This study suggests that bladder and rectal dysfunction occur more frequently with colorectal resection in rectal endometriosis compared with excision of the nodules alone.
Keywords: Rectal endometriosis, Deep endometriosis, Excision, Colorectal resection, Complications, Constipation
Abstract
Background:
To evaluate intra- and postoperative complications associated with laparoscopic management of rectal endometriosis by either colorectal segmental resection or nodule excision.
Methods:
During 39 consecutive months, 46 women underwent laparoscopic management of rectal endometriosis and were included in a retrospective comparative study. The distinguishing feature of the study is that the choice of the surgical procedure is not related to the characteristics of the nodule.
Results:
Colorectal segmental resection with colorectal anastomosis was carried out in 15 patients (37%), while macroscopically complete rectal nodule excision was performed in 31 women (63%). No intraoperative complications were recorded. In the colorectal resection group, 3 women (18%) had a bladder atony (spontaneously regressive in 2 women), 4 women (24%) experienced chronic constipation, one had an anastomosis leakage (6%), while 2 women (13%) had acute compartment syndrome with peripheral sensory disturbance. In the nodule excision group, 1 woman (4%) developed transitory right obturator nerve motor palsy. Based on both postoperative pain and improvement in quality of life, all 29 women in the excision group (100%) and 14 women in the colorectal resection group (82%) would recommend the surgical procedure to a friend suffering from the same disease.
Conclusion:
Our study suggests that carrying out colorectal segmental resection in rectal endometriosis is associated with unfavourable postoperative outcomes, such as bladder and rectal dysfunction. These outcomes are less likely to occur when rectal nodules are managed by excision. Information about complications related to both surgical procedures should be provided to patients managed for rectal endometriosis and should be taken into account when a decision is being made about the most appropriate treatment of rectal endometriosis in each case.
INTRODUCTION
Laparoscopic management of rectal endometriosis is carried out worldwide by numerous surgical teams, and several retrospective studies have been published in recent years.1–7 Readers may notice significant variations in terms of the intra- and postoperative complications related to each surgical strategy. The choice of the operative procedure depends on different parameters: size of the nodule, rectal circumference involved by the disease,8 frequency of multifocal intestinal nodules and that of other associated deep lesions,9 and on surgeons' experience and school of thought. In their daily practice, numerous surgeons chose to carry out colorectal resection in more than 90% of women presenting with symptomatic rectal endometriosis, strongly believing that the radical removal of occult endometriotic foci is the most effective way to avoid the risk of recurrences. This choice is supported by studies showing that microscopic endometriotic lesions usually exist around the main rectal nodule.10,11 We recently showed that active glandular endometrial foci are responsible for a deeper infiltration of rectal layers than that of fibrosis and smooth fibers, and thus they are likely to be left out after fibrous nodules excision.12
Alternatively, several experienced teams have chosen to perform primarily nodule excision rather than colorectal resection. As an example, rectal resection has never been performed in a very large series of woman presenting with rectal endometriosis and managed by the team of Jacques Donnez.13 This choice is based on strong arguments: surgical morbidity appears to be higher in women managed by colorectal resection,1,14,15 postoperative functional digestive symptoms seem to be less satisfactory after rectal removal,16,17 microscopic endometriotic foci may still be found on the limits of segmental resection suggesting that microscopically complete resection is always an aim but rarely a reality,18,19 and rectal resection does not avoid postoperative recurrences of pain.19 In addition, the clinical implications of leaving microscopic foci of endometriosis in the digestive tract is unknown,20 because recent data suggest that postoperative continuous medical treatment might be able to halt the progression of occult endometriotic implants and to decrease the risk of recurrences.21
A recent comparative study20 failed to reveal significant differences in the risk of recurrences between women managed by either segmental or discoid resection, whose median follow-up was 33 months. Conversely, the study revealed that segmental resection is associated with a significant risk of bladder dysfunction and a tendency towards constipation. However, patients included in the 2 groups were not totally comparable, because discoid resection was always carried out in women whose nodule size was ≤3cm, while segmental resection was performed in women with larger nodules. Consequently, authors cannot state that performing disc resection in large nodules would probably be associated with less postoperative morbidity than that observed in the group undergoing segmental resection.
The aim of our study was to evaluate intra- and postoperative complications associated with laparoscopic management of rectal endometriosis by segmental resection or nodule excision, carried out uniquely by skilled surgeons in our department. The distinguishing feature of our study is that the choice of surgical procedure is not determined by the characteristics of the nodule.
