Abstract
Kawasaki disease (KD) is an acute systemic inflammatory illness of childhood that can result in coronary artery aneurysms, myocardial infarction, and sudden death. Clinical and epidemiologic data point to an unknown infectious agent as the cause. We discovered that an oligoclonal IgA immune response is present in arterial tissue in acute KD. Synthetic versions of prevalent IgA antibodies in the KD arterial wall identify cytoplasmic inclusion bodies in acute KD ciliated bronchial epithelium and other inflamed KD tissues. Light and electron microscopic studies show that the inclusion bodies are consistent with aggregates of viral protein and RNA, and are likely formed by the KD etiologic agent. KD susceptibility is likely to be polygenic. Treatment of gammaglobulin non-responders usually consists of additional intravenous immunoglobulin, methylprednisolone, and/or infliximab. Additional data regarding KD pathogenesis are urgently needed to provide other targets for therapy for those patients at highest risk of developing coronary artery abnormalities.
Keywords: IgA, Inclusion bodies, Intravenous immunoglobulin, Coronary artery aneurysm
Introduction
Kawasaki disease (KD) is the most common cause of acquired heart disease in children in developed nations. The etiology remains unknown, but clinical and epidemiologic features support an infectious cause [1]. Immunologic and pathologic data suggest a respiratory portal of entry for the KD agent [2]. The clinical features of the illness include prolonged high fever, nonexudative conjunctival injection, erythema of the lips and oropharynx, rash, erythema and swelling of the hands and feet, and cervical lymphadenopathy. Classic diagnostic criteria for KD include fever plus four of the five other clinical features; however, some children with KD, particularly infants, can present with an incomplete form of illness manifested by fever with three or fewer other clinical signs [3]. Both classic and incomplete KD can be associated with coronary artery aneurysms, myocardial infarction, and sudden death [1]. Treatment with intravenous immunoglobulin (IVIG) and aspirin by the tenth illness day reduces the prevalence of coronary artery abnormalities in KD from 30% to 5% [4], but the mechanism of action is unknown. About 10% to 15% of KD patients do not respond to a single dose of IVIG, 2 g/kg, with aspirin; these nonresponders are at higher risk of developing coronary artery abnormalities than those who respond to initial therapy [5]. Major advances in understanding and managing KD have recently focused on the following areas: etiology, genetics, and treatment of gammaglobulin nonresponders.
Etiology
Dr. Tomisaku Kawasaki described the clinical features of KD in the Japanese-language literature in 1967 [6]. Soon thereafter, it was recognized that KD can result in coronary artery aneurysms and myocardial infarction in young children, and that prior to Dr. Kawasaki’s description of the clinical features of the illness, the same entity had been diagnosed at autopsy by pathologists and termed “infantile periarteritis nodosa” [7]. This realization was followed by extensive descriptions of the vascular pathology in KD [8, 9]. Autopsy studies of children with fatal KD further revealed that KD is a systemic inflammatory illness involving many organs and tissues [10], although arterial inflammation (particularly coronary artery inflammation) results in the most significant consequences to the patient.
Since Dr. Kawasaki’s description of the clinical features of KD, theories have abounded regarding its etiology. A household toxin, particularly rug shampoo, was proposed in the United States as a potential cause [11], but rug shampoo is not associated with KD in Japan [12], and it seems highly unlikely that the etiology of such a characteristic and striking clinical illness as KD would be different in Japan and the United States. Moreover, a household toxin as the etiology of KD is not a compelling theory, because it would be expected that the illness would recur when the child reentered the home after hospitalization for KD. Although many investigators believe that KD is an autoimmune disease, little evidence exists to support this theory. Autoimmune diseases are infrequent in very young infants, who are the most likely to develop KD. A characteristic feature of autoimmune processes is disease recurrence, whereas KD only rarely recurs. KD patients do not have higher levels of circulating autoantibodies than control children [13], and although detectable in the circulation in the late acute stage of KD, immune complexes have not been convincingly demonstrated in the tissue pathology. Several widely held views about Kawasaki disease are not well-supported by the data (Table 1). Hypotheses that implicate an endothelial cell autoantigen as the cause of the disease process do not explain many other pathologic features of KD (eg, prostatitis, bronchitis, salivary ductitis, or pancreatic ductitis).
