Abstract
α-Synuclein (α-syn) is an abundant neuronal protein expressed at the synapse. In neurodegenerative disease α-syn accumulates in the extracellular space. Astrocytes present at neural synapses are thought to contribute to synaptogenesis through cholesterol release and normally exhibit increased glial fibrillary acidid protein (GFAP) reactivity and apolipoprotein E (apoE) expression in neurodegenerative disease states. We proposed that extracellular α-syn treatment of human astrocytes would impact cholesterol levels and expression of GFAP and apolipoprotein E (apoE). Human astrocytes were treated with α-syn at different concentrations and time points to determine the effective membrane permeability of the peptide. After α-syn treatment, we analyzed apoE and cholesterol levels in the astrocyte membrane. Lastly, we performed immunoctyochemistry for GFAP in control and α-syn treated cells. Our results indicate membrane apoE was reduced and redistributed from a nuclear and membranous dominated expression to the cytosol. Cholesterol levels were also reduced in the astrocyte cell membrane. GFAP expression was sharply increased in α-syn treated cells indicating that α-syn may contribute to reactive gliosis. Our results support the conclusion that astrocytes play a role in pathological mechanisms in synucleinopathies.
Keywords: Synaptogenesis, Glia, Parkinson’s Disease, Dementia with Lewy Bodies, Alzheimer’s Disease, Lipoproteins
Article Outline
α-Synuclein (α-syn) is an abundant protein expressed in neurons. The location of this protein is in the synapse [15]. The function of α-syn is largely unknown, but the accumulation of α-syn in Parkinson’s Disease and Dementia with Lewy Bodies is seen in post-mortem brain analysis and is characteristic of the disease pathology. [31, 34].
Some evidence suggests that α-syn itself may be responsible for the destruction of neurons in Parkinson’s Disease and Dementia with Lewy Bodies [5, 16, 18]. While other evidence disputes this notion, lending credence to the idea that cell stress causes cell death [1, 14], which leads to aggregation of α-syn.
It is also known α-syn is normally released by neurons into the extracellular space at the synapse [3]. Astrocytes surround synapses and increased glial fibrillary acid protein (GFAP) expression in astrocytes, a marker for reactive gliosis, occurs after brain injury and in neurodegenerative disease [4, 17, 19, 25].
Recent evidence points to cholesterol lowering agents having the ability to diminish α-syn in cell culture and cell free assays [1, 2]. Cholesterol release from astrocytes can cause synaptogenesis in neurons [8, 23, 24, 27, 28]. Cholesterol is secreted from astrocytes in lipoproteins, a main component which is apolipoprotein E (apoE) [10], a protein exclusively synthesized in astrocytes in the human nervous system [29, 30]. The e4 allele of apoE is the most definitive genetic risk factor for late onset Alzheimer’s Disease and causes Abeta peptide accumulation [32]. Deficient apoE can increase neurodegeneration in aging [22]. α-Syn overexpression in transgenic mice was shown to cause large increases in apoE and insoluble Abeta deposits supporting the idea of interrelation between Parkinson’s Disease and Alzheimer’s Disease mechanisms and pathology [7].
Since α-syn is normally expressed in the synapse [21], is membrane permeable [6], and can be released from neurons [3], we were interested in understanding how α-syn in the extracellular space might influence human astrocytes. In this study, we analyzed apoE and cholesterol levels as well as apoE and GFAP immunocytochemistry in human astrocytes after α-syn treatment.
Human primary astrocytes (Sciencell, Carlsbad, CA) were cultured in Astrocyte Medium (Sciencell, #1801), 1% penicillin/streptomycin solution (Sciencell, #0503), 2% Fetal Bovine Serum (Sciencell), and 1% astrocytes growth supplement (Sciencell #1852). When cells were about 80% confluent, they were treated with human recombinant α-syn (Calbiochem, San Diego, CA) in identical but serum free media. For cholesterol levels assay, cells were placed in identical medium but with delipidized Fetal Bovine Serum (Valley Biomedical, Winchester, VA) 24 hours prior to serum free treatment with α-syn. These cells were chosen for their known ability to express glial fibrillary acidic protein (GFAP).