MATERIALS AND METHODS
We conducted a retrospective study including women who had undergone laparoscopic management for posterior deep endometriosis with rectal involvement, from January 2006 to April 2009. We excluded both women managed by laparotomy and those who had secondary laparoconversion at any point in the procedure. We defined “rectal endometriosis” as being deep posterior endometriosis involving muscular, submucosal or mucosal layers of the rectum, which had been assessed by MRI and endorectal ultrasound examination, which was then intraoperatively confirmed. Data on patient's age, antecedents, previous treatment for endometriosis, intraoperative disease localization, MRI, and endorectal ultrasound examination were prospectively recorded in a computer database. Intra- and postoperative complications were checked from medical charts and specific survey questionnaires focusing on postoperative pelvic pain, digestive and urinary symptoms. In accordance with French regulations, this retrospective study was exempted from IRB approval.
Over the last 5 years, these 2 surgical procedures have been performed by skilled surgeons in our Department of Gynecology and Obstetrics at Rouen University Hospital, France. Before November 2007, rectal endometriosis nodules were systematically removed using colorectal segmental resection followed by colorectal anastomosis and systematic resection of posterior vaginal fornix. This choice was justified by our desire to provide macroscopically complete removal of occult nodules, expected to minimize the risk of rectal recurrences. The surgical procedure on the digestive tract was performed by a digestive surgeon skilled in the laparoscopic approach. To prevent anastomotic leakage of digestive sutures, temporary ileostomy was often performed. During this period, only 3 patients did not benefit from this radical procedure, because they specifically asked to be managed by nodule excision. The colorectal segmental resection procedure was similar to that described in the literature by other teams, while colorectal anastomosis was performed laparoscopically using a single-use circular transanal stapler PCEA 28 device (Figure 1).2,3 Postoperative treatment by GnRH analogs and add-back therapy were systematically prescribed for 3 months to 6 months, followed by continuous contraceptive pill intake in women not intending to conceive.
Figure 1.
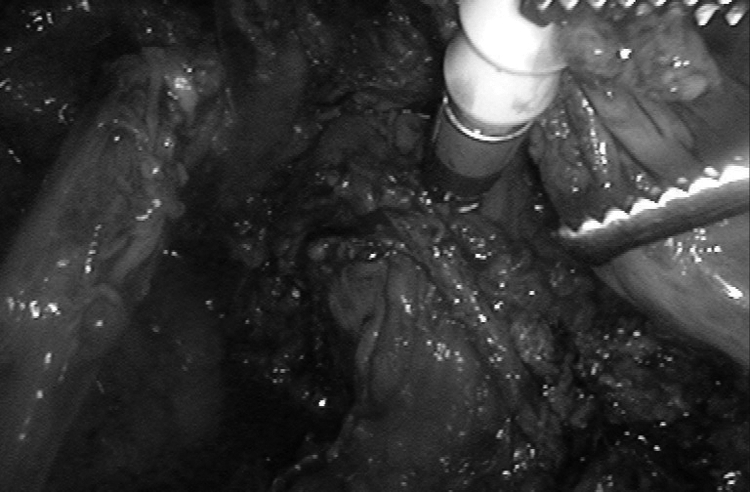
Laparoscopic colorectal anastomosis is performed laparoscopically using a single-use circular transanal stapler PCEA 28 device.
November 2007 marked a change in our choice of surgical procedure and in our thoughts about the disease,22 in response to recent literature data and exchanges with other surgical teams.1,2,16 From this date onward, we came to believe that although with nodule excision the removal of microscopic rectal implants might be incomplete,12 thorough relief of symptoms could durably be obtained by associating prolonged postoperative amenorrhea.23 Similarly, we came to believe that colorectal segmental resection was an overly complex procedure followed in some cases by pelvic nerve damage, which resulted in unpleasant functional urinary and digestive symptoms in young patients.17,19,22 Consequently, the decision was made to perform rectal nodule excision instead of colorectal resection, associated with systematic postoperative treatment by GnRH analogs followed by long-term continuous contraceptive pill intake. From November 2007 to April 2009, colorectal resection was carried out in only 7 nulliparas, because they expressed an intention to conceive a few months after surgery, and these circumstances were incompatible with prolonged postoperative treatment. Conversely, we completely stopped performing colorectal resection in women who did not intend to conceive in the future and who accepted long-term postoperative medical treatment. In the case of elderly women, the postoperative medical treatment was replaced by bilateral adnexectomy.
