The authors present a simple technique for anchoring instruments, powered lights, and micro machines through the abdominal wall.
Keywords: Lights, Engines, Natural orifice surgery, Single-port laparoscopy
Abstract
Background and Objectives:
Secured independent tools are being introduced to aid in peritoneoscopy. We present a simple technique for anchoring instruments, powered lights, and micro machines through the abdominal wall.
Methods:
We used a laparoscopic trainer, micro alligator clips with one or two 2-0 nylon tails and cables for engines and lights. The above instruments were introduced via a 12-mm or 15-mm port. Clips were placed for traction, retraction and exposure, lights for illumination, and motors for potential work. A laparoscopy port closure or suture passer was introduced percutaneously to grab and extract the tails or cables outside of the simulated abdominal cavity. The engines and lights were powered by a direct electric current (DC) plugged into exteriorized cables.
Results:
We used 2 to 3 clips for each, and engines performed well.
Conclusion:
This basic simulation adds independent instruments, lights, and engines. We replaced cannulas with threads or cables in an attempt to limit the number of ports. This technique further opens the door for innovations in wired machines in laparoscopy, single-port laparoscopy, or natural orifice surgery.
INTRODUCTION
The field of intraperitoneal operative endoscopy is embarking on the challenge to operate with one or no abdominal skin incisions. To reach this goal, a recent change has taken place in the concept that each instrument must access the abdominal cavity via an additional port.1–3 Multiple instruments, such as magnetic devices, are designed to perform this task; others are being introduced and anchored, screwed or sutured to the peritoneum and fascia. Among these instruments are lights, cameras, graspers, locomotives, knives, and wired robots. Wireless independent tools remain a goal for these innovations.4 Some devices are motorized, requiring DC power. Our experiments with Secured Independent Tools (SIT) provide a simple approach for anchoring instruments and for powering lights and micro engines.
MATERIALS AND METHODS
Our experiments were conducted in simulators. We used a laparoscopic trainer (Lap trainer with SimuVsion, Simualb Corp., Seattle, WA). We built a simulated vaginal port using cardboard at the distal end of the laparoscopy trainer. The cardboard was taped and a canula 12mm or 15mm in diameter was placed in the center of the cardboard. The cannula was used to place a laparoscope with an operating channel. We practiced with alligator clips, mini lamps, a 10cm in length by 10mm in diameter tubular container, and micro motors of 5mm and 12.8mm in diameter. The clips, micro motors, and mini lights are available in electronic supply stores.
The alligator clips have 1 or 2 tails of 2-0 nylon that are 75cm in length. We used a laparoscope with a 6-mm operating channel and a suture passer of 1.6mm in diameter (Figure 1). For the introduction, the alligator clip was put into the operating channel at the tip of the laparoscope leaving it with the jaws open (Figure 2). The alligator clip was directed to a target area to be grasped. A blunt probe was used to push the clip out of the operating channel, and with this action the jaw closed and the target was grasped. We also practiced with 2 cable motors and 4 cable tubular containers. The tubular containers were needed in situations where more than one engine was being used, and for cameras. We used color-coded cables, black holding the front, white for the back, green on the right side, and red on the left side. A port-closure needle or suture-passer needle was placed through the abdominal wall in a percutaneous manner at the chosen area to exteriorize the tails of the clip. The tail was visualized, and the port closure or suture passer grasped the tail and pulled it outside the abdominal wall; the same maneuver was repeated for additional tails or cables.
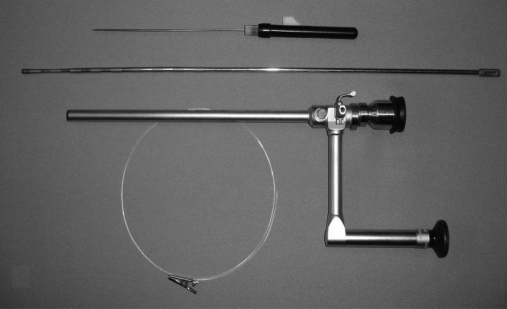
Figure 1.