Immunoblot analysis was performed with cells which were fractionated by centrifugation into soluble (cytosolic) and insoluble (membrane) fractions as previously described with slight changes [12, 26]. Briefly, cells were sonicated in PDGF buffer (HEPES 1.0 mM, Benzamidine 5 mM, 2-mercaptoethanol 2 mM, EDTA 3 mM, Magnesium sulfate 0.5, Sodium Azide 0.05%) containing 1X protease and phosphatase inhibitors (Sigma-Aldrich) and centrifuged for 10min at 5000×g. The resulting supernatent was ultracentrifuged (274,000×g, 1 hrs, 4°C), and the detergent soluble proteins were collected in the supernatant fraction. The pelleted detergent insoluble proteins were dissolved in PDGF buffer containing 1X protease and phosphotase inhibitors. Total protein concentration of each sample was determined using BCA protein assay reagents (Pierce, Rockford, IL).
Samples from detergent-soluble and -insoluble fractions were separated on 4–12% SDS-PAGE gels (NuPAGE, Invitrogen) and transferred onto 0.22 µM PVDF membranes (Schleicher & Schunell, Keene, NH) using 1X 3-[Cyclohexylamino]-1-propaneosulfonic acid (CAPS) transfer buffer containing 20% methanol. Membranes were blocked with 3% milk in PBS containing 0.1% Tween-20 (Sigma-Aldrich) (PBS-T), followed by incubation in primary antibody (1:1000) in PBS-T and 3% Bovine Serum Albumin (BSA) overnight at 4 °C. The primary antibodies used were as follows: anti-actin and anti-α-syn from Chemicon International (Temecula, CA); anti-apoE (Q-16) (Santa Cruz Biosciences, Santa Cruz, CA). Membranes were further incubated with goat anti-mouse or anti-rabbit IgG secondary antibodies conjugated to horseradish peroxidase (1:5000, American Qualex, San Clemente, CA) and visualized by enhanced chemiluminescence (ECL, NEN Life Sciences, Boston, MA) and exposed to film. For determinations of levels of immunoreactivity, ECL treated membranes were analyzed in the VersaDoc imaging system (Bio-Rad, Hercules, CA) using the Quantity One software (Bio-Rad).
Quantification of membrane cholesterol was performed using the Cholesterol/Cholesterol Ester Quantification Kit (BioVision, Mountain View, CA) colorimetric method. 15 µg of protein for each membrane cell lysate sample was assayed in triplicate for cholesterol only and not cholesterol esterase.
Immunocytochemistry was performed as follows: cells were seeded onto poly-l-lysine-coated glass coverslips, grown to 80% confluence, fixed in 4% paraformaldehyde (30 min) at 4°C and placed in PBS. Coverslips were first washed 3 times for 5 minutes each in PBS. They were then pretreated with 1% triton-X, 3% hydrogen peroxide in PBS for 15 minutes, washed again 3 times for 5 minutes in PBS and blocked in 10% serum matching the animal the secondary antibody was raised in for 1 hour and then washed again 3 times for 5 minutes each in PBS. Coverslips were then placed in primary antibody: mouse anti-GFAP and (Chemicon, Temecula, CA (1:500)) and goat anti-apoE (Q-16) (Santa Cruz) overnight in 4°C. Coverslips were washed 3 times for 5 min in PBS and then placed in biotynilated secondary antibody (1:100) (Vector Laboratories, Burlingame, CA) for 2 hours. After washing again 3 times in PBS, sections were placed in 20% diaminobenzene (DAB) (Vector Laboratories) for 20 seconds. The reaction with DAB was halted by immersing the sections in double distilled water. Coverslips were then mounted, dried and coverslipped with entillin (Fisher).
All values in the figures are expressed as the means ± SEM. To determine the statistical significance, the values were compared by two group t-tests using the Statview II statistical package for the Macintosh computer. The differences were considered to be significant if p values were less than 0.05.
