Abstract
Importance of the field
Nuclear factor kappa B (NF-κB) is activated by a variety of cancer-promoting agents. The reciprocal activation between NF-κB and inflammatory cytokines makes NF-κB important for inflammation-associated cancer development. Both the constitutive and anticancer therapeutic-induced NF-κB activation blunts the anticancer activities of the therapy. Elucidating the roles of NF-κB in cancer facilitates developing approaches for cancer prevention and therapy.
Areas covered in this review
By searching PubMed, we summarize the progress of studies on NF-κB in carcinogenesis and cancer cells' drug resistance in recent 10 years.
What the reader will gain
The mechanisms by which NF-κB activation pathways are activated; the roles and mechanisms of NF-κB in cell survival and proliferation, and in carcinogenesis and cancer cells' response to therapy; recent development of NF-κB-modulating means and their application in cancer prevention and therapy.
Take home message
NF-κB is involved in cancer development, modulating NF-κB activation pathways has important implications in cancer prevention and therapy. Due to the complexity of NF-κB roles in different cancers, careful evaluation of NF-κB's in each cancer type is crucial in this regard. More cancer cell-specific NF-κB inhibiting means are desired for improving anticancer efficacy and reducing systemic toxicity.
Keywords: apoptosis, cancer, carcinogenesis, chemoresistance, NF-κB, prevention, proliferation, therapy
1. Introduction
Cancer cells acquire a number of characteristic alterations during the course of transformation, including the capacity to proliferate autonomously, to invade surrounding tissues, and to metastasize to distant sites. In addition, cancer cells elicit an angiogenic response, evade mechanisms such as apoptosis that limit cell proliferation, and elude immune surveillance [1]. These properties are initiated in part through alterations in the cell signaling pathways that in normal cells control cell proliferation, motility, and survival. The pathways controlling survival and cell proliferation include MAPK, PI3K-Akt, and NF-κB [1]. In this review, we focus on the involvement NF-κB in cancer development and the potential of targeting NF-κB for cancer prevention and therapy.
2. NF-κB family of proteins
NF-κB is a transcription factor that consists of heterodimers or homodimers formed by the members of the NF-κB family. In mammalian cells there are five NF-κB family members: p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1), and p52 (NF-κB2). The NF-κB family of proteins are characterized by their unique structure, an N-terminal Rel homology domain (RHD) that is responsible for forming dimers, binding DNA, and associating with inhibitor of NF-κB(IκB). The p65 (RelA), RelB and c-Rel proteins harbor a C-terminal transactivation domain (TAD) that interacts with the transcription machinery that promotes gene transcription. Lacking a TAD, the homodimers of p50 or p52 serve as transcription repressors that provide a threshold for NF-κB activation [2]. A nuclear localization signal (NLS) sequence that is required for translocation of NF-κB to the nucleus is located in the middle of the NF-κB family proteins. In most quiescent normal cells the NF-κB dimers are squelched in the cytoplasm by associating IκB proteins that mask the NLS in the NF-κB proteins. There are seven members of the IκB protein family: IκBα, IκBβ, IκBγ, IκBε, BCL-3, and the precursor proteins p105 and p100, which inhibit NF-κB by squelching it in the cytoplasm [2].
3. NF-κB activation pathways
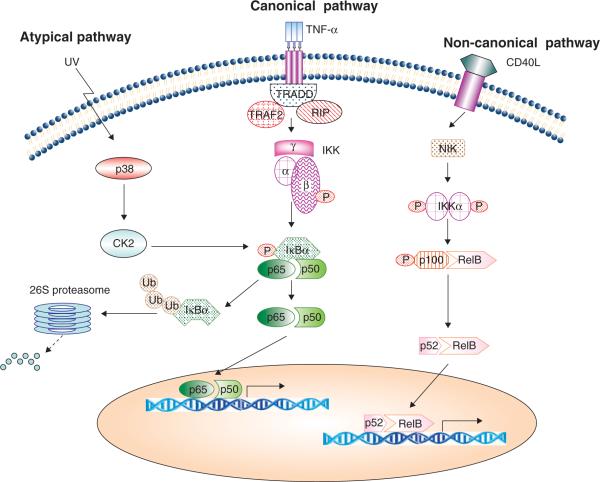
Two main NF-κB activation pathways, namely the canonical (classic) and non-canonical (non-classic), mediate NF-κB activation (Figure 1). The canonical pathway is the major pathway in most cell types and it involves p65, c-Rel and p50. This pathway consists of IKK (an IκB kinase heterodimer consisting of IKKα/IKK1, IKKβ/IKK2, and IKKγ/NEMO, with IKKβ as the catalytic subunit), IκB, and NF-κB (typically a p65/p50 heterodimer). It is often activated by proinflammatory cytokines such as IL-1β and TNF-α as well as a variety of cellular stresses [2]. The NF-κB activation pathway induced by TNF-α is the most intensively studied one, which represents a typical canonical NF-κB activation pathway. This pathway is turned on by the binding of TNF-α to TNF-α receptor (TNFR), which recruits IKK to the TNFR1 signaling complex through TRAF2 and receptor-interacting protein kinase 1 (RIP1). The K63 ubiquitination chain is added to RIP by E3 ubiquitin ligases cIAP-1 and cIAP-2 and serves as a platform for the landing of IKK. IKK is then activated through a RIP-mediated phosphorylation that involves MAPK kinase kinase 3 (MEKK3) or TGF-β-activated kinase 1 (TAK1) [3–5]. The activated catalytic subunit IKKb phosphorylates the serine residues at positions 32 and 36 in IκB, triggering polyubiquitination on IκB and rapid degradation in the proteasome. This process exposes the NLS signal on p65 and p50, ensuring nuclear translocation of NF-κB to promote gene transcription. The NF-κB transcription activity is further regulated by phosphorylation and acetylation on the p65 subunit, thereby affecting its binding to DNA or interaction with transcriptional co-activators such as CBP/p300 [2]. DNA damage induced by adriamycin, camptothecin, etoposide or ionic radiation that induces NF-κB also uses the canonical pathway. Through the DNA damage sensor kinase ataxia telangiectasia mutated (ATM), the IKK subunit NEMO/IKKγ is phosphorylated and recruited to form a complex called the PIDDsome, consisting of RIP1, p53-induced death domain (PIDD), and NEMO in the nucleus, where RIP1 triggers NEMO activation [6–8]. During this process NEMO is phosphorylated by ATM and migrates from the nucleus to the cytoplasm where it binds IKKβ. The IKKβ subunit is then activated to trigger IκB degradation, turning on the canonical NF-κB activation pathway [6,9].