The surgical procedure involved in rectal nodule excision has already been described12 and is similar to that performed by other surgical teams.1,13,24 Both pararectal spaces are opened below the lateral limits of the rectal nodule, and the rectovaginal space is reached under the inferior limit of the nodule. The nodule is then dissected away from the rectal wall, by using the Ultracision Harmonic Scalpel (Ethicon Endosurgery, Cincinnati, OH, USA) (Figure 2). The dissection is made into the thickness of the rectal wall, to remove all abnormal fibrous lesions involving the rectal layers, using a high magnification endoscopic view. Partial- or full-thickness rectal wall defects are closed laparoscopically in 1 or 2 layers by using resorbable sutures. At the end of the procedure, the site of rectal dissection is recovered by an omentum flap, which is fixed by nonresorbable sutures.12
Figure 2.
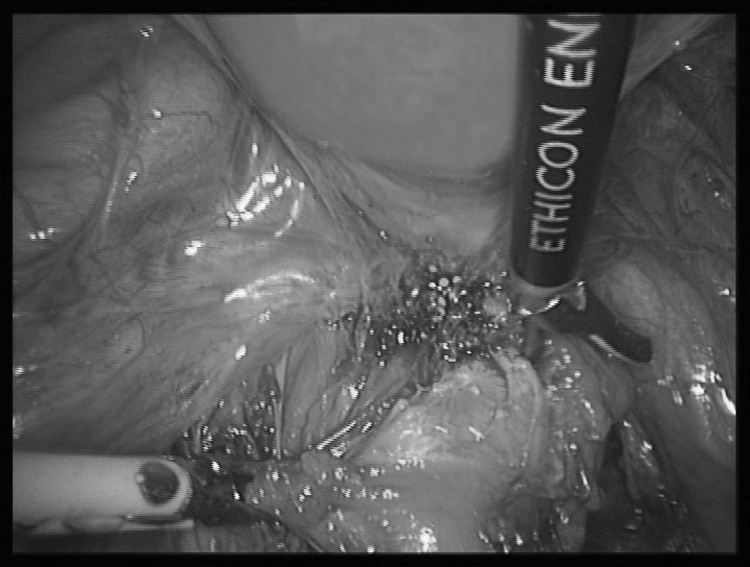
Rectal nodule excision from the anterior rectal wall, using the Ultracision Harmonic Scalpel (Ethicon Endosurgery, Cincinnati, OH, USA).
Statistical analysis was performed using Stata 9.0 Software (Stata Corporation, 4905 Lakeway Drive, TX, USA). Median values, percentiles, range, mean values, and SD were calculated for continuous variables, and percentages for the qualitative variables. Parameter distributions, stratified on surgical procedure were compared by univariate analysis (Fischer's exact test in qualitative parameters and Mann-Whitney U test in continuous variables). P < 0.05 was considered statistically significant.
RESULTS
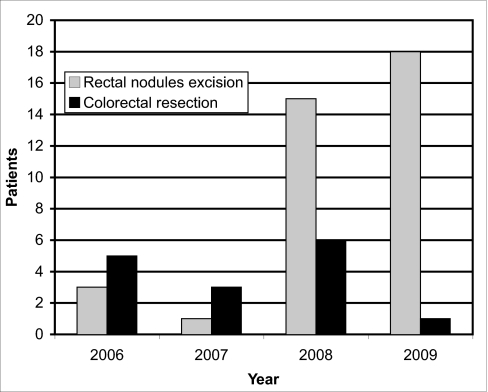
Fifty-four patients were managed for rectal endometriosis during 39 consecutive months. Eight women (15%; 4 women were managed in 2006, 2 in 2007, and 2 in 2008) were excluded from this study: in 4 cases (7.4%) the procedure was carried out entirely by laparotomy, and in another 4 patients (7.4%) laparoconversion was performed during the gynecologic stage due to major adhesions. Thus, 46 women (85%) having benefited from exclusive laparoscopic surgery were included in the study (Figure 3).
Figure 3.
Laparoscopic procedures used in the management of rectal endometriosis.
Patients' characteristics are listed in the Table 1. Colorectal segmental resection with colorectal anastomosis was carried out in 15 patients (33%), while macroscopically complete rectal nodule excision was performed in 31 women (67%). Additional surgical procedures and complications are presented in Table 2. Temporary ileostomy (from 9 to 54 days) was carried out in 14 women (30%) to avoid anastomotic leakage. Posterior vaginal fornix resection was always performed, because vaginal infiltration by advanced deep endometriosis is a consistent event.13,25 Segmental ureteral resection with anastomosis was carried out in 2 patients (4%) presenting with enlarged endometriosis nodules infiltrating both the rectum and the left ureter, while another 2 patients (4%) required cystectomy for bladder endometriosis.
Table 1.