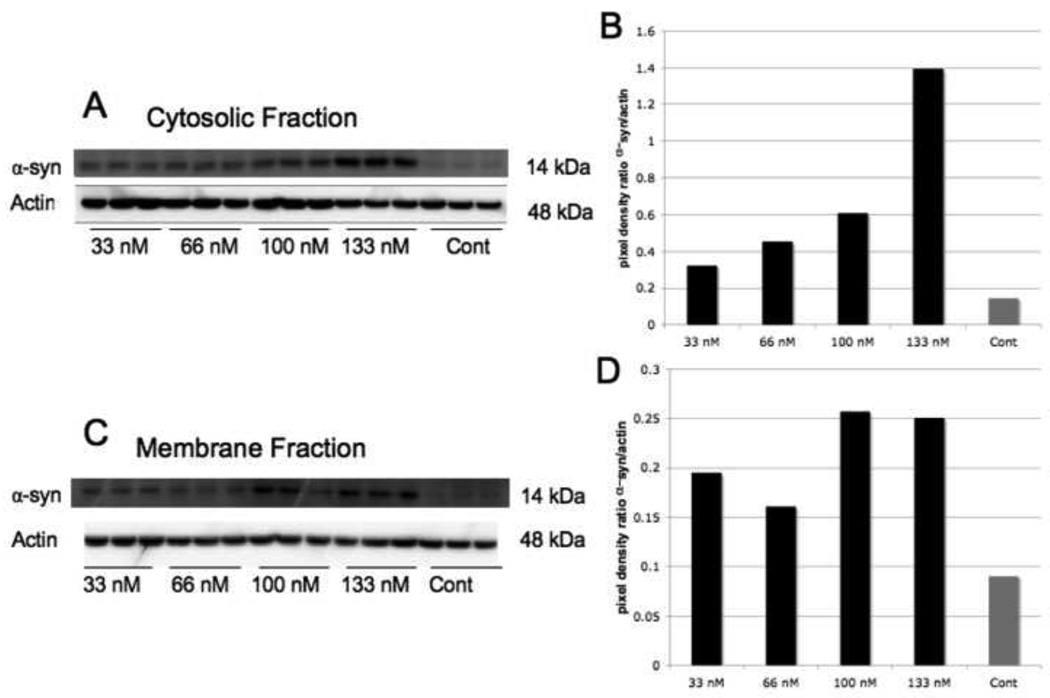
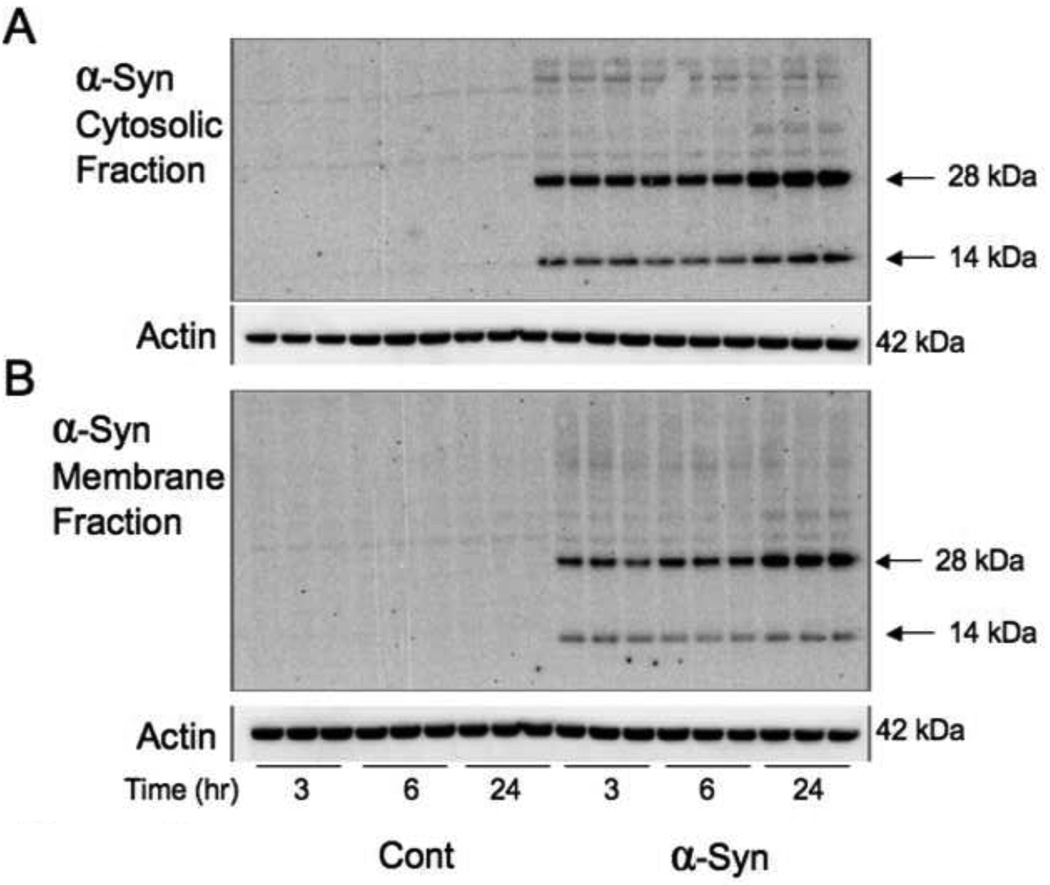
To determine the best dose response for α-syn we added 33 nM, 66 nM, 100 nM and 133 nM of recombinant α-syn to the media for 24 hours. Western blot analysis showed that the threshold level for α-syn to irrupt into the cytosol (Figures 1A, 1B) was at 133nM and 100 nM in the membrane (Figures 1C, 1D). Using 100 nM, we attempted to determine whether this dosage would be effective at earlier time points. However, when looking at dimers and monomers of α-syn, it was able to penetrate astrocytes most effectively after 24 hours in both the cytosol and membrane fractions (Figure 2).