Table 1.
A challenge to widely held views about Kawasaki disease
| Widely held view | What the data show |
|---|---|
| Findings in KD peripheral blood mirror events in target tissue |
Neutrophils and CD4 T lymphocytes predominate in peripheral blood in acute KD whereas CD8 T lymphocytes, macrophages, eosinophils, and plasma cells predominate in target tissues [22, 49, 50] |
| KD is an autoimmune disease | KD rarely recurs, is most common in young infants, autoantibodies are not more prevalent in KD patients than controls [13], immune complexes are not observed in KD tissues, KD synthetic antibodies target cytoplasmic inclusion bodies consistent with aggregates of viral proteins and RNA [24, 25•] |
| Upregulation of cytokines in KD indicates the presence of a superantigen |
Cytokines are upregulated in virtually all systemic infectious and inflammatory diseases; presence of a specific adaptive immune response in KD argues against a superantigen as the cause for cytokine upregulation [15, 17] |
| KD results from “unusual immune response” to many different etiologic agents |
No precedent exists for an acute febrile systemic illness with distinctive clinical features to be the result of multiple diverse etiologic agents. Polio, roseola, Fifth disease, and AIDS were similarly proposed to represent an “unusual immune response to many different etiologic agents” prior to identification of their causative agents, all of which were found to be single viruses or a group of closely related viruses. |
KD—Kawasaki disease.
Clinical features of KD such as prolonged fever, exanthem, enanthem, conjunctival injection, and lymphadenopathy suggest infection. Epidemiologic features, including the young age group affected, the occurrence of epidemics of illness, and the well-documented, geographic, wavelike spread of illness during epidemics [14] also point to an infectious cause. However, no known infectious agent has yet been consistently identified in KD patients. Although some investigators have speculated that a bacterial superantigen causes KD, suppression of specific immune responses as a result of massive stimulation of T lymphocytes, which is the characteristic feature of superantigen-mediated diseases such as toxic shock syndrome, is not observed in KD; in fact, adaptive immune responses seem well-developed, and clonal responses in both B lymphocytes [15, 16] and T lymphocytes [17] are well-documented. A recent study to detect bacterial superantigen genes in stools of children with KD and controls identified more superantigen genes in the stool of KD children than controls [18]. This study was seriously flawed by failure to control for antibiotic use, which is highly likely to affect bacterial flora; failure to determine whether any of the superantigens were actually produced in KD or control children and detectable either in serum or even in the stool; and failure to determine the presence or absence of antibody to the superantigens in the patients, which would have provided information regarding whether patients were susceptible or immune to the effects of the superantigens [18].
The theory that best fits the epidemiologic data is that KD is the result of infection with a very common infectious agent that results in asymptomatic infection in most individuals and KD in genetically susceptible children [19••].