Patients' Characteristics (N = 46)
| N = 46 (100%) | Rectal Nodule Excision N = 31 (67%) | Colorectal Segmental Resection + Colorectal Anastomosis N = 15 (33%) | P | |
|---|---|---|---|---|
| Age | 32.8 ± 5.8 | 33 ± 5.9 | 32.5 ± 6 | 0.76 |
| Parity | 0.94 | |||
| Nulliparous | 33 (72) | 22 (71) | 11 (73) | |
| Multiparous | 13 (28) | 9 (29) | 4 (27) | |
| Pain evaluation using 10-points analog rating scale* | ||||
| Dysmenorrhea | 7.6 ± 1.5 | 7.2 ± 1.8 | 0.60 | |
| Dyspareunia | 5.5 ± 2.9 | 5.4 ± 3.1 | 0.92 | |
| Nonmenstrual pain | 7.9 ± 2.3 | 7 ± 2.2 | 0.46 | |
| Menstrual defecation pain | 46 (100) | 31 (100) | 15 (100) | 1 |
| Endometriosis colorectal nodules (intra-operative finding) | 0.59 | |||
| 1 rectal nodule | 21 (46) | 14 (45) | 7 (47) | |
| 1 rectal nodule + 1 or more nodules involving either rectum or pelvic sigmoid colon | 25 (54) | 17 (55) | 8 (53) |
10-points analog rating scale: 0 = absent, 10 = unbearable.
Table 2.
Laparoscopic Surgical Procedures Used in the Management of Patients (N = 46)
| Surgical Procedures | N = 46 (100%) | Rectal Nodule Excision N = 31 (67%) | Colorectal Segmental Resection + Colorectal Anastomosis N = 15 (33%) | P |
|---|---|---|---|---|
| Posterior vaginal fornix resection + suture | 46 (100) | 31 (100) | 15 (100) | 1 |
| Total hysterectomy | 11 (24) | 9 (29) | 2 (13) | 0.30 |
| Bilateral adnexectomy | 5 (11) | 5 (16) | 0 | 0.16 |
| Unilateral adnexectomy | 4 (9) | 2 (6) | 2 (13) | 0.59 |
| Endometriomas cystectomy | 18 (39) | 12 (39) | 6 (40) | 1 |
| Bladder resection + suture | 2 (4) | 1 (3) | 1 (6) | 0.55 |
| Ureter resection + anastomosis | 2 (4) | 1 (3) | 1 (6) | 0.55 |
| Digestive surgery | ||||
| Temporary ileostomy | 14 (30) | 2 (6) | 12 (80) | <0.001 |
| Sigmoid colon resection above rectosigmoid junction + anastomosis | 4 (13) | * | ||
| Ileum resection + anastomosis | 2 (4) | 1 (3) | 1(6) | 0.55 |
| Appendicectomy | 2 (4) | 1 (3) | 1 (6) | 0.55 |
Colorectal resection had always required the removal of the terminal part of the sigmoid colon, located immediately above the rectosigmoid junction.
No intraoperative complications, such as uncontrolled bleeding, inadvertent ureteral or digestive injuries, were recorded.
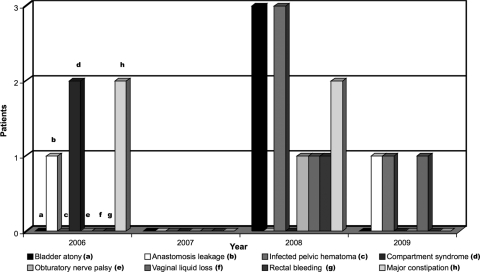
Various immediate postoperative events were observed in 15 patients (33%) (Table 3, Figure 4). Two women (4%) having undergone colorectal segmental resection, and for whom operative time averaged 6 hours, had respectively acute compartment syndrome of the peroneal muscles of the left shank (1 case), and both peroneal muscles of the right shank and posterior muscles of the right forearm (1 case). They required emergency aponeurotomy, and subsequently neither developed neurological motor sequelae. Three women (7%) managed by colorectal resection presented with bladder atony and required routine catheterization for 2, 8, and 30 weeks respectively. Four other women (9%) had postoperative pelvic hematoma associated with fever, secondarily justifying laparoscopic aspiration of hematoma followed by inflammatory syndrome relief. One patient (2%) having undergone segmental resection of the sigmoid colon above the rectosigmoid junction had acute rectorrhagia on day 1, originating from the anastomosis staple line, and required clipping of the bleeding vessel by colonoscopy for control. One woman (2%) had developed an incomplete right obturator nerve motor palsy present 18 months postop, even though repeated electromyography examination ruled out inadvertent section of the nerve. Two women total hysterectomy continuous vaginal discharge. The discharge spontaeously regressed after 3 and 6 weeks. In both cases, bladder and ureteral fistulae were ruled out by computed tomography. It is most likely that the vaginal discharge was lymphatic in origin, secondary to extensive dissection of the deep pelvis and section of small lymphatic vessels. In our sample, neither rectovaginal fistulae nor ureteral fistulae were recorded.