An operative laparoscope, a probe, and an alligator clip with a tail.
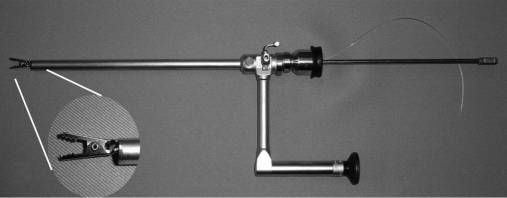
Figure 2.
The alligator clip with open jaws is mounted at the end of the operative channel.
Outside the abdomen, the tail or tails were held as marionette strings with small clamps. To open the clip, a 5-mm diameter clip applier (Thomas clip applier Imanlap, Buenos Aires, Argentina) or strong biopsy forceps was used. The alligator one-tail clip can be moved up and down by pulling or releasing the tail, and a double tail adds a side-to-side movement.
We introduced mini lamps and micro motors and tubular containers by using an operating laparoscope. These devices were held at the tip of the laparoscope with a needle holder mounted inside the operative channel. The cables were placed through the cannula parallel to the laparoscope. The tubular containers and larger micro motors required a 15-mm diameter cannula. The cables were exteriorized by using the port closure or suture passer as previously describe, and held with rubber padded clamps and plugged to DC outlets. These tools were parked against the peritoneal wall and moved to the designated position for work in the simulating area.
RESULTS
These are experiments in the simulator using the alligator graspers. We tried lights and engines for the potential use of the transabdominal plug and outlet. We also worked with 4 string tubular containers for special situations. We were able to aid in triangulation, traction, and retraction with the alligator clips. Our lights and micro engines operated well. The 4-cable tubular containers gave us additional connections for potential uses for either power or transmission signals. In addition, it helps to better control positioning and mobilization.
DISCUSSION
The advances and availability of micro devices allow us to foresee multiple applications for SIT, such as lights, cameras, engines, electrosurgery, locomotives, graspers, small hands, and other such things.1–5 Laparoscopy and minilaparoscopy require one port per instrument. The diameter of a port is larger than the diameter of the instruments used for each particular entrance. SIT uses one entrance for multiple instruments.
Securing specimens and retraction with a leash has been previously done in laparoscopy.6–12 Fausto Davila, MD, uses marionette leashes in single-port laparoscopy and in hybrid and pure culdolaparoscopy.8 We modified an internal laparoscopic magnet grasper by adding a 75-cm tail. This change was originally recommended for transvaginal cholecystectomies as a way to secure or retrieve the internal magnet in case of misplacement during natural orifice surgery.6 The tail left inside the vaginal port was held externally and removed with the magnet at the end of the procedure.11 Meanwhile in SIT, the tails or cables do not occupy the vaginal or single port, because in these cases they will be exteriorized and held outside the skin. We try to limit the number of needle entrances per device, yet some earlier tools will require more than one string or 2 or more cables for DC power. More cables or threads offer some advantages for better control, to prevent instrument loss during surgery, and to adjust to positioning and additional mobilization. Tubular 4-cable containers could hold lanterns, double engine machines, cameras, and other instrument. Several models of port-closure needle or suture passer are on the market, and others are at the experimental level therefore giving options to choose the best suited for each task, considering the necessary safety, sharpness, diameter, and strength. The surgeon or assistant working at the operating table or from a separate console could control the power-plugged devices.
The potential for electrosurgery with SIT could be achieved by minor modifications in the bipolar electrohemostasis catheter and the monopolar flexible Bugbee electrode.