Figure 1. The NF-κB activation pathways.
TNF-α is shown as a representative inducer of the canonical pathway. In this pathway, TNF receptor 1 recruits and activates IKK to form a signaling complex through TNF receptor associated death domain (TRADD), RIP, and TRAF2. IKK then phosphorylates IκB, which leads to IκB ubiquitination and degradation in the proteasome. Subsequently, the NF-κB complex (p65/p50) migrates to the nucleus and activates gene transcription. In the non-canonical pathway (represented by CD40L), NIK-mediated IKKα activation triggers the processing of p100 to create p52. Then the NF-κB complex (p52/RelB) moves to the nucleus to activate gene transcription. In the atypical pathway such as that activated by UV, IKK-independent mechanisms are involved in IκB phosphorylation.
The non-canonical pathway is activated by non-death receptor members of the TNF receptor family such as CD40, lymphotoxin beta (LTβ), and B-cell-activating factor (BAF) and some viral proteins such as LMP-1 from Epstein-Barr virus (EBV). This pathway is dependent on NF-κB-inducing kinase (NIK)-mediated activation of IKKα, which triggers cleavage of p100 to generate p52. Then p52 forms a functional complex with RelB and translocates to the nucleus to enhance gene expression [2]. Interestingly, the cIAP proteins, which promote the canonical pathways, play a negative role in the non-canonical pathway by triggering NIK ubiquitination and degradation [10]. Therefore, the canonical and non-canonical pathways could be coordinately regulated under some circumstances.
In some rare cases alternative pathways, which are called atypical pathways, have emerged to activate NF-κB in addition to the canonical and non-canonical pathways (Figure 1). For example, short wavelength UV light causes an IKK-independent NF-κB activation pathway that involves casein kinase 2 (CK2)-mediated phosphorylation and calpain-dependent IκB degradation [11]. Hydrogen peroxide has been shown to activate NF-κB activation through tyrosine phosphorylation of IκB at Tyr42, which likely involves c-Src or Syk kinases [12,13].
4. Biological functions of NF-κB
As a multifunctional factor, NF-κB is involved in a variety of physiological and pathological processes such as development, immunity, tissue homeostasis and inflammation. At the molecular and cellular level NF-κB regulates gene expression, cell apoptosis and proliferation.
4.1 Regulation of transcription
In most cases, NF-κB acts as a transcriptional activator by directly binding to the promoter to facilitate gene transcription. NF-κB-inducd gene expression is responsible for most biochemical and biological functions such as inflammation, growth, and immune response. So far more than 200 genes have been identified as NF-κB-responsive genes [14]. Thus, reagents that block gene expression at either the transcription or translation levels have been readily used to suppress NF-κB's function. Conversely, NF-κB was recently reported to suppress rather than activate gene transcription when it was induced by DNA-damaging drugs [15]. NF-κB's mechanism of transcriptional suppression is still elusive; however, interactions with transcriptional repressors or tumor suppressors such as p53 or ARF may be involved [16]. NF-κB's transcriptional suppression property is probably cell-type-specific because some of these agents-induced NF-κB was clearly transcriptionally active in different tested cells [17,18].
4.2 Regulation of apoptosis
NF-κB is generally regarded as a cell survival factor because it confers cell survival [2]. Indeed, numerous NF-κB targets such as cIAP-1, cIAP-2, TRAF1, TRAF2, Bcl-xL, XIAP, MnSOD, and IEX-1L have anti-apoptotic properties [19]. Specifically, cIAP-1 and cIAP-2 function as an apoptosis brake through directly binding and suppressing the effector caspases. The IAP proteins may form a positive feedback loop for NF-κB activation because ubiquitination of RIP by c-IAPs was thought to be important for recruitment and activation of IKK [5,20–22]. However, NF-κB could be pro-apoptotic because it activates expression of apoptosis mediators such as death receptor DR5, FAS ligand, PUMA and Bax [23–25].
4.3 Regulation of proliferation
NF-κB transactivates the expression of cyclin D1 and c-myc that promote cell proliferation. Interestingly, proinflammatory cytokines such as TNF, IL-1β and IL-8, which trigger inflammation as well as cell proliferation that is involved in carcinogenesis, are also NF-κB targets [19]. However, because NF-κB is able to suppress the proliferation factor JNK and induce the expression of the cycle suppressor p21/WAF1, it can function to inhibit cell proliferation [14].
5. NF-κB activation in cancer
NF-κB is aberrantly activated in tumor cells, contributing to the cells' advantage in survival and proliferation [14,26]. The mechanism of NF-κB activation in tumor cells is not well elucidated, but it is apparently complex and varies in different tumor types. Undoubtedly, understanding the mechanism of NF-κB activation in tumor cells will facilitate development of means for cancer prevention and therapy.
The constitutive NF-κB activation may be a result of mutations or epigenetic aberrations that affect the expression of the NF-κB subunits. Genes regulating NF-κB activity, including IκB and other genes directly or indirectly affecting NF-κB, may also be altered in tumors. For example, mutations in the IκBα gene or a reduction in IκBα protein stability results in constitutive NF-κB activation in Hodgkin's lymphoma. The mutation of Her2/Neu that is frequently active in a number of cancers, such as breast and lung cancers, is able to activate NF-κB in a CK2-dependent manner [27]. Transglutaminase (TG2) overexpression leads to constitutive activation in an IKK-independent manner [28]. Moreover, virus-derived oncoproteins such as human T-cell leukemia virus (HTLV) Tax protein, and hepatitis B virus × protein activate NF-κB and contribute to viral-infection-associated carcinogenesis [29,30].
In addition, while tumors often arise in an inflammatory environment and hypoxia presents in the tumor tissue, tumor, stromal and inflammatory cells secret proinflammatory cytokines such as TNF to establish a positive NF-κB activation loop [31,32]. Indeed, carcinogens and tumor promoters induce NF-κB. For example, the carcinogen benzo[a]pyrene is capable of activating NF-κB, at least in part through TNF autocrine action [33].
6. NF-κB in carcinogenesis
6.1 NF-κB in inflammation-associated cancer
It is estimated that approximately 15 – 20% of human cancers are strongly linked to inflammation [34]. The reciprocal activation between NF-κB and inflammatory cytokines makes NF-κB an important factor not only for inflammation but also for cancer development. However, due to the complexity of carcinogenesis and the contribution of NF-κB in different cell types, for example immune and parenchymal cells, NF-κB's complicated roles are found in different tumor models as described below.
