Abstract
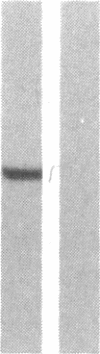
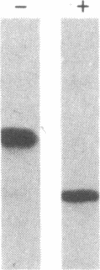
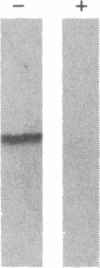
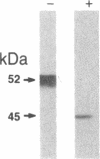
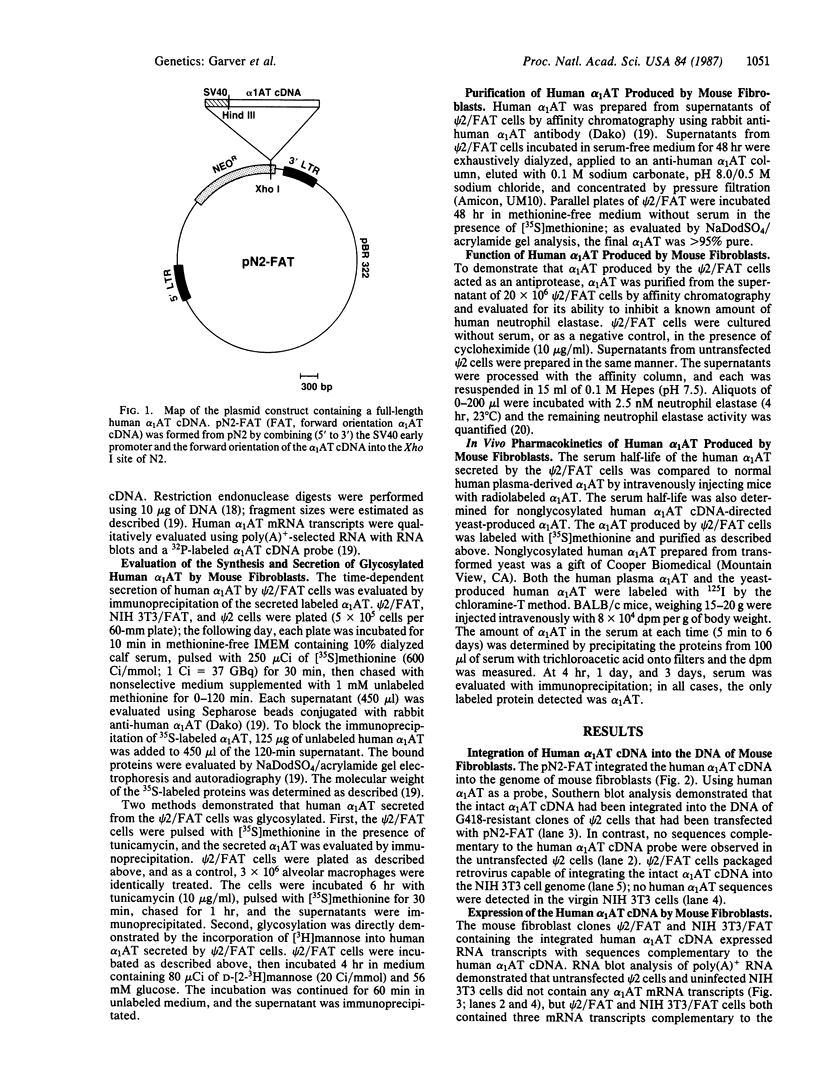
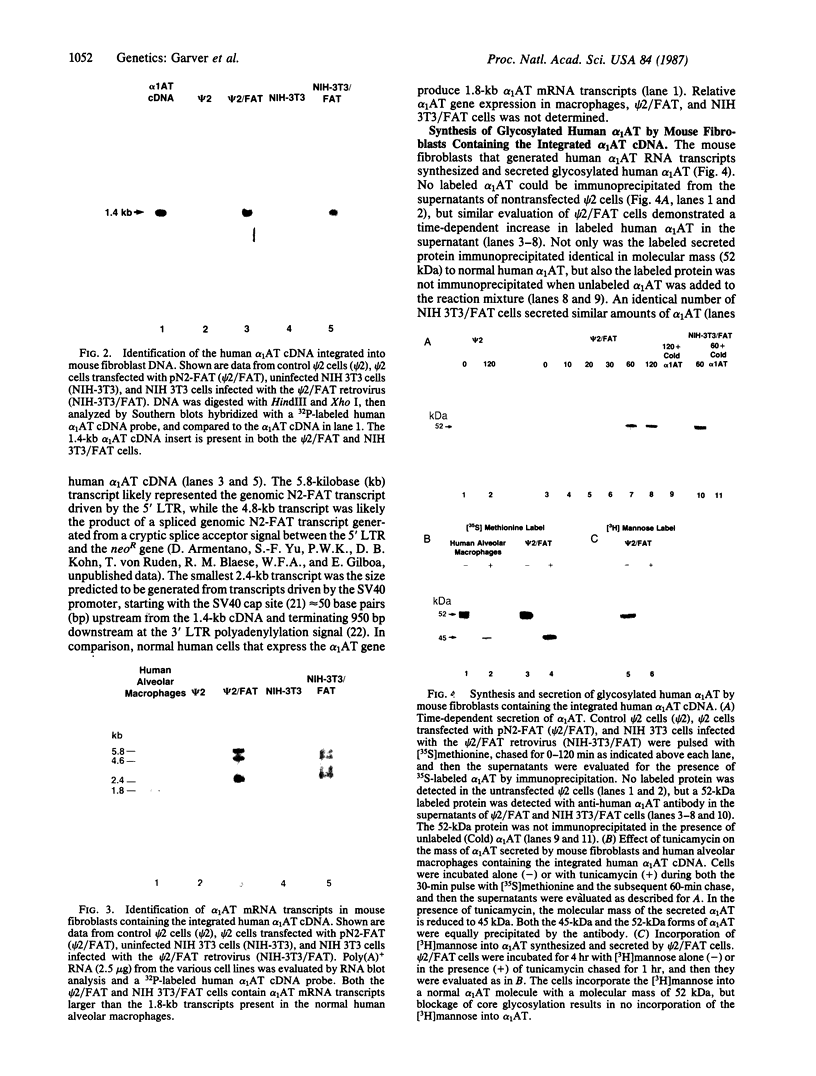
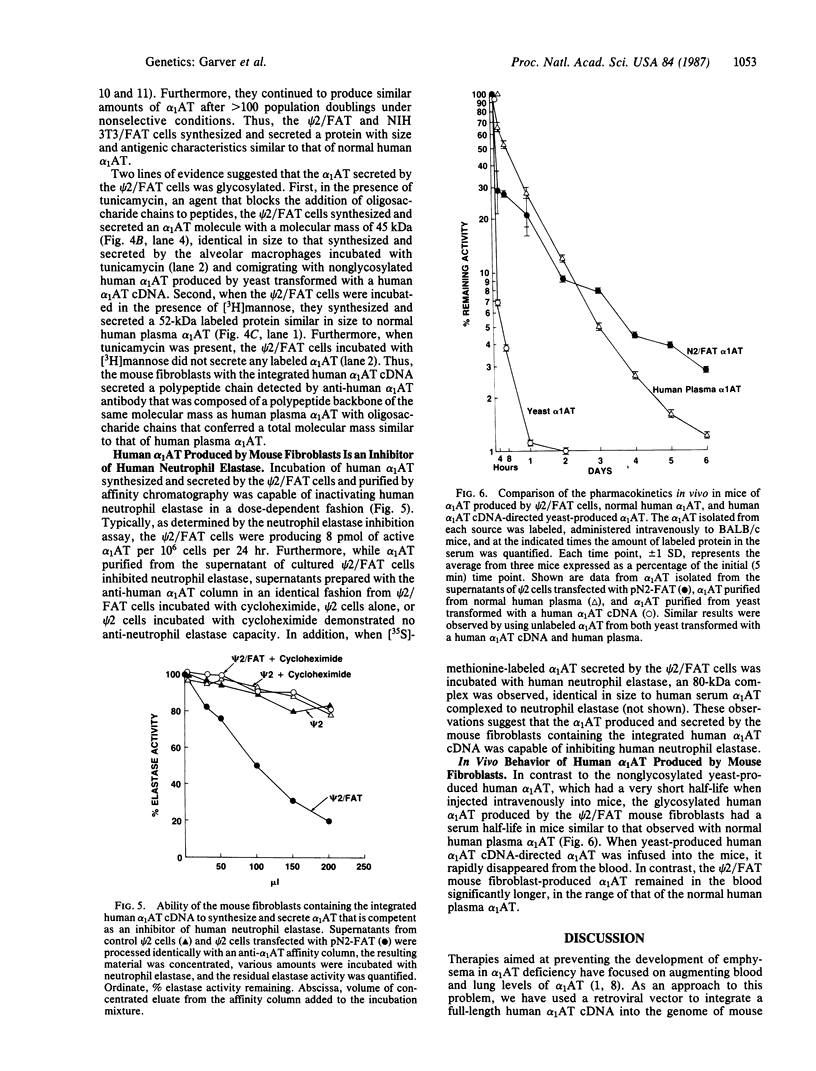
Alpha 1-Antitrypsin (alpha 1AT) deficiency is a hereditary disorder characterized by reduced serum levels of alpha 1AT, resulting in destruction of the lower respiratory tract by neutrophil elastase. As an approach to augment alpha 1AT levels in this disorder with physiologically normal human alpha 1AT, we have integrated a full-length normal human alpha 1AT cDNA into the genome of mouse fibroblasts. To accomplish this, the retroviral vector N2 was modified by inserting the simian virus 40 early promoter followed by the alpha 1AT cDNA. Southern analysis demonstrated that the intact cDNA was present in the genome of selected clones of the transfected murine fibroblasts psi 2 and infected NIH 3T3. The clones produced three mRNA transcripts (5.8, 4.8, and 2.4 kilobases) containing human alpha 1AT sequences, secreted an alpha 1AT molecule recognized by an anti-human alpha 1AT antibody, with the same molecular mass (52 kDa) as normal human alpha 1AT and that complexed with and inhibited human neutrophil