Figure 1. Dose Response Levels of α-Synuclein in Human Astrocytes Treated with α-Synuclein Peptide.
A) An immunoblot of the cytosolic fraction of human astrocytes for α-syn after cells were treated with the α-syn peptide in the media for 24 hours. B) Pixel density levels of α-syn in A when comparing to actin as a loading control. Pixel density levels revealed α-syn had the highest concentration in the cytosol when 133 nM of peptide was added to the media. C) An immunoblot of α-syn in the membrane fraction of human astrocytes after treatment with the α-syn peptide in the media for 24 hours. When comparing pixel density to the actin loading control in D, 100 nM was effective for α-syn to enter the cell membrane.
Figure 2. Time Course Levels of α-Synuclein in Human Astrocytes Treated with α-Synuclein Peptide.
Immunoblots of human astrocytes treated with 100 nM α-syn for 3, 6 and 24 hours. In A and B, two molecular weight levels are seen, the band at 14 corresponds to the protein monomer and at 28 to the protein dimer. In A, the immunoblot of the cytosolic fraction indicates that the α-syn monomer and dimer increased inside the astrocytes at 24 hours. In B, the monomer and dimer were also elevated at 24 hours when compared to the 3 and 6 hour treatment time points.
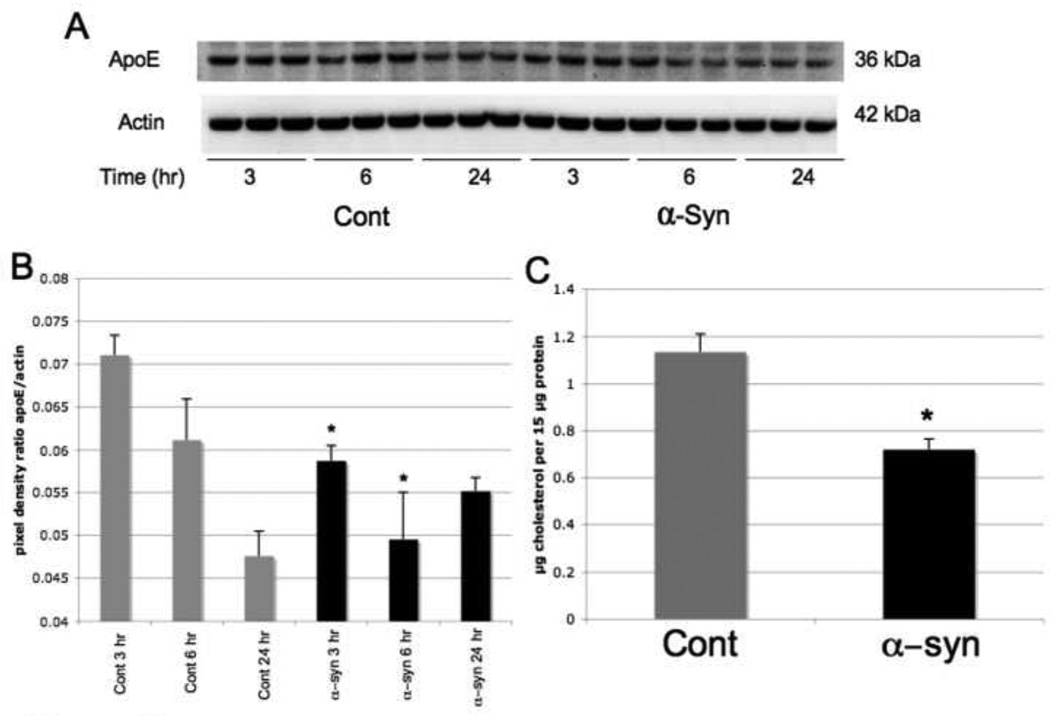