In the dextran-sulphate sodium (DSS)-induced chronic-inflammatory-colitis-associated cancer mouse model, blocking NF-κB by knocking down IKKβ in enterocytes resulted in an 80% reduction in tumor multiplicity, although there were no changes in tumor size. These results suggest that NF-κB functions during the early stages of colon cancer development [35]. The blocking of NF-κB reduced anti-apoptotic gene BCL-XL expression and increased apoptosis. The blocking of NF-κB in myeloid cells also reduced tumor multiplicity by 50%, which was associated with reduction of growth factors such as IL-6 [35]. A similar effect of NF-κB blockage on hepatocellular carcinoma (HCC) development was also seen in a multidrug resistance 2 (MDR2)-knock-out mouse model. In this model, a defect in transporting and secreting bile acids and phospholipids from hepatocytes leads to low-grade chronic hepatitis and eventually HCC. The blockage of NF-κB with a hepatocyte-specific expression of IκB super suppressor (IκB SR, an IκBα mutant that is resistant to phosphorylation and degradation) resulted in increased liver cell apoptosis and reduced HCC. The NF-κB activation and HCC development in this model is probably mediated by cytokines, including TNF-α, because administration of a TNF-α antibody suppressed nuclear RelA immunostaining in hepatocytes and reduced HCC [36]. Mucosal-associated lymphoid tissue (MALT)-derived lymphoma, another tumor that results from chronic bacterial infection and inflammation, also involves NF-κB aberrant activation that is due to overexpression of the Bcl10 and MALT genes [37].
However, a negative interplay between NF-κB and JNK is probably involved in a chemical (diethylnitrosamine, DEN)-induced HCC model, in which NF-κB in parenchymal or myeloid cells plays contradictory roles in tumor promotion. In this model the necrotic hepatocyte death promotes inflammation and regenerative proliferation that leads to HCC. NF-κB in hepatocytes blocks DEN-induced cell death, limiting liver inflammation and regenerative proliferation and thereby suppressing HCC development. However, NF-κB is required for secretion of the compensatory proliferation factors TNF-α, IL-6 and hepatocyte growth factor (HGF) from liver myeloid cells known as Kupffer cells. Thus, NF-κB in Kupffer cells plays a tumor-promoting role in this model. In the two-stage skin cancer model induced by sequential and topical application of 7,12-dimethylbenz(a)anthracene (DMBA) and phorbol ester TPA, NF-κB apparently plays a tumor-suppressing role. Blocking NF-κB in keratinocytes substantially increased the incidences of squamous cell carcinoma (SCC), suggesting NF-κB's tumor-suppressing role. In this tumor model, TNF-α-induced JNK-mediated AP1 activation is crucial for tumor promotion. NF-κB suppresses TNF-α-induced JNK activation, which explained the negative role of NF-κB tumor development in this model. As the source of TNF-α has not been identified, it remains to be determined if the myeloid or stromal cells secrete TNF-α in an NF-κB-dependent manner as seen in the DEN-induced HCC model.
The aforementioned observations strongly suggest that there are distinct roles for NF-κB in different cancer types that could be cell-, tissue- or carcinogen-specific [32,38,39]. Thus, it is crucial to characterize the function of NF-κBin each type or even subtype of cancer derived from different organs before using NF-κB as an intervention target in cancer prevention and therapy.
6.2 NF-κB in cell transformation and tumor growth
Neoplastic transformation is an important step during cancer initiation. In vivo studies have found that NF-κB contributes to the initiation and early progression of colon and liver tumors and lymphoma [32,40]. In vitro studies also have suggested a positive role for NF-κB in cell transformation induced by oncogenes such as Ras, Pim-2 and HTLV Tax in prostate and colon epithelial cells, fibroblasts, and lymphocytes [29,41–43]. In addition, neoplastic transformation of mam-mary cells induced by cigarette smoke is also dependent on NF-κB activation [44]. NF-κB protects DNA-damaged cells from apoptosis and stimulates cell proliferation, which at least partly contributes to its role in promoting cell transformation. This may involve anti-apoptotic factors such as Bcl-XL and survivin; proliferation regulators p21WAF1, cyclin D and cmyc; and growth factors including TNF-α, IL-1β, IL-6 and EGF [31,32]. Because there is a hypoxic environment in tumors and hypoxia-inducible transcription factor-1α (HIF-1α)is highly expressed in tumor cells, the NF-κB-mediated HIF-1α expression in tumors as well as in myeloid cells during hypoxic response may also contribute to tumor growth [45].
6.3 NF-κB and cancer cell invasion and metastasis
Tumor metastasis is a complicated process that involves adhesion, migration and invasion that drives cancer cells to invade and translocate to remote tissues. NF-κB activates several genes that affect cancer cell migration and invasion [14]. Epithelial–mesenchymal transition (EMT), a critical step in tumor cell invasion and metastasis, is enhanced by NF-κB. NF-κB induces EMT-related genes such as Twist, intercellular adhesion molecule-1 (ICAM-1), endothelial leukocyte adhesion molecule 1 (ELAM-1), vascular cell adhesion molecule 1 (VCAM-1), MMPs, and serine protease urokinase-type plasminogen activator (uPA) in breast cancer [46,47]. NF-κB-activated Bcl-2 expression also promotes EMT in breast cancer [48]. The tumor suppressor protein N-myc downstream-regulated gene 2 (NDRG2) suppresses fibrosarcoma and melanoma cell invasion by suppressing NF-κB-mediated MMP-9 and -2 expression and activity [49]. It was found that TNF enhanced the ability of a variety of tumor cells to adhere to the mesothelium in vitro and increased tumor migration and metastasis in vivo, partly through NF-κB-dependent induction of the chemokine receptor CXCR4 and upregulation of monocyte chemoattractant protein-1 (MCP-1), ICAM-1, and IL-8 in cancer cells [47].