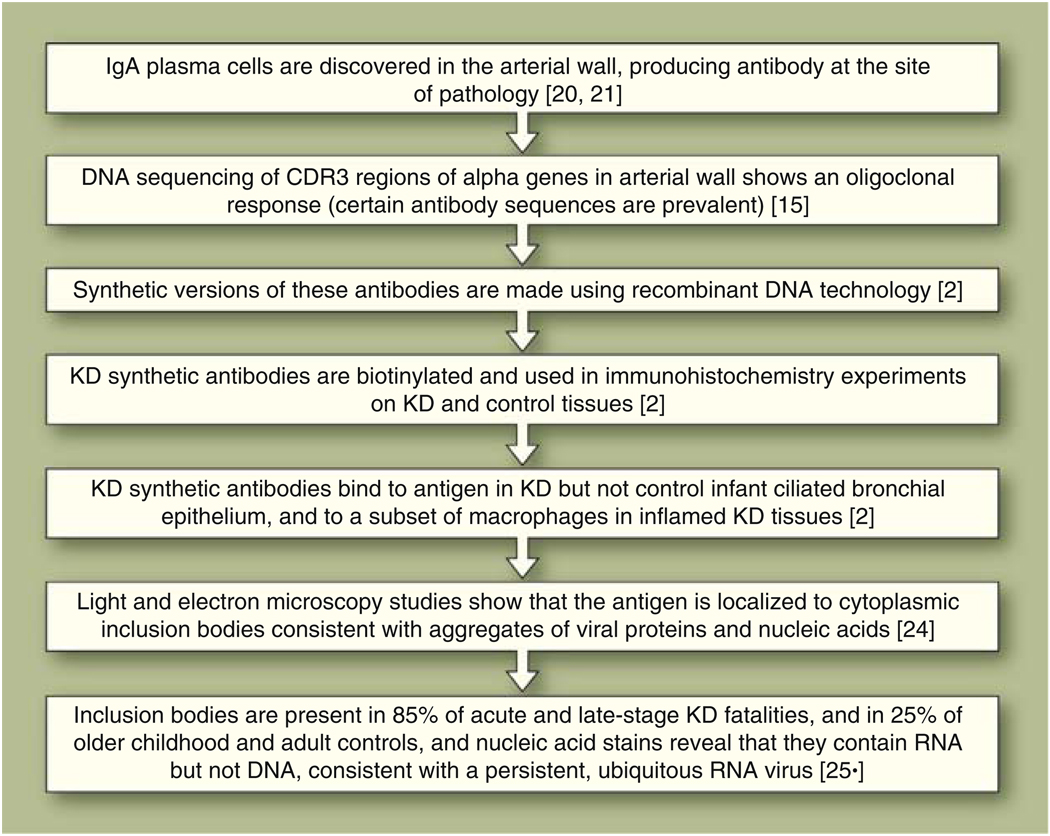
We hypothesized that KD patients make specific antibodies against the etiologic agent of the illness, because production of specific antibody is a consistent feature of infectious diseases. We discovered that IgA plasma cells infiltrated coronary artery aneurysms in acute KD [20], which led to a pathway of discovery with implications for KD etiology and pathogenesis (Fig. 1). This unusual production of IgA antibodies at a nonmucosal, nonlymphoid site suggested to us the possibility that the immune response included the direction of IgA B lymphocytes to the site of tissue pathology to allow for production of pathogen-specific antibody within inflamed KD tissues. We examined the gene sequences of the IgA antibodies in acute-stage KD arterial tissue and determined that a restricted spectrum of antibodies were produced at the site (an oligoclonal pattern), which is a characteristic feature of an antigen-driven immune response [15]. Further study showed that IgA plasma cells also were present in lung, kidney, and pancreas in patients with acute KD [21]. Macrophages and T lymphocytes, especially CD8 T lymphocytes, were also present in inflamed arterial tissue [22]. Neutrophils, the most prevalent cell type in the peripheral blood in acute KD, are much less frequently observed in inflamed KD tissues [8]. The combination of a CD8 T lymphocyte and IgA plasma cell infiltrate in inflamed KD tissues suggests an immune response to an intracellular pathogen (eg, a virus) that enters at a mucosal site.
Fig. 1.