Table 3.
Postoperative Complications Recorded in the Sample (N = 46)
| Complication | N = 46 (100%) | Rectal Nodule Excision N = 31 (67%) | Colorectal Segmental Resection + Colorectal Anastomosis N = 15 (33%) | P |
|---|---|---|---|---|
| Bladder atony > 1 week* | 3 (7) | 0 | 3 (20) | 0.03* |
| Rectovaginal fistulae | 0 | 0 | 0 | 1 |
| Anastomosis/rectal suture leakage | 1 (2) | 0 | 1 (6) | 0.34 |
| Pelvic hematoma requiring secondary surgical procedure | 4 (9) | 3 (10) | 1 (6) | 0.61 |
| Compartment syndromes requiring aponeurotomy | 2 (4) | 0 | 2 (13) | 0.11 |
| Obturator nerve palsy | 1 (2) | 1 (3) | 0 | 1 |
| Postoperative vaginal liquid loss | 2 (2) | 2 (6) | 0 | 1 |
| Rectal bleeding | 1 (2) | 1 (3) | 0 | 1 |
| Major constipation > 1 month* | 4 (9) | 0 | 4 (27) | 0.008* |
Statistically significant.
Figure 4.
Distribution of postoperative complications depending on the time of the surgery.
Delayed complications were reported in 5 patients (11%), all of whom had been managed by colorectal segmental resection. One woman (2%) had peritonitis due to colorectal anastomosis leakage occurring 6 weeks after ileostomy closure. She was managed by a temporary colostomy and secondary suture of the rectum, with a favorable outcome. Four other patients (9%) developed severe constipation (less than 1 stool/5 days), and only one was partially relieved by endoscopic dilatations of colorectal anastomosis.
All patients were asked whether they would recommend the surgical procedure to a friend suffering from the same disease. Based on both postoperative pain and improvement in quality of life, all 31 women in the nodule excision group (100%) and 12 women in the colorectal resection group (80%) answered “yes,” while 2 women in the colorectal resection group (13%) responded negatively for reasons of severe postoperative constipation or left shank compartment syndrome (P=0.09).
To identify independent factors related to delayed constipation and bladder atony, a logistic regression model would have been required. However, the absence of these unfavorable outcomes in the group undergoing nodule excision renders it statistically impossible to estimate brute and adjusted odds ratio.
DISCUSSION
The expected rate of complications represents vital information that should be made available to patients before carrying out complex and difficult surgical procedures, such as rectal endometriosis removal.1 Our study suggests that carrying out colorectal segmental resection is associated with unfavorable postoperative outcomes, such as bladder and rectal dysfunction, which are less likely to occur when rectal nodules are managed by excision.
The main strength of this study is represented by the absence of a relationship between the size or number of rectal nodules and the choice of surgical procedure. We had systematically proposed colorectal segmental resection before November 2007, as do a majority of surgeons who have published their results in the literature.2–4,8,9,14,15,26 Now, we systematically propose rectal nodule excision to avoid postoperative constipation, frequent stools, or dysuria. We are aware that our excision of microscopic foci is not complete,12 but we favor good functional outcomes over radical resection,17 as other experienced surgeons have done for many years.1,24 In addition, the recent randomized study of Seracchioli et al21 may be an argument for conservative surgical procedures, because it suggests that long-term postoperative amenorrhea prevents recurrences due to endometriotic foci left behind.
Fanfani et al20 have recently published a most interesting comparative study, whose objective and results were in the main similar to our own. However, with regards to this Italian study, it must be emphasized that the women who benefited from discoid resection had smaller nodules (<3cm) compared with those of their controls, and this difference may confound both intra- and postoperative outcomes. Conversely, our study probably provides a more accurate comparison of outcomes, because the size and number of nodules have never been criteria in the choice of surgical procedure.
Despite the systematic resection of posterior vaginal fornix, no postoperative rectovaginal fistulae were observed in our sample. Although absent in a few reports,5 this complication is reported by many authors, with a rate varying from 2.3% following rectal nodule excision1 to 10% after colorectal segmental resection.3 In our opinion, the absence of rectovaginal fistulae in our sample could be attributed to the particular care taken to thoroughly close the vaginal wound by laparoscopic suture using resorbable stitches, to separate vaginal suture and the rectal wound by fat tissue such as omentum,14 mesorectum, and not to hesitate performing temporary ileostomies to protect the anastomotic line.