CONCLUSION
Some minimally invasive surgeons are looking beyond traditional laparoscopy to gradually reduce the size and numbers of abdominal ports till the eventual zero.8 To achieve some of such a task with no additional abdominal ports, we are working on the SIT concept of instruments and engines for peritoneoscopy by replacing trocars and cannulas with threads for nonmotorized tools and narrow cables to power engines and lights. The future is focusing on wireless and battery operated micro robot instruments, but today's available and affordable motorized technology is with wired DC power. SIT allows one to operate with multiple secured tools in single-port or natural orifice laparoscopy. The unobstructed entrance could be used for the placement of other rigid, flexible, or large instruments needed in different procedures and for the extraction of specimens.
Contributor Information
Daniel A. Tsin, The Mount Sinai Hospital of Queens, Long Island City, New York, USA..
Fausto Davila, Universidad Autonoma de Mexico, Poza Rica, Veracruz, Mexico..
Guillermo Dominguez, Hospital de Clinicas, Universidad de Buenos Aires, Buenos Aires, Argentina..
Panagiotis Manolas, The Mount Sinai Hospital of Queens, Long Island City, New York, USA..
References:
- 1. Rentschler ME, Dumpert J, Platt SR, Farritor SM, Oleynikov D. Natural orifice Surgery with an endoluminal mobile robot. Surg Endosc. 2007;21:1212–1215 [DOI] [PubMed] [Google Scholar]
- 2. Cadeddu J, Fernandez R, Desai M, et al. Novel magnetically guided intra-abdominal camera to facilitate laparoendoscopic single-site surgery: initial human experience. Surg Endosc. 2009;23(8):1894–1899 Epub 2009 May 9. [DOI] [PubMed] [Google Scholar]
- 3. Fowler DL, Hu T, Nadkarni T, Allen PK, Hogle NJ. Initial trial of a stereoscopic, insertable, remotely controlled camera for minimal access surgery. Surg Endosc. 2010;24(1):9–15 Epub 2009 Jun 11 [DOI] [PubMed] [Google Scholar]
- 4. Forgione A. In vivo micro robotics for natural orifice transluminal surgery. Current status and future perspectives. Surg Oncol. 2009;18:121–129 [DOI] [PubMed] [Google Scholar]
- 5. Maeno T, Hino T. Miniature five-fingered robot hand driven by shape memory alloy activator. Proceedings of 12 IASTED International Conference. 2006 [Google Scholar]
- 6. Dominguez G, Durand L, DeRosa J, Danguise E, Arozema C, Ferrina P. Retraction and triangulation with neodymium magnetic forceps for a single-pot laparoscopy cholecystectomy. Surg Endosc. 2009;23:1660–1666 [DOI] [PubMed] [Google Scholar]
- 7. Ghezzi F, Cromi A, Fasola M, Bolis PF. One trocar salpingectomy for the treatment of tubal pregnancy: A marionette like technique. BJOG. 2005;112:1417–1419 [DOI] [PubMed] [Google Scholar]
- 8. Fausto D, Tsin DA, Dominguez G, Davila U, Jesús R, Arteche AG. Transvaginal cholecystectomy without abdominal ports. JSLS. 2009;13:213–216 [PMC free article] [PubMed] [Google Scholar]
- 9. Duh QY, Way LW. Laparoscopic jejunostomy using T-fasteners as retractors and anchors. Arch Surg. 1993;128:105–108 [DOI] [PubMed] [Google Scholar]
- 10. Tsin DA, Colombero LT. Laparoscopic Leash: A simple technique to prevent specimen loss during laparoscopy. Obst Gynecol. 1999;94:628–629 [DOI] [PubMed] [Google Scholar]
- 11. Tsin DA, Dominguez G, Jesus R, Aguilar S, Davila F. Transvaginal cholecystectomy using magnetic graspers. JMIG. 2008;15:S111–S111 [Google Scholar]
- 12. Tsin DA, Colombero LT, Lambeck J, Manolas P. Minilaparoscopy assisted natural orifice surgery. JSLS. 2007;11:24–29 [PMC free article] [PubMed] [Google Scholar]