Pathway of discovery from IgA plasma cells to inclusion bodies. KD—Kawasaki disease
To determine what antigen is targeted by the KD-specific IgA antibodies we identified in KD arterial tissue, we produced synthetic versions of the antibodies and used them in immunohistochemistry experiments on KD and control tissues. The synthetic antibodies identified specific antigen in acute KD ciliated bronchial epithelium, but not in control infant bronchial epithelium, and in a subset of macrophages in acute KD tissues [2]. Synthetic antibodies made using IgA gene sequences more prevalent in arterial tissue showed stronger binding to the antigen than did antibodies made using IgA gene sequences less prevalent in the tissue, consistent with an antigen-driven immune response [23]. Antibodies with different complementarity-determining region 3 gene sequences showed binding to the same antigen, confirming the presence of an antigen-driven immune response [23]. Light and electron microscopic study of the antigen-positive tissues revealed that the antigen resides in cytoplasmic inclusion bodies that are consistent with aggregates of viral proteins and nucleic acids [24].
These results support the hypothesis that KD is the result of infection with a previously unidentified ubiquitous respiratory virus that infects ciliated bronchial epithelium and is taken up by tissue macrophages, which enter the circulation and allow the agent to access its target tissues [19••]. We hypothesized that such a ubiquitous agent would be occasionally identified in asymptomatic controls. Therefore, we tested young childhood and adult control lungs and identified cytoplasmic inclusion bodies in bronchial epithelium in 25% of these controls [25•]. Detection of the inclusion bodies in such a high percentage of older childhood and adult controls strongly suggests that the agent can result in persistent infection [25•]. We also tested late-stage KD fatalities to determine whether inclusion bodies could be detected after the acute stage of illness. Cytoplasmic inclusion bodies were detected in 85% of acute and late-stage KD fatalities, again strongly suggesting a persistent infectious etiologic agent. Because KD inclusion bodies were identified using a single monoclonal KD synthetic antibody, these results are not consistent with a theory that multiple disparate pathogens give rise to KD, but rather support the hypothesis that a single agent or a group of very closely related agents causes the illness. Light microscopic studies using nucleic acid stains showed that the inclusion bodies contain RNA but not DNA [25•]. These findings point to a previously unidentified, ubiquitous persistent RNA virus as the etiologic agent of KD [19••, 25•]. High-throughput sequencing of KD tissue complementary DNA (cDNA) with comparison of sequences to computer databases is in progress in several laboratories, in an attempt to find specific sequences of the etiologic agent of KD.
Genetics
Clearly, genetics play a role in susceptibility to KD, because Asian children have the highest attack rates of KD of any ethnic or racial group, with Japanese children having about tenfold increased risk of KD compared with Caucasian children [26, 27]. Early studies of genetic susceptibility to KD generally included small numbers of patients and findings were often not reproducible in other groups of children with KD. More recently, genomewide linkage analysis has been performed to determine potential KD susceptibility loci [28]. These studies led to identification of a polymorphism in the gene ITPKC, which encodes a negative regulator of T-lymphocyte activation, as a KD susceptibility gene and one associated with an increased risk of coronary artery abnormalities in KD in both Japanese and US populations [29•]. However, this gene could only account for a small fraction of KD cases, and it is highly likely that multiple genes are involved in KD susceptibility [30••]. Additional candidate genes were identified recently [31], and it is likely that the field of KD genetics will yield a great deal of new information over the next several years.
Treatment of IVIG Nonresponders
It is truly remarkable that despite the unknown etiology of KD, and therefore a lack of availability of specific therapy, effective therapy consisting of IVIG and aspirin has been developed. This treatment has been shown to reduce the risk of coronary artery abnormalities following the illness from 30% in untreated patients to 5% in those who receive a single 10–12 hr infusion of IVIG, 2 g/kg, with aspirin, 80–100 mg/kg/d, given orally in 4 divided doses within the first 10 days of illness onset [4, 32]. Adding an intravenous dose of corticosteroid to IVIG and aspirin for primary therapy of KD has not been shown to be beneficial [33••]. The mechanism of action of IVIG in KD remains unknown, although many theories have been proposed, including reduction of circulating cytokine levels and provision of specific antibody to the etiologic agent of the disease. Unfortunately, 10–15% of patients with acute KD do not respond to a single dose of IVIG with aspirin, and continue to manifest fever and elevated acute-phase reactants such as serum C-reactive protein and neutrophil count (the sedimentation rate cannot be used to monitor disease activity after IVIG treatment because IVIG therapy increases the sedimentation rate). Other children with KD may show an initial apparent response to IVIG with aspirin, but then have recurrence of fever after a short afebrile period. Prolonged or recurrent fever is the single best predictor of the development of coronary artery abnormalities in KD [34]. The optimal treatment for such patients with “refractory” KD has not been determined. Refractory KD patients are at higher risk for the development of coronary artery abnormalities [5], and effective therapy for these patients is urgently needed. Careful monitoring of fever and performance of serial echocardiograms are important aspects of the management of patients with refractory KD [5].