6.4 NF-κB and tumor angiogenesis
Angiogenesis, the formation of new blood vessels, is important for tumor progression. Tumor angiogenesis is dependent on proinflammatory cytokines, chemokines and growth factors such as MCP-1, IL-8, TNF-α and VEGF secreted by macrophages and other inflammatory cells. NF-κB in these cells plays a pivotal role in secreting the angiogenesis factors [50,51]. Constitutive NF-κB activation in cancer cells also triggers autocrine of angiogenic chemokines, and NF-κB inhibition substantially suppresses tumor growth and angiogenesis [52]. Also, stromal cell-derived factor 1 alpha (SDF-1α) enhances tumor angiogenesis in human basal cell carcinoma by upregulating several angiogenesis-associated genes, at least partly via NF-κB [53]. Furthermore, the recruitment of bone marrow-derived cells (BMDCs) to tumors for vasculogenesis is essential for tumor angiogenesis. NF-κB-mediated IL-8 and angiogenin expression is involved in this process [54]. However, it was surprisingly noticed that NF-κB inhibition leads to an increase in B16-BL6 tumor angiogenesis in IκB SR transgenic mice [55]. However, due to the potential off-target effect of IκB SR overexpression [17,56,57], this observation needs to be evaluated with other NF-κB-blocking methods. Nevertheless, NF-κB's possible anti-angiogenesis role in some cancer types should not be neglected [58].
7. NF-κB in cancer cells' response to therapy
Inducing tumor cell apoptosis is one of the main mechanisms underlying anticancer chemo- and radiotherapy. Because NF-κB is constitutively activated in many cancer cells, chemotherapeutic agents and radiation activate NF-κB, and both constitutive and therapy-induced NF-κB activation is generally anti-apoptotic, blocking NF-κB has been tested and found to sensitize cancer cells to radiotherapy and a variety of chemotherapeutics in numerous tumor cell types [59,60]. As discussed above, induction of anti-apoptotic factors is one of the main mechanisms involving NF-κB in cancer cells' resistance to therapy. The induction of Bcl-2 family members such as Bcl2, Bcl-xL and the IAP family members cIAP1, cIAP2, XIAP and cFLIP blunts both the intrinsic and extrinsic apoptosis pathways. By inducing manganese superoxide dismutase or ferritin heavy chain, NF-κB suppresses reactive oxygen species (ROS) that are often induced by anticancer therapeutic agents to trigger cancer cell death [61]. NF-κB also suppresses the sustained JNK activation that is apoptotic [62].
The tumor suppressor p53 and its family members play an important role in therapy-induced cancer cell death and proliferation inhibition. NF-κB suppresses p53 functions through distinct mechanisms. NF-κB inhibits the p53 response to DNA damage by inducing expression of the E3 ubiquitin ligase Hdm2 (Mdm2 in mice) that destabilize p53 [63,64]. Furthermore, NF-κB attenuates the function of p53 family members through direct interactions with the promoter. For example, RelA binds and suppresses p73's transcriptional activity [65]. Thus, simultaneously inhibiting NF-κB and activating p53 could be an efficient way to enhance cancer cells' sensitivity to chemotherapeutics [66].
In addition, other mechanisms involving NF-κB also may be involved in cancer cells' resistance to chemotherapy. For example, NF-κB activates expression of multidrug resistance 1 (MDR1), and MDR1 functions to blunt the anticancer activity of therapeutics by efflux of the drugs from cancer cells [67].
Although there is abundant evidence to support NF-κB's important role in cancer cells' resistance to therapy, other reports suggest that NF-κB is required for killing cancer cells [15,68]. This may be partly explained by the fact that NF-κB induces apoptotic factors DR5, FASL and Bax or that some therapeutic-induced NF-κB suppresses expression of anti-apoptotic gene such as Bcl-XL in cells [15,23,24].It is noteworthy that controversial observations were reported regarding IκB SR-mediated NF-κB suppression in cancer cells' response to chemotherapy [69,70], which may be associated with cell types and the approaches to gene delivery. Indeed, we recently found that different approaches, that is IκB SR over-expression or knockdown of RelA or IKKβ, exerted distinct effects, suggesting that the gene target or approach affect the anticancer outcomes [17]. It is possible that some of the NF-κB-independent mechanisms caused by IκB SR may alleviate the pro-apoptotic effect of NF-κB blockage [56,57,71].
8. Approaches targeting NF-κB for cancer therapy
Because NF-κB is commonly activated in cancer cells and is generally involved in cancer cells' survival, blocking NF-κB is expected to reduce the survival threshold. NF-κB inhibition alone is generally insufficient for inducing pronounced apoptosis in cancer cells. Thus, NF-κB inhibition is being tested mainly for use with chemo- and radiotherapy. The canonical pathway has received the most attention in this regard. Different points in this pathway can be targeted for modulating NF-κB activity. In recent years, much effort has been invested in developing and characterizing NF-κB-blocking agents, including naturally occurring and synthetic compounds that are summarized in a recent review [72]. The main targeted actions in the NF-κB signaling pathway include: IKK activation, IκB degradation and NF-κB nuclear translocation and DNA binding. Promising progress has been made using these NF-κB inhibiting approaches, and hopefully will bring more NF-κB inhibitors to clinical trials.
8.1 IKK inhibitors
Due to its central role in NF-κB activation, IKK has been a major molecular target for NF-κB inhibition. The list of IKK inhibitors developed and tested in anticancer therapy is rapidly increasing. These inhibitors include BAY-11-7082, BAY-11-7085 [73], MLN120B [74], BMS-345541 [75], SC-514 [76] and CHS828 [77]. These compounds can either directly bind and inhibit the IKK kinase activity or indirectly inhibit IKK activation by blocking upstream signaling that leads to IKK activation. Combining IKK inhibitors with a variety of chemotherapeutics has been examined and sensitization was achieved in both in vitro and in vivo systems [72].
8.2 Proteasome inhibitors
Inhibiting the activity of proteasomes blocks NF-κB activation during the process of IκB protein degradation. Bortezomib, a reversible 26S proteasome inhibitor, is the first NF-κB blocking drug approved by the FDA and the European Medicines Agency for the treatment of multiple myeloma [78]. Preclinical studies show that bortezomib has manageable side effects when used as a single agent. Bortezomib also has been tested for combined therapy with other anticancer drugs, such as DNA-damage-inducing agents, in a variety of malignant tumors including lung, breast, colon, bladder, ovary and prostate cancers and achieved better responses [79]. Clinical trials have demonstrated a high anticancer efficacy when combining bortezomib and EGFR/HER2-targeting agents such as trastuzumab (Herceptin, a monoclonal antibody against HER2) in breast cancer, cetuximab (a chimeric mouse–human antibody targeted against EGFR) in NSCLC or head and neck cancers [80,81], and erlotinib in nonsmall cell lung cancer [82]. New proteasome inhibitors such as RP-171, NPI-0052 and CEP-18770 (carfilzomib) are being examined in vitro and in early-phase clinical trials [72].