A noticeable decrease in ApoE levels was discerned in the membrane fraction after α-syn treatment (Figure 3A, 3B). The levels were diminished by 19% at 3 hours and 21% at 6 hours after treatment. However, levels of apoE as a ratio of actin equilibrated back towards normal at 24 hours. To determine whether effects of apoE levels and distribution coincided with a change in cholesterol, we analyzed cholesterol levels in the membrane fraction of astrocytes after α-syn treatement. Cholesterol levels were reduced by 35% when cells were treated with α-syn (100 nM for 24 hrs) (Figure 3C).
Figure 3. ApoE and Cholesterol Expression Levels in the Membrane Fraction of Human Astrocytes Treated with α-Synuclein.
A) Membrane fraction of human astrocytes treated with α-syn (100 nM) analyzed by immunoblot for apoE at 3, 6 and 24 hours treatment. When the average blot pixel density for each time point was compared to actin in B, apoE was considerably reduced when cells were treated with α-syn at 3 and 6 hours (p < .05) but returned back to normal and was slightly increased when compared to controls at 24 hours. In C, the cholesterol level in the membrane fraction of cells treated with 100 nM α-syn for 24 hours was significantly decreased (p < .05).
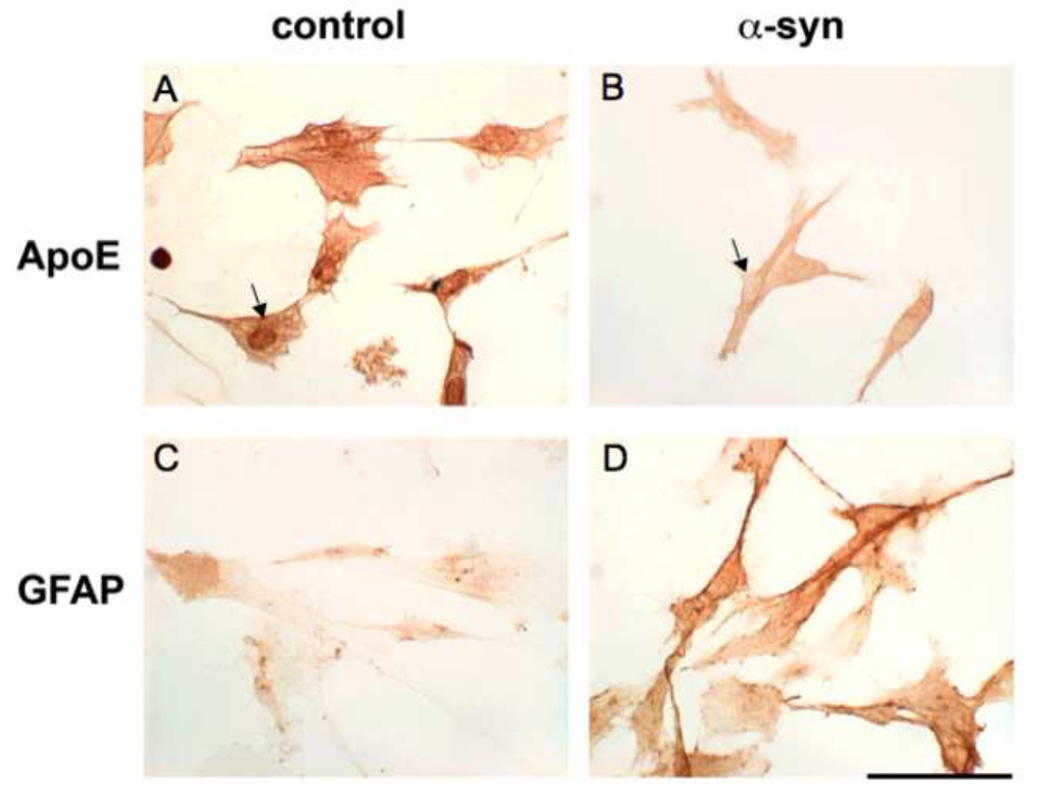
In control cells, apoE was expressed more in nuclear adjacent membranous compartments (Figure 4A). But when treated with α-syn at the particular concentration and time (100 nM for 24 hours), apoE staining diminished and migrated to the cytosol (Figure 4B). GFAP immunocytochemistry was present in control cells (Figure 4C). But when cells were treated with α-syn, GFAP staining increased dramatically (Figure 4D) indicating astrocytic reactivity to α-syn treatment (100 nM for 24 hrs).