Despite systematic complex dissection of pelvic deep spaces, no ureteral fistulae were recorded. The incidence of this complication during surgery for rectal endometriosis averages 1%.3,14 It is often due to ureteral damage from heat transferred to the ureteral wall, as the surgeon manipulates bipolar or monopolar current close to the ureter.27 We consider therefore that the Ultracision Harmonic Scalpel (Ethicon Endosurgery, Cincinnati, USA) is a useful instrument with which to carry out coagulation and section of tissues surrounding ureters, due to a limitation of the thermal effect at 2mm around the forceps.28
Transitory urinary retention or dysuria, which is generally the result of bladder parasympathetic nerve damage from uterosacral ligament section,1,4 may concern up to 16% of patients managed for rectal endometriosis.2 Although we often section uterosacral ligament insertions on the torus due to frequent infiltration by endometrial disease, care is taken to spare bladder nerves, particularly on the side corresponding to the ligament least affected by the disease. On the other hand, compared with rectal nodule excision, performing colorectal resection requires a more enlarged and deeper dissection of pararectal spaces that could lead to more frequent nerve injuries.
Postoperative compartment syndrome and peroneal nerve injury have rarely been reported by gynecologic surgeons, making it difficult to accurately estimate the risk.29 In a large sample of 192 women managed by colorectal resection for endometriosis, Mereu et al14 recorded 3 patients with persistent postoperative peripheral sensory disturbances. The 2 patients presenting with compartment syndrome in our study underwent long procedures, and their positioning on the table required candy cane stirrups. Our report might be of interest to surgeons carrying out complex laparoscopic procedures that require long operative times and combine laparoscopic and vaginal routes involving a change in patient positioning on the operative table. As regards motor palsy of the right obturator nerve, this appears to be usually associated with prolonged hip flexion stretching the nerve at the bony obturator foramen.29 However, we observed this complication in a woman undergoing rectal nodule excision and laparoscopic hysterectomy, who only had moderate hip flexion and abduction for approximately 3 hours.
Delayed postoperative complications were mainly represented by severe constipation requiring additional endoscopic procedures and radiological and physiological examinations. Persistent constipation was incompletely relieved by endoscopic dilatation of the colorectal anastomosis in one case, but “persisted in 3 patients” in (20% of the segmental resection group) respectively 39, 36, and 10 months after the surgery. Dubernard et al3 also reported that constipation increased in 24% of patients managed by colorectal resection. In a comparative study, Fanfani et al20 observed 4 cases of postoperative constipation following segmental resection versus only 1 case related to discoid excision, but the difference was not statistically significant. In our study, severe constipation was only associated with colorectal resection, and might be explained by the section of pelvic nerves due to extensive dissection around the rectum or colorectal anastomosis stenosis.5,30,31
Contrary to rectal cancer, rectal endometriosis does not threaten patients' lives; however, it is usually extremely damaging to a woman's health and well being. In this article, we chose to focus on the intra- and postoperative complications resulting from the surgical procedure used in the management of rectal endometriosis, because we believe that treating endometriosis should not mean replacing pain with other unpleasant symptoms. We also are aware that a definitive recommendation must take into account the long-term risk of recurrence associated with each surgical procedure. To date, expected recurrence rates related to each surgical procedure appear to be close,20 a comparative study focusing on the risk of recurrences would require several hundreds of patients, with a follow-up of several years.22 To our knowledge, no such randomized or prospective comparative study will be available within the next few years.
Because there are no prospective comparative studies and that recommendations concerning the management of rectal endometriosis are mainly based on retrospective series, we believe that surgeons should let their patients make an informed choice. For instance, they should be aware of the fact that both colorectal resection and rectal nodule excision have been used for many years by experienced teams, with good results in the relief of painful symptoms. They should understand that colorectal resection allows a more extensive excision of microscopic implants occurring on the digestive tract, and that nodule excision is probably incomplete in many cases. Nonetheless, colorectal resection does not avoid recurrences in all cases, and it is very likely that the risk of recurrences could be reduced by postoperative therapeutic amenorrhea. On the other hand, rectal nodule excision allows significant improvement in painful and digestive symptoms, even though microscopic implants are not thoroughly removed in half the cases. It is also very likely that postoperative complications and unpleasant digestive and urinary symptoms would be less frequent when nodule excision is performed instead of colorectal resection.