8.3 NF-κB nuclear translocation and DNA binding inhibitors
Restraining NF-κB in the cytoplasm after IκB degradation is another strategy for blocking NF-κB. SN-50, a peptide of 41 amino acid residues consisting of the p50 NLS sequence blocking NF-κB activation by inhibition of the nuclear transport machinery, substantially sensitized cisplatin's anticancer activity in ovarian cancer cells [83].
8.4 Anti-inflammatory drugs
NSAIDs, including sulindac, aspirin, ibuprofen, indomethacin, and COX-2 inhibitors, are potential NF-κB blockers. They function by either suppressing the inflammatory cell response to indirectly suppress NF-κB, or by directly suppressing NF-κB at key points along the NF-κB activation pathway. Combining these drugs with anticancer agents has been examined extensively for chemoprevention or chemosensitization [84,85]. Naturally occurring anti-inflammatory compounds such as epigallocatechin gallate (EGCG), eicosapentaenoic acid (EPA), curcumin (diferuloylmethane), and luteolin are also able to block NF-κB, making them another group of NF-κB-blocking agents for cancer prevention and therapy. These compounds block NF-κB at distinct steps of the pathway. For example, apigenin and anacardic acid inhibit IKK, resveratrol inhibits p65 phosphorylation, epicatechin inhibits p65 translocation to the nucleus and celestrol inhibits NF-κB's DNA binding [86–88]. It is of note that these chemicals are mainly antioxidants and their anticancer activity may be due to regulating the redox status of the cell. However, the modulation of redox may contribute to NF-κB blockage. For example, we found that luteolin blocks TNF-α-induced NF-κB through superoxide in lung cancer cells [89].Blocking NF-κB by luteolin shifts TNF-α-induced cancer cell survival to apoptosis. Because TNF-α is involved in inflammation-associated carcinogenesis, the blockage of NF-κB by luteolin may convert TNF-α from a tumor promoter to a tumor suppressor [90,91], making luteolin a potential chemopreventive agent [91].
8.5 Gene therapy targeting NF-κB
Gene therapy that directly targets a key component of the NF-κB activation pathway is a more specific approach than the aforementioned NF-κB-blocking agents. One approach is overexpression of IκB SR with a plasmid or viral vector [70]. RNA interference, which specifically eliminates gene expression, is another widely tested approach for blocking NF-κB. The application of siRNA molecules directed against IKKα, IKKβ and the upstream regulatory kinase TAK1 has been used in many studies [88]. Additionally, oligodeoxynucleotide-based NF-κB blocking was found to be effective for chemosensitization [92]. However, use of gene therapy in a clinical setting is awaiting the development of specific and efficient means for targeted delivery of genes to cancer cells.
Despite some inhibitors being designed to specifically target NF-κB pathway mediators, many NF-κB inhibiting compounds are also potent in interfering with other pathways. Interestingly, some of these effects could be beneficial for cancer therapy. For example, Hsp90 inhibitors suppress both NF-κB and Akt, and Akt contributes to cancer cells' survival and proliferation by both NF-κB-dependent and -independent mechanisms [93–95]. Concurrent blocking NF-κB and Akt achieves a synergistic anticancer activity [96].It should be noted that anticancer chemicals targeting the same molecule may have a distinct involvement with NF-κB. One example is the recently developed smac mimetics that potently kill cancer cells through autocrine TNF-α. NF-κB activation by different smac mimetics appears to be due to different mechanisms, and therefore distinct roles for NF-κB (pro- or anti-apoptotic) in cancer cell killing were observed [97–99]. Thus, a combination of anticancer therapeutics and NF-κB blocking methods for cancer therapy should be evaluated individually with regard to each drug.
9. Expert opinion
NF-κB is generally regarded as a cell survival signal in most cell types and is involved in cancer development in various organs. Thus, suppressing NF-κB could be a molecular target for cancer prevention [26]. However, due to the complex roles and mechanisms of NF-κB in carcinogenesis, careful evaluation of NF-κB's role in the pathogenesis of each cancer type is crucial before employing NF-κB inhibition approaches for cancer prevention. For example, NF-κB in different organs could be either tumor-promoting (i.e., colon and liver) or -suppressing (skin and liver), which is at least partly due to the functional interplay between the immune cells and the parenchymal cells, and between different signaling pathways that are simultaneously activated during inflammation. Specifically, NF-κB in immune cells plays a critical role in cancer promotion; thus it could be a major target for cancer prevention. However, because NF-κB is required for physiological immune functions of the body, sustained and systematic immune suppression causes severe consequences associated with immunodeficiency. Thus, currently available NF-κB-suppressing drugs are not suitable in cancer prevention, and directly targeting NF-κB for cancer prevention is still a challenge. An alternative approach is to target the upstream pathways for persistent NF-κB activation, such as proinflammatory cytokines or the cause of inflammation such as microbial infection in the tumor site organs. However, prolonged use of anti-inflammatory drugs can also cause non-tolerable adverse effects [50]. Naturally occurring compounds having NF-κB-suppressing properties are of great interest in relieving inflammation and preventing cancer [91,100].It is desirable to develop approaches that deliver NF-κB inhibition more specifically to transformed cells and immune cells residing in tumor-prone microenvironments.
Because NF-κB contributes to proliferation and survival in most cancer cells and cancer therapy is of a relatively shorter duration, NF-κB-inhibiting drugs can be administered intermittently, thereby greatly relieving the concern with immunosuppression caused by long-lasting NF-κB inhibition. Thus, targeting NF-κB could be a useful strategy for cancer therapy. Various NF-κB inhibitors targeting different components of the NF-κB activation pathway, that is, IKK or NF-κB subunits, are under development for cancer therapy. Also, genetic methods such as overexpression of the IκB SR have been tested for cancer therapy. Again, due to the important functions of NF-κB in normal cells, more selective methods of inhibiting NF-κB in tumor cells are desired for reducing systemic toxicity. Additionally, due to the insufficiency of mere NF-κB inhibition in inducing pronounced apoptosis in cancer cells, it is more likely that NF-κB inhibitors will be used as an adjuvant along with chemo- or radiotherapy. It is remarkable that because both the constitutive and induced NF-κB activation by therapeutics or radiation blunts the anticancer activities of the therapeutic agents, blocking NF-κB may circumvent this side effect and therefore achieve a synergistic anticancer activity. Because NF-κB is required for apoptosis in some tumors, caution should be taken when selecting some therapeutic drugs in combination with NF-κB blockers for cancer therapy. Each NF-κB blocking approach needs to be validated for therapy in each cancer type. For example, overexpressing the IκB SR is potent in blocking NF-κB, but it does not exhibit an anticancer activity in lung cancer cells [17,69], which may be due to its non-specific effect that that blocks cancer cell death [17,56,57]. Further studies on the mechanisms for constitutive and therapy-induced NF-κB activation in various human cancers are required when using NF-κB blocking in cancer therapy. Specifically, the involvement of the non-canonical and atypical pathways in each cancer type needs to be determined. Nevertheless, with the recognition of NF-κB's critical role in malignant phenotypes of cancer, great effort is being invested to develop NF-κB inhibitors for use in cancer therapy. It is expected that along with the progress in elucidating NF-κB activation mechanisms in tumors, more NF-κB-targeting drugs will be available for clinical trials in the coming years.