Figure 4. ApoE and GFAP Expression in Human Astrocytes after α-Synuclein Treatment.
A and B reveal immunocytochemistry of apoE in human astrocytes after 24 hours treatment with 100 nM α-syn. ApoE was diminished in α-syn treated cells in B when compared with controls in A. Additionally, apoE redistribution was visualized in α-syn treated cells in B. In control astrocytes, apoE demonstrated nuclear dominant expression, while the cytoplasm had higher expression than the nucleus in α-syn treated astrocytes (arrows). Additionally, GFAP expression in human astrocytes treated with α-syn at 100 nM for 24 hours exhibited characteristics of reactive gliosis as evidenced by the large increase of expression in D when compared with control cells in C. (scale bar – 20 µm).
In the experiments presented here we provide initial evidence for the effects of extracellular α-syn on human astrocytes. α-Syn accumulates in the extracellular space in brains of patients with Parkinson’s Disease and Dementia with Lewy Bodies [34]. When astrocytes were treated with α-syn, we visualized apoE redistribution, membrane cholesterol reduction and GFAP reactivity.
Increased GFAP reactivity is the hallmark of reactive gliosis, an effect shown to be increased in Parkinson’s Disease [19, 25]. Reactive gliosis also occurs in astrocytes in other neurodegenerative diseases, within an hour after injury to neural tissue and after stroke [4, 17]. Here we also show for the first time that α-syn can directly cause GFAP reactivity in human astrocytes in vitro. These results indicate α-syn may contribute to gliosis as seen in injury and neurodegenerative disease.
Our results also show a reduction and redistribution of apoE and a reduction of cholesterol in the astrocyte cell membrane after treatment with α-syn. ApoE synthesis is known to increase in astrocytes after injury [13, 20] and was also shown to be secreted from astrocytes and redistributed to neurons in Parkinson’s Disease and Alzheimer’s Disease [9]. Previous studies have shown that deficient apoE is implicated in neurodegenerative disease [7, 11, 22]. It is possible that the redistribution and reduction seen in the immunocytochemical studies here could be astrocyte secretion of apoE instigated by extracellular α-syn.
We also show reduction in astrocyte membrane cholesterol levels. Cholesterol release from astrocytes is believed to increase synaptogenesis [8, 23, 24, 27, 28] and synapse loss is a known byproduct of neurodegenerative disease [33]. The fact that these results demonstrate α-syn treatment of astrocytes causes disruptions in apoE and cholesterol levels suggests a possible role for α-syn in astrocyte function and supports the notion that astrocytes are involved in neurodegenerative disease.
Future studies on the mechanisms involved in the role of astrocytes in neurodegenerative disease will help understand these processes.
Acknowledgments
The authors would like to acknowledge the Stein Institute for Research on Aging, Don and Marilyn Short Fellowship for Research in Parkinson’s Disease at the University of California, San Diego (to AOK) and the National Institutes of Health: NIH grants AG AG10435, AG022074, AG18440, and AG5131 (to EM).