At this point it is inappropriate to state that recurrences in women treated by long-term postoperative amenorrhea are more frequent than in women who have undergone rectal nodule excision (due to the growth of microscopic implants left on the digestive tract) compared with those who have undergone colorectal resection. By providing all relevant information, the surgeon involves patients and their families in defining the aims, the means, and the strategy of rectal endometriosis management. Elderly women over 40 years or women over 35 with no intention to get pregnant in the future could be more likely than young women to require rectal nodule excision followed by prolonged postoperative amenorrhea. Because no randomized controlled trials are available in the field of rectal endometriosis surgery, we believe that comparative studies like ours are useful in helping one to decide on the most appropriate course of treatment in each individual case, according to patient's willing and informed choice.
Contributor Information
Horace Roman, Department of Gynecology and Obstetrics, University Hospital Charles Nicolle, Rouen, France..
Francisc Rozsnayi, Department of Gynecology and Obstetrics, University Hospital, Targu Mures, Romania..
Lucian Puscasiu, Department of Gynecology and Obstetrics, University Hospital, Targu Mures, Romania..
Benoit Resch, Department of Gynecology and Obstetrics, University Hospital Charles Nicolle, Rouen, France..
Hend Belhiba, Department of Radiology, University Hospital Charles Nicolle, Rouen, France..
Benoit Lefebure, Department of Digestive Surgery, University Hospital Charles Nicolle, Rouen, France..
Michel Scotte, Department of Digestive Surgery, University Hospital Charles Nicolle, Rouen, France..
Francis Michot, Department of Digestive Surgery, University Hospital Charles Nicolle, Rouen, France..
Loïc Marpeau, Department of Gynecology and Obstetrics, University Hospital Charles Nicolle, Rouen, France..
Jean Jacques Tuech, Department of Digestive Surgery, University Hospital Charles Nicolle, Rouen, France..
References:
- 1. Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal. BJOG. 2007;114:1278–1282 [DOI] [PubMed] [Google Scholar]
- 2. Darai E, Thomassin I, Barranger E, et al. Feasibility and clinical outcome of laparoscopic colorectal resection for endometriosis. Am J Obstet Gynecol. 2005;192:394–400 [DOI] [PubMed] [Google Scholar]
- 3. Dubernard G, Piketty M, Rouzier R, Houry S, Bazot M, Darai E. Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod. 2006;21:1243–1247 [DOI] [PubMed] [Google Scholar]
- 4. Dubernard G, Rouzier R, David-Montefiore E, Bazot M, Daraï E. Urinary complications after surgery for posterior deep infiltrating endometriosis are related to the extent of dissection and to uterosacral ligaments resection. J Minim Invasive Gynecol. 2008;15:235–240 [DOI] [PubMed] [Google Scholar]
- 5. Possover M, Diebolder H, Plaul K, Schneider A. Laparascopically assisted vaginal resection of rectovaginal endometriosis. Obstet Gynecol. 2000;96:304–307 [DOI] [PubMed] [Google Scholar]
- 6. Redwine DB, Wright JT. Laparoscopic treatment of complete obliteration of the cul-de-sac associated with endometriosis: long-term follow-up of en bloc resection. Fertil Steril. 2001;76:358–365 [DOI] [PubMed] [Google Scholar]
- 7. Duepree HJ, Senagore AJ, Delaney CP, Marcello PW, Brady KM, Falcone T. Laparoscopic resection of deep pelvic endometriosis with rectosigmoid involvement. J Am Coll Surg. 2002;195:754–758 [DOI] [PubMed] [Google Scholar]
- 8. Abrão MS, Podgaec S, Dias JA, Jr., Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15:280–285 [DOI] [PubMed] [Google Scholar]
- 9. Chapron C, Chopin N, Borghese B, et al. Deeply infiltrating endometriosis: pathogenetic implications of the anatomical distribution. Hum Reprod. 2006;21:1839–1845 [DOI] [PubMed] [Google Scholar]
- 10. Remorgida V, Ragni N, Ferrero S, Anserini P, Torelli P, Fulcheri E. How complete is full thickness disc resection of bowel endometriotic lesions? A prospective surgical and histological study. Hum Reprod. 2005;20:2317–2320 [DOI] [PubMed] [Google Scholar]