Acknowledgements
We apologise to our colleagues whose work was unable to be cited due to the space limit. The authors thank V Fisher for editing the manuscript.
Declaration of interest The research in this laboratory is supported in part by grants from the National Cancer Institute, NIH (R03CA125796) and Department of Energy Low Dose Radiation Research Program (DE-FG02-09ER64783).
Bibliography
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18(18):2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 3.Devin A, Cook A, Lin Y, et al. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12(4):419–29. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Lin Y, Guo Z, et al. The essential role of MEKK3 in TNF-induced NF-κB activation. Nat Immunol. 2001;2(7):620–4. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 5.Chen ZJ. Ubiquitin signalling in the NF-κB pathway. Nat Cell Biol. 2005;7(8):758–65. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-κB activation in response to DNA damage. Cell. 2005;123(6):1079–92. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 2007;14(3):400–10. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 8.Wu ZH, Mabb A, Miyamoto S. PIDD: a switch hitter. Cell. 2005;123(6):980–2. doi: 10.1016/j.cell.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Wu CJ, Conze DB, Li T, et al. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-κB activation. Nat Cell Biol. 2006;8(4):398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 10.Zarnegar BJ, Wang Y, Mahoney DJ, et al. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9(12):1371–8. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato T, Jr, Delhase M, Hoffmann A, Karin M. CK2 Is a C-terminal IκB kinase responsible for NF-κB activation during the UV response. Mol Cell. 2003;12(4):829–39. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 12.Tergaonkar V, Bottero V, Ikawa M, et al. IκB kinase-independent IκBα degradation pathway: functional NF-κB activity and implications for cancer therapy. Mol Cell Biol. 2003;23(22):8070–83. doi: 10.1128/MCB.23.22.8070-8083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30(1):43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6(3):203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Campbell KJ, Rocha S, Perkins ND. Active repression of antiapoptotic gene expression by RelA(p65) NF-κB. Mol Cell. 2004;13(6):853–65. doi: 10.1016/s1097-2765(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 16.Janssens S, Tschopp J. Signals from within: the DNA-damage-induced NF-κB response. Cell Death Differ. 2006;13(5):773–84. doi: 10.1038/sj.cdd.4401843. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Wang X, Bai L, et al. Blockage of NF-κB by IKKβ- or RelA-siRNA rather than the NF-κB super-suppressor IkBa mutant potentiates adriamycin-induced cytotoxicity in lung cancer cells. J Cell Biochem. 2008;105(2):554–61. doi: 10.1002/jcb.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur GM, Lewis J, Yang Q, et al. The death domain kinase RIP has an essential role in DNA damage-induced NF-κB activation. Genes Dev. 2003;17(7):873–82. doi: 10.1101/gad.1062403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 20.Ea CK, Deng L, Xia ZP, et al. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22(2):245–57. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133(4):693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 22.Varfolomeev E, Goncharov T, Fedorova AV, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J Biol Chem. 2008;283(36):24295–9. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh NP, Nagarkatti M, Nagarkatti PS. Role of dioxin response element and nuclear factor-κB motifs in 2,3,7,8-tetrachlorodibenzop-dioxin-mediated regulation of Fas and Fas ligand expression. Mol Pharmacol. 2007;71(1):145–57. doi: 10.1124/mol.106.028365. [DOI] [PubMed] [Google Scholar]
- 24.Shou Y, Li N, Li L, et al. NF-κB-mediated up-regulation of Bcl-XS and Bax contributes to cytochrome c release in cyanide-induced apoptosis. J Neurochem. 2002;81(4):842–52. doi: 10.1046/j.1471-4159.2002.00880.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, Qiu W, Dudgeon C, et al. PUMA is directly activated by NF-κB and contributes to TNF-α-induced apoptosis. Cell Death Differ. 2009;16(9):1192–202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar FH, Li Y. NF-κB: a potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–9. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- 27.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-κB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62(22):6770–8. [PubMed] [Google Scholar]
- 28.Mann AP, Verma A, Sethi G, et al. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-κB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66(17):8788–95. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 29.Sun SC, Yamaoka S. Activation of NF-κB by HTLV-I and implications for cell transformation. Oncogene. 2005;24(39):5952–64. doi: 10.1038/sj.onc.1208969. [DOI] [PubMed] [Google Scholar]
- 30.Tang H, Oishi N, Kaneko S, Murakami S. Molecular functions and biological roles of hepatitis B virus × protein. Cancer Sci. 2006;97(10):977–83. doi: 10.1111/j.1349-7006.2006.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 32.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441(7092):431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 33.Podechard N, Lecureur V, Le Ferrec E, et al. Interleukin-8 induction by the environmental contaminant benzo(a) pyrene is aryl hydrocarbon receptor-dependent and leads to lung inflammation. Toxicol Lett. 2008;177(2):130–7. doi: 10.1016/j.toxlet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248(3):171–83. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 35.Greten FR, Eckmann L, Greten TF, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Pikarsky E, Porat RM, Stein I, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 37.Ruland J, Duncan GS, Elia A, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell. 2001;104(1):33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 38.Perkins ND. NF-κB: tumor promoter or suppressor? Trends Cell Biol. 2004;14(2):64–9. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Chen F, Castranova V. Nuclear factor-κB, an unappreciated tumor suppressor. Cancer Res. 2007;67(23):11093–8. doi: 10.1158/0008-5472.CAN-07-1576. [DOI] [PubMed] [Google Scholar]