The authors would like to acknowledge Xavier Vagus for help with manuscript preparation and the Stein Institute for Research on Aging, Don and Marilyn Short Fellowship for Research in Parkinson’s Disease at the University of California, San Diego for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bar-On P, Crews L, Koob AO, et al. Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson's disease. J Neurochem. 2008;105:1656–1667. doi: 10.1111/j.1471-4159.2008.05254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-On P, Rockenstein E, Adame A, et al. Effects of the cholesterol-lowering compound methyl-beta-cyclodextrin in models of alpha-synucleinopathy. J Neurochem. 2006;98:1032–1045. doi: 10.1111/j.1471-4159.2006.04017.x. [DOI] [PubMed] [Google Scholar]
- 3.Borghi R, Marchese R, Negro A, et al. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- 4.Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 5.Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, et al. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J Neurochem. 2006;97:1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- 7.Gallardo G, Schluter OM, Sudhof TC, et al. A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci. 2008;11:301–308. doi: 10.1038/nn2058. [DOI] [PubMed] [Google Scholar]
- 8.Goritz C, Mauch DH, Nagler K, et al. Role of glia-derived cholesterol in synaptogenesis: new revelations in the synapse-glia affair. J Physiol Paris. 2002;96:257–263. doi: 10.1016/s0928-4257(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 9.Han SH, Hulette C, Saunders AM, et al. Apolipoprotein E is present in hippocampal neurons without neurofibrillary tangles in Alzheimer's disease and in age-matched controls. Exp Neurol. 1994;128:13–26. doi: 10.1006/exnr.1994.1108. [DOI] [PubMed] [Google Scholar]
- 10.Han X. The role of apolipoprotein E in lipid metabolism in the central nervous system. Cell Mol Life Sci. 2004;61:1896–1906. doi: 10.1007/s00018-004-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi H, Campenot RB, Vance DE, et al. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho GJ, Hashimoto M, Adame A, et al. Altered p59Fyn kinase expression accompanies disease progression in Alzheimer's disease: implications for its functional role. Neurobiol Aging. 2005;26:625–635. doi: 10.1016/j.neurobiolaging.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Ignatius MJ, Gebicke-Harter PJ, Skene JH, et al. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci U S A. 1986;83:1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ischiropoulos H. Oxidative modifications of alpha-synuclein. Ann N Y Acad Sci. 2003;991:93–100. doi: 10.1111/j.1749-6632.2003.tb07466.x. [DOI] [PubMed] [Google Scholar]
- 15.Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 16.Kirik D, Bjorklund A. Modeling CNS neurodegeneration by overexpression of disease-causing proteins using viral vectors. Trends Neurosci. 2003;26:386–392. doi: 10.1016/S0166-2236(03)00164-4. [DOI] [PubMed] [Google Scholar]
- 17.Latov N, Nilaver G, Zimmerman EA, et al. Fibrillary astrocytes proliferate in response to brain injury: a study combining immunoperoxidase technique for glial fibrillary acidic protein and radioautography of tritiated thymidine. Dev Biol. 1979;72:381–384. doi: 10.1016/0012-1606(79)90127-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee MK, Stirling W, Xu Y, et al. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis PD. Parkinsonism--neuropathology. Br Med J. 1971;3:690–692. doi: 10.1136/bmj.3.5776.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 21.Maroteaux L, Campanelliand JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masliah E, Mallory M, Ge N, et al. Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol. 1995;136:107–122. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- 23.Mauch DH, Nagler K, Schumacher S, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 24.Mutka AL, Lusa S, Linder MD, et al. Secretion of sterols and the NPC2 protein from primary astrocytes. J Biol Chem. 2004;279:48654–48662. doi: 10.1074/jbc.M405345200. [DOI] [PubMed] [Google Scholar]
- 25.Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol. 1991;50:743–755. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Petrucelli L, Dickson D, Kehoe K, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 27.Pfrieger FW. Role of cholesterol in synapse formation and function. Biochim Biophys Acta. 2003;1610:271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 28.Pfrieger FW. Roles of glial cells in synapse development. Cell Mol Life Sci. 2009;66:2037–2047. doi: 10.1007/s00018-009-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirier J, Davignon J, Bouthillier D, et al. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 30.Poirier J, Hess M, May PC, et al. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res. 1991;11:97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- 31.Spillantini MG, Schmidt ML, Lee VM, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 32.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi K, Matsumoto K, Takayama K, et al. NACP, a presynaptic protein, immunoreactivity in Lewy bodies in Parkinson's disease. Neurosci Lett. 1997;239:45–48. doi: 10.1016/s0304-3940(97)00891-4. [DOI] [PubMed] [Google Scholar]