- 11. Kavallaris A, Köhler C, Kühne-Heid R, Schneider A. Histopathological extent of rectal invasion by rectovaginal endometriosis. Hum Reprod. 2003;18:1323–1327 [DOI] [PubMed] [Google Scholar]
- 12. Roman H, Opris I, Resch B, Tuech JJ, Sabourin JC, Marpeau L. Histopathologic features of endometriotic rectal nodules and implications on management by rectal nodule excision. Fertil Steril. 2009;92:1250–1252 [DOI] [PubMed] [Google Scholar]
- 13. Donnez J, Nisolle M, Gillerot S, Smets M, Bassil S, Casanas-Roux F. rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynecol. 1997;104:1014–1018 [DOI] [PubMed] [Google Scholar]
- 14. Mereu L, Ruffo G, Landi S, et al. Laparoscopic treatment of deep endometriosis with segmental colorectal resection: short-term morbidity. J Minim Invasive Gynecol. 2007;14:463–469 [DOI] [PubMed] [Google Scholar]
- 15. Daraï E, Bazot M, Rouzier R, Houry S, Dubernard G. Outcome of laparoscopic colorectal resection for endometriosis. Curr Opin Obstet Gynecol. 2007;19:308–313 [DOI] [PubMed] [Google Scholar]
- 16. Ret Davalos ML, De Cicco C, D'Hoore A, De Decker B, Koninckx PR. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33–38 [DOI] [PubMed] [Google Scholar]
- 17. Roman H, Loisel C, Resch B, et al. Delayed functional outcomes associated with surgical management of rectal endometriosis with rectal involvement: giving patients an informed choice. Hum Reprod. 2010;25:890–899 [DOI] [PubMed] [Google Scholar]
- 18. Anaf V, El Nakadi I, De Moor V, Coppens E, Zalcman M, Noel JC. Anatomic significance of a positive barium enema in deep infiltrating endometriosis of the large bowel. World J Surg. 2009;33:822–827 [DOI] [PubMed] [Google Scholar]
- 18a. Roman H, Puscasiu L, Kouteich K, et al. Laparoscopic management of deep endometriosis with rectal affect. Chirurgia. 2007;102:421–428 [PubMed] [Google Scholar]
- 19. Vercellini P, Crosignani PG, Abbiati A, Somigliana E, Viganò P, Fedele L. The effect of surgery for symptomatic endometriosis: the other side of the story. Hum Reprod Update. 2009;15:177–188 [DOI] [PubMed] [Google Scholar]
- 20. Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444–449 [DOI] [PubMed] [Google Scholar]
- 21. Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril. 2010;93:52–56 [DOI] [PubMed] [Google Scholar]
- 22. Roman H, Bourdel N. Against the systematic use of segmental resection in colorectal endometriosis: do not replace the pain by unpleasant digestive symptoms! Gynecol Obstet Fertil. 2009;37:3583–3562 [DOI] [PubMed] [Google Scholar]
- 23. Roman H. Guidelines for the management of painful endometriosis. J Gynecol Obstet Biol Reprod. 2007;36:141–150 [DOI] [PubMed] [Google Scholar]
- 24. Donnez J, Squifflet J. Laparoscopic excision of deep endometriosis. Obstet Gynecol Clin N Am. 2004;31:567–580 [DOI] [PubMed] [Google Scholar]
- 25. Matsuzaki S, Houlle C, Botchorishvili R, Pouly JL, Mage G, Canis M. Excision of the posterior vaginal fornix is necessary to ensure complete resection of rectovaginal endometriotic nodules of more than 2 cm in size. Fertil Steril. 2009;91:1314–1315 [DOI] [PubMed] [Google Scholar]
- 26. Ruffo G, Scopelliti F, Scioscia M, Ceccaroni M, Mainardi P, Minelli L. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63–67 [DOI] [PubMed] [Google Scholar]
- 27. Ostrzenski A, Radolinski B, Ostrzenska KM. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794–799 [DOI] [PubMed] [Google Scholar]
- 28. Harold KL, Pollinger H, Matthews BD, Kercher KW, Sing RF, Heniford BT. Comparison of ultrasonic energy, bipolar thermal energy, and vascular clips for the hemostasis of small-, medium-, and large-sized arteries. Surg Endosc. 2003;17:1228–1230 [DOI] [PubMed] [Google Scholar]
- 29. Barnett JC, Hurd WW, Rogers RM, Jr, Williams NL, Shapiro SA. Laparoscopic positioning and nerve injuries. J Minimal Invasive Gynecol. 2007;14:664–672 [DOI] [PubMed] [Google Scholar]
- 30. Landi S, Ceccaroni M, Perutelli A, et al. Laparoscopic nerve-sparing complete excision of deep endometriosis: is it feasible? Hum Reprod. 2006;21:774–781 [DOI] [PubMed] [Google Scholar]
- 31. Lee WY, Takahashi T, Pappas T, Mantyh CR, Ludwig KA. Surgical autonomic denervation results in altered colonic motility: an explanation for low anterior resection syndrome? Surgery. 2008;143:778–783 [DOI] [PubMed] [Google Scholar]