About 80% of children who do not respond or have an incomplete response to a first infusion of IVIG respond to a second dose of IVIG, 2 g/kg [35, 36]. This is our preferred therapy for those who do not respond to an initial dose of IVIG with aspirin. Other therapeutic options for nonresponders include intravenous pulse steroid infusion and infliximab. Because KD resolves spontaneously with or without therapy, it is critical that studies of new therapies for IVIG nonresponders include a comparison group treated with standard therapies and that they include sufficient numbers of patients, which generally requires a multicenter collaborative trial.
Steroid therapy is commonly used in the treatment of other forms of vasculitis and was used throughout Japan as primary therapy for KD before IVIG was found to be effective treatment [37].Therateofcoronaryartery abnormalities in KD patients treated at several different centers in Japan with prednisolone appeared to be similar (about 25%) to that observed in those treated with aspirin alone [32, 37], and with the availability of IVIG therapy, primary treatment of KD with steroids in Japan has been abandoned in all but a few centers. However, intravenous methylprednisolone has become a commonly administered therapy for IVIG nonresponders and has been associated with apparent therapeutic success in many individual cases [38–41]. Some investigators have reported delayed recurrence of fever in patients treated with intravenous methylprednisolone [38], and others have reported transient dilatation of the coronary artery during pulse steroid therapy [41]. However, this therapy appears to be generally well-tolerated and effective in the subgroup of patients with refractory KD.
Because some patients with acute KD have elevated serum tumor necrosis factor α (TNF-α) levels [42] and such patients may be at increased risk of developing coronary artery abnormalities [43], investigators postulated that infliximab, a chimeric murine/human IgG1 monoclonal antibody that binds specifically to human TNF-α, might be effective therapy in KD. An initial report of apparent efficacy of this treatment in a group of refractory KD patients [44] was followed by a multicenter trial in which a second IVIG infusion was compared with infliximab for initial IVIG nonresponders [45]. This study showed that the two therapies were safe and well-tolerated, and that most patients treated with either therapy appeared to respond. A recent report indicated that 2.3% of US patients with KD received infliximab for IVIG-resistant KD in 2006 [46].
Rarely, particularly refractory KD patients have been treated with other immunosuppressive therapies including cyclophosphamide [36] or methotrexate [47], or with plasma exchange [48]; it is difficult to draw conclusions from these rare individual cases. Additional studies of KD pathogenesis are needed to provide additional therapeutic targets for IVIG nonresponders.
Conclusions
KD is a systemic inflammatory disease of childhood that is the most common cause of acquired heart disease in children in developed nations. It can result in coronary artery aneurysms, myocardial infarction, and sudden death. KD is likely the result of infection with a ubiquitous, persistent, previously unrecognized RNA virus that enters through the respiratory tract and infects ciliated bronchial epithelium, is taken up by macrophages, and enters the circulation. Most individuals are likely infected asymptomatically, and the clinical features of KD occur in a very small subset of genetically predisposed individuals. The presence of the agent in a very small percentage of circulating monocyte/macrophages allows for delivery of the pathogen to its target tissues (eg, the coronary arteries). IgA B lymphocytes and CD8 T lymphocytes participate in an antigen-driven immune response, which is ultimately successful in eradicating the pathogen (IVIG likely contributes to its eradication); however, the coronary arteries can be damaged before the pathogen is eradicated.
