI] [PubMed] [Google Scholar]
- Roeleveld K, Stegeman DF, Falck B, Stålberg EV. Motor unit size estimation: confrontation of surface EMG with macro EMG. Electroencephalogr Clin Neurophysiol. 1997a;105:181–188. doi: 10.1016/s0924-980x(97)96670-4. [DOI] [PubMed] [Google Scholar]
- Roeleveld K, Stegeman DF, Vingerhoets HM, Van Oosterom A. Motor unit potential contribution to surface electromyography. Acta Physiol Scand. 1997b;160:175–183. doi: 10.1046/j.1365-201X.1997.00152.x. [DOI] [PubMed] [Google Scholar]
- Roeleveld K, Stegeman DF, Vingerhoets HM, Van Oosterom A. The motor unit potential distribution over the skin surface and its use in estimating the motor unit location. Acta Physiol Scand. 1997c;161:465–472. doi: 10.1046/j.1365-201X.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- Segal RL, Song AW. Nonuniform activity of human calf muscles during an exercise task. Arch Phys Med Rehabil. 2005;86:2013–2017. doi: 10.1016/j.apmr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Staudenmann D, Kingma I, Daffertshofer A, Stegeman DF, van Dieën JH. Heterogeneity of muscle activation in relation to force direction: A multi-channel surface electromyography study on the triceps surae muscle. J Electromyogr Kinesiol. 2009;19:882–895. doi: 10.1016/j.jelekin.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Stålberg E, Antoni L. Electrophysiological cross section of the motor unit. J Neurol Neurosurg Psychiatry. 1980;43:469–474. doi: 10.1136/jnnp.43.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålberg E, Dioszeghy P. Scanning EMG in normal muscle and in neuromuscular disorders. Electroencephalogr Clin Neurophysiol. 1991;81:403–416. doi: 10.1016/0013-4694(91)90001-k. [DOI] [PubMed] [Google Scholar]
- Stålberg E. Macro EMG, a new recording technique. J Neurol Neurosurg Psychiatry. 1980;43:475–482. doi: 10.1136/jnnp.43.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira TMM, Windhorst U, Merletti R. Is the stabilization of quiet upright stance in humans driven by synchronized modulations of the activity of medial and lateral gastrocnemius muscles? J Appl Physiol. 2010;108:85–97. doi: 10.1152/japplphysiol.00070.2009. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Ammerman J, Jann B. Organization of responses in human lateral gastrocnemius muscle to specified body perturbations. J Electromyogr Kinesiol. 1998;8:11–21. doi: 10.1016/s1050-6411(97)00001-1. [DOI] [PubMed] [Google Scholar]
- Wuerker RB, McPhedran AM, Henneman E. Properties of motor units in a heterogeneous pale muscle (m. gastrocnemius) of the cat. J Neurophysiol. 1965;28:85–99. doi: 10.1152/jn.1965.28.1.85. [DOI] [PubMed] [Google Scholar]