- 40.Wang LC, Okitsu CY, Zandi E. Tumor necrosis factor α-dependent drug resistance to purine and pyrimidine analogues in human colon tumor cells mediated through IKK. J Biol Chem. 2005;280(9):7634–44. doi: 10.1074/jbc.M413384200. [DOI] [PubMed] [Google Scholar]
- 41.Kim BY, Gaynor RB, Song K, et al. Constitutive activation of NF-κB in Ki-ras-transformed prostate epithelial cells. Oncogene. 2002;21(29):4490–7. doi: 10.1038/sj.onc.1205547. [DOI] [PubMed] [Google Scholar]
- 42.Hammerman PS, Fox CJ, Cinalli RM, et al. Lymphocyte transformation by Pim-2 is dependent on nuclear factor-κB activation. Cancer Res. 2004;64(22):8341–8. doi: 10.1158/0008-5472.CAN-04-2284. [DOI] [PubMed] [Google Scholar]
- 43.Hanson JL, Hawke NA, Kashatus D, Baldwin AS. The nuclear factor κB subunits RelA/p65 and c-Rel potentiate but are not required for Ras-induced cellular transformation. Cancer Res. 2004;64(20):7248–55. doi: 10.1158/0008-5472.CAN-03-3898. [DOI] [PubMed] [Google Scholar]
- 44.Anto RJ, Mukhopadhyay A, Shishodia S, et al. Cigarette smoke condensate activates nuclear transcription factor-κB through phosphorylation and degradation of IκBα: correlation with induction of cyclooxygenase-2. Carcinogenesis. 2002;23(9):1511–8. doi: 10.1093/carcin/23.9.1511. [DOI] [PubMed] [Google Scholar]
- 45.Rius J, Guma M, Schachtrup C, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453(7196):807–11. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Huber MA, Beug H, Wirth T. Epithelial-mesenchymal transition: NF-κB takes center stage. Cell Cycle. 2004;3(12):1477–80. doi: 10.4161/cc.3.12.1280. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Belguise K, Kersual N, et al. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9(4):470–8. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim A, Kim MJ, Yang Y, et al. Suppression of NF-κB activity by NDRG2 expression attenuates the invasive potential of highly malignant tumor cells. Carcinogenesis. 2009;30(6):927–36. doi: 10.1093/carcin/bgp072. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15(2):425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt D, Textor B, Pein OT, et al. Critical role for NF-κB-induced JunB in VEGF regulation and tumor angiogenesis. EMBO J. 2007;26(3):710–9. doi: 10.1038/sj.emboj.7601539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakamoto K, Maeda S, Hikiba Y, et al. Constitutive NF-κB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res. 2009;15(7):2248–58. doi: 10.1158/1078-0432.CCR-08-1383. [DOI] [PubMed] [Google Scholar]
- 53.Chu CY, Cha ST, Lin WC, et al. Stromal cell-derived factor-1α (SDF-1α CXCL12)-enhanced angiogenesis of human basal cell carcinoma cells involves ERK1/2-NF-κB/interleukin-6 pathway. Carcinogenesis. 2009;30(2):205–13. doi: 10.1093/carcin/bgn228. [DOI] [PubMed] [Google Scholar]
- 54.Chan DA, Kawahara TL, Sutphin PD, et al. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell. 2009;15(6):527–38. doi: 10.1016/j.ccr.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kisseleva T, Song L, Vorontchikhina M, et al. NF-κB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116(11):2955–63. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou M, Gu L, Zhu N, et al. Transfection of a dominant-negative mutant NF-κB inhibitor (IkBm) represses p53-dependent apoptosis in acute lymphoblastic leukemia cells: interaction of IkBm and p53. Oncogene. 2003;22(50):8137–44. doi: 10.1038/sj.onc.1206911. [DOI] [PubMed] [Google Scholar]
- 57.Aguilera C, Hoya-Arias R, Haegeman G, et al. Recruitment of IκBα to the hes1 promoter is associated with transcriptional repression. Proc Natl Acad Sci USA. 2004;101(47):16537–42. doi: 10.1073/pnas.0404429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabruyn SP, Griffioen AW. A new role for NF-κB in angiogenesis inhibition. Cell Death Differ. 2007;14(8):1393–7. doi: 10.1038/sj.cdd.4402156. [DOI] [PubMed] [Google Scholar]
- 59.Baud V, Karin M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8(1):33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed KM, Li JJ. ATM-NF-κB connection as a target for tumor radiosensitization. Curr Cancer Drug Targets. 2007;7(4):335–42. doi: 10.2174/156800907780809769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamata H, Honda S, Maeda S, et al. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120(5):649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 62.Tang G, Minemoto Y, Dibling B, et al. Inhibition of JNK activation through NF-κB target genes. Nature. 2001;414(6861):313–7. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 63.Tergaonkar V, Pando M, Vafa O, et al. p53 stabilization is decreased upon NFκB activation: a role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1(5):493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 64.Yang PM, Huang WC, Lin YC, et al. Loss of IKKβ activity increases p53 stability and p21 expression leading to cell cycle arrest and apoptosis. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00712.x. published online Feb 20 2009, doi: 10.1111/j.1582-4934.2009.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cianfrocca R, Muscolini M, Marzano V, et al. RelA/NF-κB recruitment on the bax gene promoter antagonizes p73-dependent apoptosis in costimulated T cells. Cell Death Differ. 2008;15(2):354–63. doi: 10.1038/sj.cdd.4402264. [DOI] [PubMed] [Google Scholar]
- 66.Dey A, Verma CS, Lane DP. Updates on p53: modulation of p53 degradation as a therapeutic approach. Br J Cancer. 2008;98(1):4–8. doi: 10.1038/sj.bjc.6604098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ros JE, Schuetz JD, Geuken M, et al. Induction of Mdr1b expression by tumor necrosis factor-α in rat liver cells is independent of p53 but requires NF-κB signaling. Hepatology. 2001;33(6):1425–31. doi: 10.1053/jhep.2001.24667. [DOI] [PubMed] [Google Scholar]
- 68.Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-κB in p53-mediated programmed cell death. Nature. 2000;404(6780):892–7. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- 69.Ganapathi R, Vaziri SA, Tabata M, et al. Inhibition of NF-κB and proteasome activity in tumors: can we improve the therapeutic potential of topoisomerase I and topoisomerase II poisons. Curr Pharm Des. 2002;8(22):1945–58. doi: 10.2174/1381612023393549. [DOI] [PubMed] [Google Scholar]
- 70.Lee CT, Seol JY, Lee SY, et al. The effect of adenovirus-IκBα transduction on the chemosensitivity of lung cancer cell line with resistance to cis-diamminedichloroplatinum(II) (cisplatin) and doxorubicin(adriamycin) Lung Cancer. 2003;41(2):199–206. doi: 10.1016/s0169-5002(03)00227-7. [DOI] [PubMed] [Google Scholar]