The identification of cytoplasmic inclusion bodies consistent with aggregates of viral proteins and nucleic acids in bronchial epithelium of KD patients using a KD synthetic antibody derived from prevalent IgA sequences in the acute KD arterial wall has several implications. First, the antigen-driven IgA immune response in acute KD appears to be targeting a microbial, most likely viral, antigen rather than an autoantigen. Second, proteins and nucleic acids of the agent are likely present within the inclusion bodies. Third, the identification of the inclusion bodies in 85% of KD patients using a single synthetic monoclonal antibody strongly implies that KD results from infection with a single etiologic agent or a group of closely related agents. Identification of the proteins and nucleic acids within the inclusion bodies could lead to discovery of the etiologic agent of KD.
Many genes influence susceptibility to infection with specific pathogens, and it is likely that a group of different genes in combination leads to increased susceptibility to KD. Studies over the next 3–5 years should provide additional information about genetic susceptibility to KD. Additional data regarding the pathogenesis of KD are urgently needed to provide new therapeutic targets for IVIG nonresponders.
Footnotes
Disclosure No potential conflict of interest relevant to this article was reported.
Contributor Information
Anne H. Rowley, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Morton 4-685B, 310 East Superior Street, Chicago, IL 60611, USA, a-rowley@northwestern.edu
Stanford T. Shulman, The Division of Infectious Diseases, The Children’s Memorial Hospital, 2300 Children’s Plaza #20, Chicago, IL 60614, USA, sshulman@northwestern.edu
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 2.Rowley AH, Baker SC, Shulman ST, et al. Detection of antigen in bronchial epithelium and macrophages in acute Kawasaki disease by use of synthetic antibody. J Infect Dis. 2004;190:856–865. doi: 10.1086/422648. [DOI] [PubMed] [Google Scholar]
- 3.Rowley AH. Incomplete (atypical) Kawasaki disease. Pediatr Infect Dis J. 2002;21:563–565. doi: 10.1097/00006454-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 5.Freeman AF, Shulman ST. Refractory Kawasaki disease. Pediatr Infect Dis J. 2004;23:463–464. doi: 10.1097/01.inf.0000125893.66941.e0. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children] Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 7.Landing BH, Larson EJ. Are infantile periarteritis nodosa with coronary artery involvement and fatal mucocutaneous lymph node syndrome the same? Comparison of 20 patients from North America with patients from Hawaii and Japan. Pediatrics. 1977;59:651–662. [PubMed] [Google Scholar]
- 8.Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: I. Pathology and morphogenesis of the vascular changes. Jpn Circ J. 1979;43:633–643. doi: 10.1253/jcj.43.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: II. Distribution and incidence of the vascular lesions. Jpn Circ J. 1979;43:741–748. doi: 10.1253/jcj.43.741. [DOI] [PubMed] [Google Scholar]
- 10.Amano S, Hazama F, Kubagawa H, et al. General pathology of Kawasaki disease. On the morphological alterations corresponding to the clinical manifestations. Acta Pathol Jpn. 1980;30:681–694. [PubMed] [Google Scholar]
- 11.Fatica NS, Ichida F, Engle MA, Lesser ML. Rug shampoo and Kawasaki disease. Pediatrics. 1989;84:231–234. [PubMed] [Google Scholar]
- 12.Ohga K, Yamanaka R, Kinumaki H, et al. Kawasaki disease and rug shampoo. Lancet. 1983;1:930. doi: 10.1016/s0140-6736(83)91358-2. [DOI] [PubMed] [Google Scholar]
- 13.Nash MC, Shah V, Reader JA, Dillon MJ. Anti-neutrophil cytoplasmic antibodies and anti-endothelial cell antibodies are not increased in Kawasaki disease. Br J Rheumatol. 1995;34:882–887. doi: 10.1093/rheumatology/34.9.882. [DOI] [PubMed] [Google Scholar]