elastase. The psi 2 produced alpha 1AT was glycosylated, and when infused intravenously into mice, it had a serum half-life similar to normal alpha 1AT purified from human plasma and markedly longer than that of nonglycosylated human alpha 1AT cDNA-directed yeast-produced alpha 1AT. These studies demonstrate the feasibility of using a retroviral vector to insert the normal human alpha 1AT cDNA into non-alpha 1AT-producing cells, resulting in the synthesis and secretion of physiologically "normal" human alpha 1AT.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. doi: 10.1073/pnas.77.7.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek J. E., Klein H. G., Holland P. V., Crystal R. G. Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981 Nov;68(5):1158–1165. doi: 10.1172/JCI110360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Janoff A., White R., Carp H., Harel S., Dearing R., Lee D. Lung injury induced by leukocytic proteases. Am J Pathol. 1979 Oct;97(1):111–136. [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Black L. F. Alpha1-antitrypsin and its deficiency. Am Rev Respir Dis. 1974 Aug;110(2):176–194. doi: 10.1164/arrd.1974.110.2.176. [DOI] [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mega T., Lujan E., Yoshida A. Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. II. Structure of oligosaccharides. J Biol Chem. 1980 May 10;255(9):4057–4061. [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornex J. F., Chytil-Weir A., Martinet Y., Courtney M., LeCocq J. P., Crystal R. G. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986 Jun;77(6):1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltini C., Hance A. J., Ferrans V. J., Basset F., Bitterman P. B., Crystal R. G. Accurate quantification of cells recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1984 Oct;130(4):650–658. doi: 10.1164/arrd.1984.130.4.650. [DOI] [PubMed] [Google Scholar]
- Shimada T., Nienhuis A. W. Only the promoter region of the constitutively expressed normal and amplified human dihydrofolate reductase gene is DNase I hypersensitive and undermethylated. J Biol Chem. 1985 Feb 25;260(4):2468–2474. [PubMed] [Google Scholar]
- Straus S. D., Fells G. A., Wewers M. D., Courtney M., Tessier L. H., Tolstoshev P., Lecocq J. P., Crystal R. G. Evaluation of recombinant DNA-directed E.coli produced alpha 1-antitrypsin as an anti-neutrophil elastase for potential use as replacement therapy of alpha 1-antitrypsin deficiency. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1177–1184. doi: 10.1016/0006-291x(85)91739-5. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]