- 71.Dreyfus DH, Nagasawa M, Gelfand EW, Ghoda LY. Modulation of p53 activity by I-κBα: evidence suggesting a common phylogeny between NF-κB and p53 transcription factors. BMC Immunol. 2005;6(1):12. doi: 10.1186/1471-2172-6-12. Published online 21 June 2005, doi:10.1186/1471-2172-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen HM, Tergaonkar V. NFκB signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis. 2009;14(4):348–63. doi: 10.1007/s10495-009-0315-0. [DOI] [PubMed] [Google Scholar]
- 73.Garcia MG, Alaniz L, Lopes EC, et al. Inhibition of NF-κB activity by BAY 11-7082 increases apoptosis in multidrug resistant leukemic T-cell lines. Leuk Res. 2005;29(12):1425–34. doi: 10.1016/j.leukres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Hideshima T, Chauhan D, Kiziltepe T, et al. Biologic sequelae of IκB kinase (IKK) inhibition in multiple myeloma: therapeutic implications. Blood. 2009;113(21):5228–36. doi: 10.1182/blood-2008-06-161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang J, Amiri KI, Burke JR, et al. BMS-345541 targets inhibitor of κB kinase and induces apoptosis in melanoma: involvement of nuclear factor κB and mitochondria pathways. Clin Cancer Res. 2006;12(3 Pt 1):950–60. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choo MK, Sakurai H, Kim DH, Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-α-promoted metastasis by suppressing nuclear factor-κB signaling in murine colon cancer cells. Oncol Rep. 2008;19(3):595–600. [PubMed] [Google Scholar]
- 77.Ravaud A, Cerny T, Terret C, et al. Phase I study and pharmacokinetic of CHS-828, a guanidino-containing compound, administered orally as a single dose every 3 weeks in solid tumours: an ECSG/EORTC study. Eur J Cancer. 2005;41(5):702–7. doi: 10.1016/j.ejca.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 78.Dingli D, Rajkumar SV. Emerging therapies for multiple myeloma. Oncology (Williston Park) 2009;23(5):407–15. [PubMed] [Google Scholar]
- 79.Gasparian AV, Guryanova OA, Chebotaev DV, et al. Targeting transcription factor NFκB: comparative analysis of proteasome and IKK inhibitors. Cell Cycle. 2009;8(10):1559–66. doi: 10.4161/cc.8.10.8415. [DOI] [PubMed] [Google Scholar]
- 80.Cardoso F, Durbecq V, Laes JF, et al. Bortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER-2-positive breast cancer cells in a synergistic manner. Mol Cancer Ther. 2006;5(12):3042–51. doi: 10.1158/1535-7163.MCT-06-0104. [DOI] [PubMed] [Google Scholar]
- 81.Sloss CM, Wang F, Liu R, et al. Proteasome inhibition activates epidermal growth factor receptor (EGFR) and EGFR-independent mitogenic kinase signaling pathways in pancreatic cancer cells. Clin Cancer Res. 2008;14(16):5116–23. doi: 10.1158/1078-0432.CCR-07-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lynch TJ, Fenton D, Hirsh V, et al. A randomized Phase 2 study of erlotinib alone and in combination with bortezomib in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(8):1002–9. doi: 10.1097/JTO.0b013e3181aba89f. [DOI] [PubMed] [Google Scholar]
- 83.Mabuchi S, Ohmichi M, Nishio Y, et al. Inhibition of NFκB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004;279(22):23477–85. doi: 10.1074/jbc.M313709200. [DOI] [PubMed] [Google Scholar]
- 84.Baron JA. Aspirin and NSAIDs for the prevention of colorectal cancer. Rec Results Cancer Res. 2009;181:223–9. doi: 10.1007/978-3-540-69297-3_21. [DOI] [PubMed] [Google Scholar]
- 85.Bank A, Yu J, Zhang L. NSAIDs downregulate Bcl-X(L) and dissociate BAX and Bcl-X(L) to induce apoptosis in colon cancer cells. Nutr Cancer. 2008;60(Suppl 1):98–103. doi: 10.1080/01635580802381261. [DOI] [PubMed] [Google Scholar]
- 86.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269(2):378–87. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72(11):1439–52. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 88.Sethi G, Sung B, Aggarwal BB. Nuclear factor-κB activation: from bench to bedside. Exp Biol Med (Maywood) 2008;233(1):21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 89.Ju W, Wang X, Shi H, et al. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-κB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol. 2007;71(5):1381–8. doi: 10.1124/mol.106.032185. [DOI] [PubMed] [Google Scholar]
- 90.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29(11):1275–88. doi: 10.1111/j.1745-7254.2008.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8(7):634–46. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uetsuka H, Haisa M, Kimura M, et al. Inhibition of inducible NF-κB activity reduces chemoresistance to 5-fluorouracil in human stomach cancer cell line. Exp Cell Res. 2003;289(1):27–35. doi: 10.1016/s0014-4827(03)00223-4. [DOI] [PubMed] [Google Scholar]
- 93.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics – an update. Expert Opin Emerg Drugs. 2005;10(1):137–49. doi: 10.1517/14728214.10.1.137. [DOI] [PubMed] [Google Scholar]
- 94.Lewis J, Devin A, Miller A, et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-κB activation. J Biol Chem. 2000;275(14):10519–26. doi: 10.1074/jbc.275.14.10519. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Ju W, Renouard J, et al. 17-allylamino-17-demethoxy-geldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-κB pathway. Cancer Res. 2006;66(2):1089–95. doi: 10.1158/0008-5472.CAN-05-2698. [DOI] [PubMed] [Google Scholar]
- 96.Wang X, Chen W, Lin Y. Sensitization of TNF-induced cytotoxicity in lung cancer cells by concurrent suppression of the NF-κB and Akt pathways. Biochem Biophys Res Commun. 2007;355(3):807–12. doi: 10.1016/j.bbrc.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 97.Vince JE, Wong WW, Khan N, et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell. 2007;131(4):682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 98.Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell. 2007;131(4):669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 99.Bai L, Chen W, Wang X, et al. IKKβ-mediated nuclear factor-κB activation attenuates smac mimetic-induced apoptosis in cancer cells. Mol Cancer Ther. 2009;8(6):1636–45. doi: 10.1158/1535-7163.MCT-09-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhandarkar SS, Arbiser JL. Curcumin as an inhibitor of angiogenesis. Adv Exp Med Biol. 2007;595:185–95. doi: 10.1007/978-0-387-46401-5_7. [DOI] [PubMed] [Google Scholar]