- 14.Yanagawa H, Nakamura Y, Kawasaki T, Shigematsu I. Nationwide epidemic of Kawasaki disease in Japan during winter of 1985–86. Lancet. 1986;2:1138–1139. doi: 10.1016/s0140-6736(86)90541-6. [DOI] [PubMed] [Google Scholar]
- 15.Rowley AH, Shulman ST, Spike BT, et al. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001;166:1334–1343. doi: 10.4049/jimmunol.166.2.1334. [DOI] [PubMed] [Google Scholar]
- 16.Lee HH, Shin JS, Kim DS. Immunoglobulin V(H) chain gene analysis of peripheral blood IgM-producing B cells in patients with Kawasaki disease. Yonsei Med J. 2009;50:493–504. doi: 10.3349/ymj.2009.50.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi IH, Chwae YJ, Shim WS, et al. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997;159:481–486. [PubMed] [Google Scholar]
- 18.Suenaga T, Suzuki H, Shibuta S, et al. Detection of multiple superantigen genes in stools of patients with Kawasaki disease. J Pediatr. 2009;155:266–270. doi: 10.1016/j.jpeds.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 19. Rowley AH, Baker SC, Orenstein JM, Shulman ST. Searching for the cause of Kawasaki disease—cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol. 2008;6:394–401. doi: 10.1038/nrmicro1853. This article reviews etiologic, pathologic, and immunologic studies in KD, including the discovery that viral-like cytoplasmic inclusion bodies are present in KD tissues, and proposes a model of KD pathogenesis.
- 20.Rowley AH, Eckerley CA, Jack HM, et al. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997;159:5946–5955. [PubMed] [Google Scholar]
- 21.Rowley AH, Shulman ST, Mask CA, et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis. 2000;182:1183–1191. doi: 10.1086/315832. [DOI] [PubMed] [Google Scholar]
- 22.Brown TJ, Crawford SE, Cornwall ML, et al. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–943. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 23.Rowley AH, Shulman ST, Garcia FL, et al. Cloning the arterial IgA antibody response during acute Kawasaki disease. J Immunol. 2005;175:8386–8391. doi: 10.4049/jimmunol.175.12.8386. [DOI] [PubMed] [Google Scholar]
- 24.Rowley AH, Baker SC, Shulman ST, et al. Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis. 2005;192:1757–1766. doi: 10.1086/497171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rowley AH, Baker SC, Shulman ST, et al. RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001582. This article describes a study demonstrating the presence of RNA-containing cytoplasmic inclusion bodies in 85% of acute and late-stage KD fatalities, and in 25% of adult controls, consistent with the hypothesis that KD is the result of infection with a previously unidentified, ubiquitous, persistent RNA virus.
- 26.Holman RC, Curns AT, Belay ED, et al. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003;112(3 Pt 1):495–501. doi: 10.1542/peds.112.3.495. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura Y, Yashiro M, Uehara R, et al. Epidemiologic features of Kawasaki disease in Japan: results from the nationwide survey in 2005–2006. J Epidemiol. 2008;18:167–172. doi: 10.2188/jea.JE2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onouchi Y, Tamari M, Takahashi A, et al. A genomewide linkage analysis of Kawasaki disease: evidence for linkage to chromosome 12. J Hum Genet. 2007;52:179–190. doi: 10.1007/s10038-006-0092-3. [DOI] [PubMed] [Google Scholar]
- 29. Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. This study shows an association between a polymorphism of ITPKC that results in inefficient downregulation of T-lymphocyte activation and susceptibility both to KD and to the development of coronary artery abnormalities.
- 30. Onouchi Y. Molecular genetics of Kawasaki disease. Pediatr Res. 2009;65(5 Pt 2):46R–54R. doi: 10.1203/PDR.0b013e31819dba60. This article is an outstanding review of the history of genetics studies in KD, and a discussion of future research in this area.
- 31.Burgner D, Davila S, Breunis WB, et al. A genome-wide association study identifies novel and functionally related susceptibility loci for Kawasaki disease. PLoS Genet. 2009;5:e1000319. doi: 10.1371/journal.pgen.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 33. Newburger JW, Sleeper LA, McCrindle BW, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356:663–675. doi: 10.1056/NEJMoa061235. This study demonstrated a lack of efficacy of intravenous methylprednisolone with IVIG and aspirin as compared with IVIG and aspirin alone as primary therapy of KD.
- 34.Asai T. Evaluation method for the degree of seriousness in Kawasaki disease. Acta Paediatr Jpn. 1983;25:170–175. [Google Scholar]
- 35.Sundel RP, Burns JC, Baker A, et al. Gamma globulin re-treatment in Kawasaki disease. J Pediatr. 1993;123:657–659. doi: 10.1016/s0022-3476(05)80972-2. [DOI] [PubMed] [Google Scholar]
- 36.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 37.Kusakawa S, Tatara K. Efficacies and risks of aspirin in the treatment of the Kawasaki disease. Prog Clin Biol Res. 1987;250:401–413. [PubMed] [Google Scholar]
- 38.Furukawa T, Kishiro M, Akimoto K, et al. Effects of steroid pulse therapy on immunoglobulin-resistant Kawasaki disease. Arch Dis Child. 2008;93:142–146. doi: 10.1136/adc.2007.126144. [DOI] [PubMed] [Google Scholar]
- 39.Lang BA, Yeung RS, Oen KG, et al. Corticosteroid treatment of refractory Kawasaki disease. J Rheumatol. 2006;33:803–809. [PubMed] [Google Scholar]
- 40.Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr. 1996;128:146–149. doi: 10.1016/s0022-3476(96)70447-x. [DOI] [PubMed] [Google Scholar]
- 41.Hashino K, Ishii M, Iemura M, et al. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001;43:211–217. doi: 10.1046/j.1442-200x.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 42.Maury CP, Salo E, Pelkonen P. Elevated circulating tumor necrosis factor-alpha in patients with Kawasaki disease. J Lab Clin Med. 1989;113:651–654. [PubMed] [Google Scholar]
- 43.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 44.Burns JC, Mason WH, Hauger SB, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146:662–667. doi: 10.1016/j.jpeds.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Burns JC, Best BM, Mejias A, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153:833–838. doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Son MB, Gauvreau K, Ma L, et al. Treatment of Kawasaki disease: analysis of 27 US pediatric hospitals from 2001 to 2006. Pediatrics. 2009;124:1–8. doi: 10.1542/peds.2008-0730. [DOI] [PubMed] [Google Scholar]
- 47.Lee TJ, Kim KH, Chun JK, Kim DS. Low-dose methotrexate therapy for intravenous immunoglobulin-resistant Kawasaki disease. Yonsei Med J. 2008;49:714–718. doi: 10.3349/ymj.2008.49.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mori M, Imagawa T, Katakura S, et al. Efficacy of plasma exchange therapy for Kawasaki disease intractable to intravenous gamma-globulin. Mod Rheumatol. 2004;14:43–47. doi: 10.1007/s10165-003-0264-3. [DOI] [PubMed] [Google Scholar]
- 49.Terai M, Kohno Y, Niwa K, et al. Imbalance among T-cell subsets in patients with coronary arterial aneurysms in Kawasaki disease. Am J Cardiol. 1987;60:555–559. doi: 10.1016/0002-9149(87)90304-3. [DOI] [PubMed] [Google Scholar]
- 50.Terai M, Yasukawa K, Honda T, et al. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. Pediatr Infect Dis J. 2002;21:777–781. doi: 10.1097/00006454-200208000-00015. [DOI] [PubMed] [Google Scholar]